Current and Emerging Sedation Practices for Colonoscopy: A Narrative Review of Pharmacological Agents, High-Risk Populations, and Safety Considerations
Abstract
1. Introduction
2. Materials and Methods
3. Mechanisms of Pain During Colonoscopy
3.1. Physiological Basis of Pain Perception
- Mesenteric Traction: Excessive looping of the colonoscope can stretch the mesentery, activating mechanoreceptors in the visceral peritoneum. This form of pain is often described as deep and poorly localized [5],
- Neurogenic Inflammation: In some patients, repeated stimulation of nociceptors may lead to the release of inflammatory mediators such as substance P and calcitonin gene-related peptide (CGRP). These neuropeptides enhance pain sensitivity by promoting local inflammation and neuronal excitability [6,7].
3.2. Neural Pathways and Pain Modulation
- Visceral Afferent Pathways: The primary sensory inputs from the colon travel via the pelvic and splanchnic nerves to spinal segments T10-L2 and S2-S4. From there, they ascend to the brainstem and thalamus, where they are relayed to higher cortical centers involved in pain perception [8],
- Spinal and Supraspinal Processing: Within the spinal cord, nociceptive signals undergo modulation by excitatory and inhibitory neurotransmitters. In some individuals, hyperexcitability of dorsal horn neurons may contribute to heightened pain responses, a phenomenon known as central sensitization [9],
- Cortical and Subcortical Processing: The anterior cingulate cortex, insula, and prefrontal cortex play key roles in integrating pain-related information with emotional and cognitive factors. Anxiety and anticipation of pain can lead to increased activity in these regions, enhancing pain perception [4,5,10].
3.3. Factors Influencing Pain Severity
- Patient-Related Factors:
- Sex Differences: Women generally report higher pain scores than men, potentially due to hormonal influences on visceral sensitivity [10],
- Age and Comorbidities: Older adults may have reduced visceral sensitivity, whereas patients with IBS, IBD, or prior abdominal surgeries may experience heightened pain due to altered pain processing. Patients with IBD, such as Crohn’s disease and ulcerative colitis, have inflamed mucosa with heightened sensitivity, making them more prone to pain during endoscopic contact. The presence of ulcers, erosions, or prior radiation damage can further increase pain perception due to exposure of nerve endings and altered mucosal integrity. Patients with chronic opioid use may experience increased pain during the colonoscopy due to opioid-induced hyperalgesia [3,10,11].
- Colonic Anatomical Variability:
- Colon Length and Mobility: Redundant or elongated colons, commonly seen in women and elderly patients, can increase the risk of looping and mesenteric traction [12],
- Endoscopic Technique:
- Scope Insertion and Loop Formation: Excessive looping of the colonoscope within the sigmoid or transverse colon can generate unnecessary stretching forces, exacerbating pain [12],
- Insufflation Type: While air insufflation is traditionally used, CO2 insufflation has been found to cause less discomfort due to its rapid absorption from the bowel [13],
- Polypectomy and biopsy: It causes localized tissue trauma, leading to transient pain and an inflammatory response. Post-polypectomy coagulation syndrome occurs due to thermal injury extending into the colonic wall, resulting in transmural burns that can cause delayed visceral pain and mimic peritonitis [11],
- Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), which involve deeper tissue resection, can activate pain pathways more intensely than standard biopsy procedures [11].
4. Sedation and Anesthesia for Colonoscopy
4.1. General Considerations
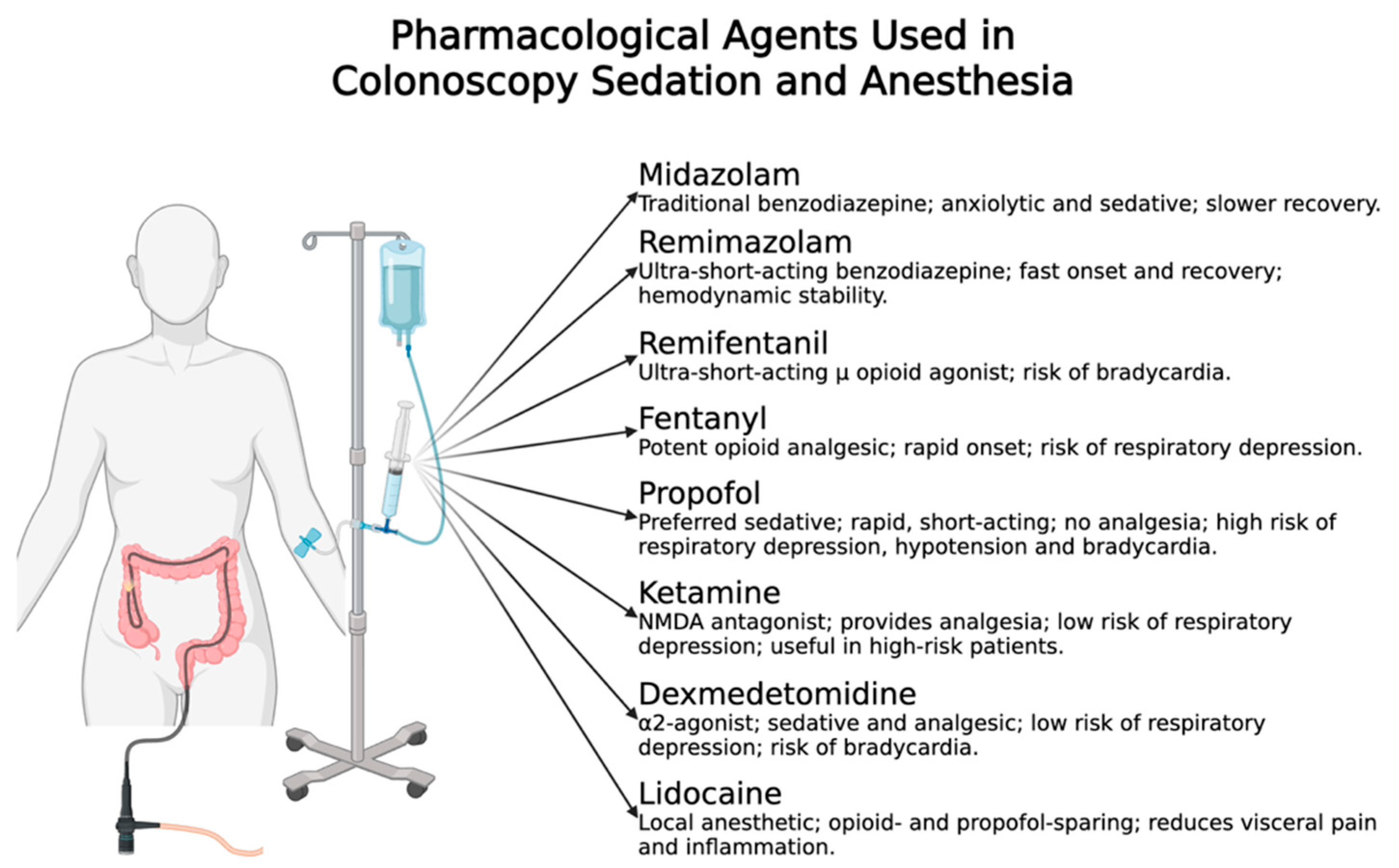
4.2. Pharmacological Agents Used in Colonoscopy Sedation and Anesthesia
4.2.1. Benzodiazepines
Midazolam
Remimazolam
4.2.2. Opioids
Fentanyl
Remifentanil
4.2.3. Propofol
- Respiratory depression and apnea (most common dose-limiting effect)
- Hypotension and bradycardia (secondary to systemic vasodilation)
- Injection site pain (mitigated by co-administration of lidocaine) [22].
4.2.4. Ketamine
4.2.5. Dexmedetomidine
4.2.6. Lidocaine
4.3. Comparative Efficacy and Safety of Sedation Regimens
4.3.1. Hemodynamic Stability
4.3.2. Respiratory Safety
4.3.3. Recovery Profile and Cognitive Outcomes
4.3.4. Analgesia, Procedural Conditions, and Satisfaction
4.3.5. Conclusion and Ideal Clinical Scenarios
- For healthy, low-risk patients (ASA I-II), propofol monotherapy remains a highly effective option, providing rapid onset, excellent procedural conditions, and high patient satisfaction.
- For patients with significant cardiovascular risk, remimazolam emerges as the preferred agent due to its superior hemodynamic stability and significantly reduced risk of hypotension and bradycardia.
- For patients with severe respiratory disease (e.g., OSA, COPD), a regimen based on dexmedetomidine, remimazolam, or a propofol-ketamine combination offers the greatest margin of safety due to minimal respiratory depression.
- To achieve optimal procedural conditions for the endoscopist, especially during complex procedures, a combination of propofol with a short-acting opioid (fentanyl/remifentanil) or ketamine provides the most stable field and highest endoscopist satisfaction.
- The traditional midazolam-fentanyl regimen, given its less favorable safety and recovery profile, should be considered a second-line option when modern alternatives are unavailable or contraindicated.
5. Anesthesia-Related Complications in Colonoscopy: Risks, Management, and Advances in Sedation Strategies
6. High-Risk Patient Populations
6.1. Obesity and Obstructive Sleep Apnea (OSA)
6.2. Cardiovascular Diseases
6.3. Respiratory Diseases
6.4. Elderly and Frail Patients
7. Patient Monitoring During Colonoscopy
7.1. Basic Physiological Monitoring
7.2. Depth of Sedation Monitoring
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| BIS | Bispectral Index |
| BMI | Body Mass Index |
| CO2 | Carbon Dioxide |
| CGRP | Calcitonin Gene-Related Peptide |
| COPD | Chronic Obstructive Pulmonary Disease |
| EMR | Endoscopic Mucosal Resection |
| ESD | Endoscopic Submucosal Dissection |
| GI | Gastrointestinal |
| MAC | Minimum alveolar concentration |
| MOAA/S | Modified Observer’s Assessment of Alertness/Sedation |
| NMDA | N-Methyl-D-Aspartate |
| OIH | Opioid-Induced Hyperalgesia |
| OSA | Obstructive Sleep Apnea |
| PSI | Patient State Index |
| TCI | Target-Controlled Infusion |
References
- Kanth, P.; Inadomi, J.M. Screening and Prevention of Colorectal Cancer. BMJ 2021, 374, n1855. [Google Scholar] [CrossRef]
- Trummel, J.M.; Chandrasekhara, V.; Kochman, M.L. Anesthesia for Colonoscopy and Lower Endoscopic Procedures. Anesthesiol. Clin. 2017, 35, 679–686. [Google Scholar] [CrossRef]
- Trevisani, L. Colonoscopy, Pain and Fears: Is It an Indissoluble Trinomial? World J. Gastrointest. Endosc. 2014, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; La, J.H.; Schwartz, E.S.; Gebhart, G.F. Irritable Bowel Syndrome: Methods, Mechanisms, and Pathophysiology. Neural and Neuro-Immune Mechanisms of Visceral Hypersensitivity in Irritable Bowel Syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1085–G1098. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Hongo, M.; Fukudo, S. Visceral Hypersensitivity in Irritable Bowel Syndrome. J. Gastro. Hepatol. 2011, 26, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef]
- Gebhart, G.F. Visceral Pain—Peripheral Sensitisation. Gut 2000, 47, iv54–iv55. [Google Scholar] [CrossRef]
- Mayer, E.A.; Gebhart, G.F. Basic and Clinical Aspects of Visceral Hyperalgesia. Gastroenterology 1994, 107, 271–293. [Google Scholar] [CrossRef]
- Naliboff, B.D.; Munakata, J.; Chang, L.; Mayer, E.A. Toward a Biobehavioral Model of Visceral Hypersensitivity in Irritable Bowel Syndrome. J. Psychosom. Res. 1998, 45, 485–492. [Google Scholar] [CrossRef]
- Benson, S.; Kattoor, J.; Kullmann, J.S.; Hofmann, S.; Engler, H.; Forsting, M.; Gizewski, E.R.; Elsenbruch, S. Towards Understanding Sex Differences in Visceral Pain: Enhanced Reactivation of Classically-Conditioned Fear in Healthy Women. Neurobiol. Learn Mem. 2014, 109, 113–121. [Google Scholar] [CrossRef]
- Hurtado-Lorenzo, A.; Honig, G.; Weaver, S.A.; Larkin, P.B.; Heller, C. Chronic Abdominal Pain in IBD Research Initiative: Unraveling Biological Mechanisms and Patient Heterogeneity to Personalize Treatment and Improve Clinical Outcomes. Crohn’s Colitis 360 2021, 3, otab034. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 153, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Memon, M.A.; Memon, B.; Yunus, R.M.; Khan, S. Carbon Dioxide Versus Air Insufflation for Elective Colonoscopy: A Meta-Analysis and Systematic Review of Randomized Controlled Trials. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Dossa, F.; Dubé, C.; Tinmouth, J.; Sorvari, A.; Rabeneck, L.; McCurdy, B.R.; Dominitz, J.A.; Baxter, N.N. Practice Recommendations for the Use of Sedation in Routine Hospital-Based Colonoscopy. BMJ Open Gastroenterol. 2020, 7, e000348. [Google Scholar] [CrossRef] [PubMed]
- Goudra, B.; Gouda, G.; Mohinder, P. Recent Developments in Drugs for GI Endoscopy Sedation. Dig. Dis. Sci. 2020, 65, 2781–2788. [Google Scholar] [CrossRef]
- Lee, A.; Shirley, M. Remimazolam: A Review in Procedural Sedation. Drugs 2021, 81, 1193–1201. [Google Scholar] [CrossRef]
- Gupta, K.; Nagappa, M.; Prasad, A.; Abrahamyan, L.; Wong, J.; Weingarten, T.N.; Chung, F. Risk Factors for Opioid-Induced Respiratory Depression in Surgical Patients: A Systematic Review and Meta-Analyses. BMJ Open 2018, 8, e024086. [Google Scholar] [CrossRef]
- Kumar, K.; Kirksey, M.A.; Duong, S.; Wu, C.L. A Review of Opioid-Sparing Modalities in Perioperative Pain Management: Methods to Decrease Opioid Use Postoperatively. Anesth. Analg. 2017, 125, 1749–1760. [Google Scholar] [CrossRef]
- Niccum, B.; Moninuola, O.; Miller, K.; Khalili, H. Opioid Use Among Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 895–907.e4. [Google Scholar] [CrossRef]
- Akcaboy, Z.N.; Akcaboy, E.Y.; Albayrak, D.; Altinoren, B.; Dikmen, B.; Gogus, N. Can Remifentanil Be a Better Choice than Propofol for Colonoscopy during Monitored Anesthesia Care? Acta Anesthesiol. Scand. 2006, 50, 736–741. [Google Scholar] [CrossRef]
- Moerman, A.T.; Herregods, L.L.; De Vos, M.M.; Mortier, E.P.; Struys, M.M.R.F. Manual versus Target-Controlled Infusion Remifentanil Administration in Spontaneously Breathing Patients. Anesth. Analg. 2009, 108, 828–834. [Google Scholar] [CrossRef]
- Deegan, R.J. Propofol: A Review of the Pharmacology and Applications of an Intravenous Anesthetic Agent. Am. J. Med. Sci. 1992, 304, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Cuiabano, I.S.; de Miranda Garbin, P.; Módolo, N.S.P.; do Nascimento, P. Safety and Efficacy of Target-Controlled Infusion versus Intermittent Bolus Administration of Propofol for Sedation in Colonoscopy: A Randomized Controlled Trial. Braz. J. Anesthesiol. 2023, 73, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Barrett, W.; Buxhoeveden, M.; Dhillon, S. Ketamine: A Versatile Tool for Anesthesia and Analgesia. Curr. Opin. Anesthesiol. 2020, 33, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Dexmedetomidine: Present and Future Directions. Korean J. Anesthesiol. 2019, 72, 323–330. [Google Scholar] [CrossRef]
- Kavousi, E.; Shariefnia, H.R.; Pourfakhr, P.; Khajavi, M.; Behseresht, A. Dexmedetomidine versus Propofol in Combination with Fentanyl for Sedation-Analgesia in Colonoscopy Procedures: A Randomized Prospective Study. Middle East J. Dig. Dis. 2021, 13, 328–332. [Google Scholar] [CrossRef]
- Beaussier, M.; Delbos, A.; Maurice-Szamburski, A.; Ecoffey, C.; Mercadal, L. Perioperative Use of Intravenous Lidocaine. Drugs 2018, 78, 1229–1246. [Google Scholar] [CrossRef]
- Gross, J.B.; Caldwell, C.B.; Shaw, L.M.; Laucks, S.O. The Effect of Lidocaine on the Ventilatory Response to Carbon Dioxide. Anesthesiology 1983, 59, 521–525. [Google Scholar] [CrossRef]
- Forster, C.; Vanhaudenhuyse, A.; Gast, P.; Louis, E.; Hick, G.; Brichant, J.-F.; Joris, J. Intravenous Infusion of Lidocaine Significantly Reduces Propofol Dose for Colonoscopy: A Randomised Placebo-Controlled Study. Br. J. Anesth. 2018, 121, 1059–1064. [Google Scholar] [CrossRef]
- Chudziński, K.; Szułdrzyński, K.; Jankowski, M.; Adamczyk, K.; Sokół-Kobielska, E. Comparison of Fentanyl, Ketamine, and Lidocaine Combined with Propofol Anesthesia in Patients with Crohn Disease Undergoing Colonoscopy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2024, 30, e944116-1. [Google Scholar] [CrossRef]
- Ulmer, B.J.; Hansen, J.J.; Overley, C.A.; Symms, M.R.; Chadalawada, V.; Liangpunsakul, S.; Strahl, E.; Mendel, A.M.; Rex, D.K. Propofol versus Midazolam/Fentanyl for Outpatient Colonoscopy: Administration by Nurses Supervised by Endoscopists. Clin. Gastroenterol. Hepatol. 2003, 1, 425–432. [Google Scholar] [CrossRef]
- Vargo, J.J.; Niklewski, P.J.; Williams, J.L.; Martin, J.F.; Faigel, D.O. Patient Safety during Sedation by Anesthesia Professionals during Routine Upper Endoscopy and Colonoscopy: An Analysis of 1.38 Million Procedures. Gastrointest. Endosc. 2017, 85, 101–108. [Google Scholar] [CrossRef]
- Koo, B.-W.; Na, H.-S.; Park, S.-H.; Bang, S.; Shin, H.-J. Comparison of the Safety and Efficacy of Remimazolam and Propofol for Sedation in Adults Undergoing Colonoscopy: A Meta-Analysis of Randomized Controlled Trials. Medicina 2025, 61, 646. [Google Scholar] [CrossRef] [PubMed]
- Ahmer, W.; Imtiaz, S.; Alam, D.M.; Ahmed, K.; Sajid, B.; Yousuf, J.; Asnani, S.; Fahim, M.A.A.; Ali, R.; Mansoor, M.; et al. Remimazolam versus Propofol for Sedation in Gastrointestinal Endoscopy and Colonoscopy within Elderly Patients: A Meta-Analysis of Randomized Controlled Trials. Eur. J. Clin. Pharmacol. 2024, 80, 493–503. [Google Scholar] [CrossRef]
- Kan, Z.; Min, W.; Dai, Y.; Zhang, P. Intravenous Esketamine as an Adjuvant for Sedation/Analgesia Outside the Operating Room: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2024, 15, 1287761. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J.E.; Tanner, J.W.; Lichtenstein, G.R.; Metz, D.C.; Katzka, D.A.; Ginsberg, G.G.; Kochman, M.L. A Randomized, Controlled, Double-Blind Trial of Patient-Controlled Sedation with Propofol/Remifentanil Versus Midazolam/Fentanyl for Colonoscopy. Anesth. Analg. 2008, 106, 434. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, J.; Hu, Z.; Hou, Q.; Hu, Q.; Dai, Z.; Zhou, W.; Qi, D.; Li, Y.; Wang, Q.; et al. The Efficacy and Safety of Remimazolam in Painless Colonoscopy: A Prospective, Randomized Clinical Trial. Front. Med. 2024, 11, 1434767. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Nakamura, M.; Watanabe, O.; Yamamura, T.; Funasaka, K.; Ohno, E.; Kawashima, H.; Miyahara, R.; Goto, H.; Hirooka, Y. Dexmedetomidine Provides Less Body Motion and Respiratory Depression during Sedation in Double-Balloon Enteroscopy than Midazolam. SAGE Open Med. 2017, 5, 2050312117729920. [Google Scholar] [CrossRef]
- Chang, Y.; Huang, Y.-T.; Chi, K.-Y.; Huang, Y.-T. Remimazolam versus Propofol for Procedural Sedation: A Meta-Analysis of Randomized Controlled Trials. PeerJ 2023, 11, e15495. [Google Scholar] [CrossRef]
- Tang, R.; Huang, Y.; Zhang, Y.; Ma, X.; Yu, H.; Song, K.; Ren, L.; Zhao, B.; Wang, L.; Zheng, W. Efficacy and Safety of Sedation with Dexmedetomidine in Adults Undergoing Gastrointestinal Endoscopic Procedures: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2023, 14, 1241714. [Google Scholar] [CrossRef]
- Thompson, R.; Seck, V.; Riordan, S.; Wong, S. Comparison of the Effects of Midazolam/Fentanyl, Midazolam/Propofol, and Midazolam/Fentanyl/Propofol on Cognitive Function After Gastrointestinal Endoscopy. Surg. Laparosc. Endosc. Percutaneous Tech. 2019, 29, 441. [Google Scholar] [CrossRef]
- Li, T.; Han, W.; Yang, X.; Wang, Y.; Peng, L.; He, L.; Hu, L.; Liu, J.; Xia, M.; Wang, S. Effects of Different Injection Rates of Propofol on Postoperative Cognition in Elderly Patients Undergoing Laparoscopic Inguinal Hernia Repair. Drug Des. Dev. Ther. 2023, 17, 1741–1752. [Google Scholar] [CrossRef]
- Li, J.; Liao, L.; Shao, C.; Yang, Y.; Tang, Y.; Wei, Q.; Xu, L. Comparison of Remimazolam Tosylate and Propofol in Hemodynamic Stability, Postoperative Cognitive Function, and Recovery in General Anesthesia Combined with Regional Nerve Blocks: A Retrospective Cohort Study. BMC Anesthesiol. 2025, 25, 126. [Google Scholar] [CrossRef]
- Horill, S.; Zhou, X.; Zhou, X.-K.; Dong, H.; Jin, W. Perioperative Neurocognitive Disorders and Remimazolam: A Narrative Review of the Currently Available Evidence. JCA Adv. 2024, 1, 100041. [Google Scholar] [CrossRef]
- Wang, Y.; Bu, X.; Zhao, N.; Wang, S.; Wang, X.; Ge, Y.; Yi, H. Dexmedetomidine Effect on Delirium in Elderly Patients Undergoing General Anesthesia: A Protocol for Systematic Review and Meta-Analysis. Medicine 2021, 100, e27782. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shi, J.; Liu, C.; Zhang, J.; Liu, Y.; Wu, Y.; Han, X.; Dai, H.; Wu, J.; Bo, L.; et al. The Effect of Low-Dose Dexmedetomidine on Perioperative Neurocognitive Dysfunction in Elderly Patients Undergoing Endoscopic Retrograde Cholangiopancreatography (ERCP): A Randomized, Controlled, Double-Blind Trial. Drug Des. Dev. Ther. 2024, 18, 3715–3725. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, A.; Frangopoulos, C.; Shaffer, L.E.T. Patient Satisfaction with Propofol for Outpatient Colonoscopy: A Prospective, Randomized, Double-Blind Study. Dis. Colon Rectum 2017, 60, 1102–1108. [Google Scholar] [CrossRef]
- Basarigidad, A.; Killedar, S. Comparative Study of Safety and Efficacy between Propofol-Fentanyl Versus Propofol-Dexmeditomidine Combination For Sedation in Upper Gastro-Intestinal (GI) Endoscopic Procedures—A Prospective Randomised Study. Acad. Anesthesiol. Int. 2019, 4, 67–70. [Google Scholar] [CrossRef]
- Song, H.-Y.; Shen, L.-J.; Sun, W.; Zhang, L.-D.; Liang, J.-G.; Zhang, G.-X.; Lu, X.-Q. Comparison of Patient-Controlled Analgesia and Sedation (PCAS) with Remifentanil and Propofol versus Total Intravenous Anesthesia (TIVA) with Midazolam, Fentanyl, and Propofol for Colonoscopy. Medicine 2024, 103, e37411. [Google Scholar] [CrossRef]
- Khajavi, M.; Emami, A.; Etezadi, F.; Safari, S.; Sharifi, A.; Shariat Moharari, R. Conscious Sedation and Analgesia in Colonoscopy: Ketamine/Propofol Combination Has Superior Patient Satisfaction Versus Fentanyl/Propofol. Anesth. Pain Med. 2013, 3, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Suzuki, H.; Hosoe, N.; Ogata, H.; Kanai, T.; Yahagi, N. Dexmedetomidine vs Propofol for Gastrointestinal Endoscopy: A Meta-Analysis. United Eur. Gastroenterol. J. 2017, 5, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.; Turnbull, D.; Haboubi, H.; Leeds, J.S.; Healey, C.; Hebbar, S.; Collins, P.; Jones, W.; Peerally, M.F.; Brogden, S.; et al. British Society of Gastroenterology Guidelines on Sedation in Gastrointestinal Endoscopy. Gut 2024, 73, 1–27. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Fisher, D.A.; Maple, J.T.; Ben-Menachem, T.; Cash, B.D.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; Fukami, N.; et al. Complications of Colonoscopy. Gastrointest. Endosc. 2011, 74, 745–752. [Google Scholar] [CrossRef]
- Wernli, K.J.; Brenner, A.T.; Rutter, C.M.; Inadomi, J.M. Risks Associated with Anesthesia Services During Colonoscopy. Gastroenterology 2016, 150, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Sneyd, J.R.; Absalom, A.R.; Barends, C.R.M.; Jones, J.B. Hypotension during Propofol Sedation for Colonoscopy: A Retrospective Exploratory Analysis and Meta-Analysis. Br. J. Anesth. 2022, 128, 610–622. [Google Scholar] [CrossRef]
- Saugel, B.; Sessler, D.I. Perioperative Blood Pressure Management. Anesthesiology 2021, 134, 250–261. [Google Scholar] [CrossRef]
- Chen, M.; Lu, Y.; Liu, H.; Fu, Q.; Li, J.; Wu, J.; Shangguan, W. The Propofol-Sparing Effect of Intravenous Lidocaine in Elderly Patients Undergoing Colonoscopy: A Randomized, Double-Blinded, Controlled Study. BMC Anesthesiol. 2020, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Nongnuang, K.; Limprasert, N.; Munjupong, S. Can Intravenous Lidocaine Definitely Attenuate Propofol Requirement and Improve Outcomes among Colonoscopic Patients under Intravenous Sedation?: A Double-Blinded, Randomized Controlled Trial. Medicine 2022, 101, e30670. [Google Scholar] [CrossRef]
- Fostier, M.; Delhez, Q.; Januleviciute, G.; Bairy, L. Propofol-Based Deep Sedation for Colonoscopy: Does Sufentanil, Alfentanil or Ketamine Help? A Propensity Score Weighted Retrospective Study. PeerJ 2025, 13, e19146. [Google Scholar] [CrossRef]
- Barbosa, E.C.; Espírito Santo, P.A.; Baraldo, S.; Meine, G.C. Remimazolam versus Propofol for Sedation in Gastrointestinal Endoscopic Procedures: A Systematic Review and Meta-Analysis. Br. J. Anesth. 2024, 132, 1219–1229. [Google Scholar] [CrossRef]
- Lieber, S.R.; Heller, B.J.; Martin, C.F.; Howard, C.W.; Crockett, S. Complications of Anesthesia Services in Gastrointestinal Endoscopic Procedures. Clin. Gastroenterol. Hepatol. 2020, 18, 2118–2127.e4. [Google Scholar] [CrossRef]
- Qadeer, M.A.; Lopez, A.R.; Dumot, J.A.; Vargo, J.J. Hypoxemia during Moderate Sedation for Gastrointestinal Endoscopy: Causes and Associations. Digestion 2011, 84, 37–45. [Google Scholar] [CrossRef]
- Bhananker, S.M.; Posner, K.L.; Cheney, F.W.; Caplan, R.A.; Lee, L.A.; Domino, K.B. Injury and Liability Associated with Monitored Anesthesia Care: A Closed Claims Analysis. Anesthesiology 2006, 104, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.A.; Cannesson, M.; Bui, C.C.M. Opioid Free Anesthesia: Feasible? Curr. Opin. Anesthesiol. 2020, 33, 512–517. [Google Scholar] [CrossRef]
- Dalal, R.S.; Palchaudhuri, S.; Snider, C.K.; Lewis, J.D.; Mehta, S.J.; Lichtenstein, G.R. Exposure to Intravenous Opioids Is Associated with Future Exposure to Opioids in Hospitalized Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 2269–2278.e3. [Google Scholar] [CrossRef]
- Cooper, G.S.; Kou, T.D.; Rex, D.K. Complications Following Colonoscopy with Anesthesia Assistance: A Population-Based Analysis. JAMA Intern. Med. 2013, 173, 551–556. [Google Scholar] [CrossRef]
- Wu, F.; Smith, M.R.; Mueller, A.L.; Klapman, S.A.; Everett, L.L.; Houle, T.; Kuo, B.; Hobai, I.A. Association of Glucagon-like Peptide Receptor 1 Agonist Therapy with the Presence of Gastric Contents in Fasting Patients Undergoing Endoscopy under Anesthesia Care: A Historical Cohort Study. Can. J. Anesth. 2024, 71, 958–966. [Google Scholar] [CrossRef]
- Amornyotin, S. Sedation-Related Complications in Gastrointestinal Endoscopy. World J. Gastrointest. Endosc. 2013, 5, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, P.; Mouton-Faivre, C.; Castells, M.C.; Hepner, D.L. Anesthesia in the Patient with Multiple Drug Allergies: Are All Allergies the Same? Curr. Opin. Anesthesiol. 2011, 24, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Reimers, A.; Odin, P.; Ljung, H. Drug-Induced Cognitive Impairment. Drug Saf. 2025, 48, 339–361. [Google Scholar] [CrossRef]
- Kong, H.; Xu, L.-M.; Wang, D.-X. Perioperative Neurocognitive Disorders: A Narrative Review Focusing on Diagnosis, Prevention, and Treatment. CNS Neurosci. Ther. 2022, 28, 1147–1167. [Google Scholar] [CrossRef]
- Tian, L.; Luan, H.; Zhu, P.; Zhang, Z.; Bao, H. A Randomized Controlled Trial for Measuring Effects on Cognitive Functions of Adding Ketamine to Propofol during Sedation for Colonoscopy. Medicine 2020, 99, e21859. [Google Scholar] [CrossRef]
- Zheng, L.; Ye, M.; Ma, J.; Jin, C.; Yang, Y.; Li, H.; Zheng, R.; Wang, Y. Effects of Adding Adjuvants to Propofol on the Post-Anesthesia Cognitive Function in Patients Undergoing Gastroscopy/Colonoscopy: A Systematic Review and Meta-Analysis. Expert Opin. Drug Saf. 2024, 23, 995–1005. [Google Scholar] [CrossRef]
- Lin, S.; Wei, Y.; Zhuo, Y.; Que, S.; Jin, X.; Yao, Y.; Qian, B. Comparing Cognitive Recovery of Remimazolam versus Propofol in Elderly Patients Undergoing Colonoscopy: A Randomized Controlled Trial. Clin. Interv. Aging 2024, 19, 2133–2143. [Google Scholar] [CrossRef]
- Sundararaman, L.; Goudra, B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J. Clin. Med. 2024, 13, 4635. [Google Scholar] [CrossRef] [PubMed]
- Laffin, A.E.; Kendale, S.M.; Huncke, T.K. Severity and Duration of Hypoxemia during Outpatient Endoscopy in Obese Patients: A Retrospective Cohort Study. Can. J. Anesth. 2020, 67, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, J.; Hao, R.; Wang, S.; Chen, M.; Liu, H.; Qi, L.; Hao, Z. The Efficacy and Safety of Remimazolam Besylate Combined with Esketamine for Outpatient Colonoscopy: A Prospective, Randomized, Controlled Clinical Trial. Drug Des. Dev. Ther. 2023, 17, 2875–2887. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, C.; Wang, X.; Qi, B.; Zheng, L.; Zhao, Y.; Yu, W. Efficacy of High-Flow Nasal Oxygen in Preventing Hypoxia during Gastrointestinal Endoscopy: A Retrospective Cohort Study. BMC Anesthesiol. 2025, 25, 287. [Google Scholar] [CrossRef]
- Cukierman, D.S.; Perez, M.; Guerra-Londono, J.J.; Carlson, R.; Hagan, K.; Ghebremichael, S.; Hagberg, C.; Ge, P.S.; Raju, G.S.; Rhim, A.; et al. Nasal Continuous Positive Pressure versus Simple Face Mask Oxygenation for Adult Obese and Obstructive Sleep Apnea Patients Undergoing Colonoscopy under Propofol-Based General Anesthesia without Tracheal Intubation: A Randomized Controlled Trial. J. Clin. Anesth. 2023, 89, 111196. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, Q.; Li, K.; Zeng, Y.; Chen, Z.; Liu, S. Effect of End-Expiratory Carbon Dioxide Monitoring on Painless Colonoscopy Procedures in Obstructive Sleep Apnea Patients. Perioper. Med. 2025, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, X.; Bai, N.; Li, L.; Zhang, M.; Cheng, Z.; Guo, Q. Safety and Efficacy of Remimazolam Besylate in Patients Undergoing Colonoscopy: A Multicentre, Single-Blind, Randomized, Controlled, Phase III Trial. Front. Pharmacol. 2022, 13, 900723. [Google Scholar] [CrossRef]
- Aminnejad, R.; Hormati, A.; Shafiee, H.; Alemi, F.; Hormati, M.; Saeidi, M.; Ahmadpour, S.; Sabouri, S.M.; Aghaali, M. Comparing the Efficacy and Safety of Dexmedetomidine/Ketamine with Propofol/Fentanyl for Sedation in Colonoscopy Patients: A Doubleblinded Randomized Clinical Trial. CNS Neurol. Disord. Drug Targets 2022, 21, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, E.; Absalom, A.R. Pharmacokinetic and Pharmacodynamic Changes in the Elderly: Impact on Anesthetics. Anesthesiol. Clin. 2023, 41, 549–565. [Google Scholar] [CrossRef]
- Fondeur, J.; Escudero Mendez, L.; Srinivasan, M.; Hamouda, R.K.; Ambedkar, B.; Arzoun, H.; Sahib, I.; Mohammed, L. Dexmedetomidine in Prevention of Postoperative Delirium: A Systematic Review. Cureus 2022, 14, e25639. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists. Practice Guidelines for Moderate Procedural Sedation and Analgesia 2018: A Report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology 2018, 128, 437–479. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists Practice Guidelines for Sedation and Analgesia by Non-Anesthesiologists. Anesthesiology 2002, 96, 1004–1017. [CrossRef] [PubMed]
- Wadhwa, V.; Gupta, K.; Vargo, J.J. Monitoring Standards in Sedation and Analgesia: The Odyssey of Capnography in Sedation for Gastroenterology Procedures. Curr. Opin. Anesthesiol. 2019, 32, 453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Zhang, Y.; Yang, X.; Wu, J. The Effect of Capnography on the Incidence of Hypoxia during Sedation for EGD and Colonoscopy in Mildly Obese Patients: A Randomized, Controlled Study. BMC Anesthesiol. 2023, 23, 188. [Google Scholar] [CrossRef]
- Baytaş, V.; Vural, Ç.; Özçelik, M.; Torres, R.T.; Saunders, R.; Alkış, N. Patient Safety during Propofol Sedation before and after Implementation of Capnography Monitoring. J. Clin. Med. 2023, 12, 5959. [Google Scholar] [CrossRef]
- Sargin, M.; Uluer, M.S.; Şimşek, B. The Effect of Bispectral Index Monitoring on Cognitive Performance Following Sedation for Outpatient Colonoscopy: A Randomized Controlled Trial. Sao Paulo Med. J. 2019, 137, 305–311. [Google Scholar] [CrossRef]
- Miyake, K.; Higuchi, H.; Miyake, S.; Nishioka, Y.; Fujimoto, M.; Kurita, E.; Kawase, A.; Wakasugi, Y.; Miyawaki, T. Evaluation of Sedation Levels Using SedLine During Intravenous Sedation for Dental Procedures: A Case-Series Study. Anesth. Prog. 2023, 70, 85–87. [Google Scholar] [CrossRef]
- Ramsay, M.A.E.; Newman, K.B.; Jacobson, R.M.; Richardson, C.T.; Rogers, L.; Brown, B.J.; Hein, H.A.T.; Vol, E.B.D.; Daoud, Y.A. Sedation Levels During Propofol Administration for Outpatient Colonoscopies. Bayl. Univ. Med. Cent. Proc. 2014, 27, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.; Wyler, D.; Torjman, M.C.; Trinh, T.; Li, L.; Mehta, A.; Fitchett, E.; Kastenberg, D.; Mahla, M.; Romo, V. High Incidence of Burst Suppression during Propofol Sedation for Outpatient Colonoscopy: Lessons Learned from Neuromonitoring. Anesthesiol. Res. Pract. 2020, 2020, 7246570. [Google Scholar] [CrossRef]
- Hoshijima, H.; Higuchi, H.; Sato Boku, A.; Shibuya, M.; Morimoto, Y.; Fujisawa, T.; Mizuta, K. Patient Satisfaction with Deep versus Light/Moderate Sedation for Non-Surgical Procedures: A Systematic Review and Meta-Analysis. Medicine 2021, 100, e27176. [Google Scholar] [CrossRef]
- Ramsay, M.A.E.; Savege, T.M.; Simpson, B.R.J.; Goodwin, R. Controlled Sedation with Alphaxalone-Alphadolone. Br. Med. J. 1974, 2, 656–659. [Google Scholar] [CrossRef]
- Sessler, C.N.; Gosnell, M.S.; Grap, M.J.; Brophy, G.M.; O’Neal, P.V.; Keane, K.A.; Tesoro, E.P.; Elswick, R.K. The Richmond Agitation–Sedation Scale. Am. J. Respir. Crit. Care Med. 2002, 166, 1338–1344. [Google Scholar] [CrossRef]
- Chernik, D.A.; Gillings, D.; Laine, H.; Hendler, J.; Silver, J.M.; Davidson, A.B.; Schwam, E.M.; Siegel, J.L. Validity and Reliability of the Observer’s Assessment of Alertness/Sedation Scale: Study with Intravenous Midazolam. J. Clin. Psychopharmacol. 1990, 10, 244–251. [Google Scholar]
- Heo, J.; Jung, M.K.; Lee, H.S.; Cho, C.M.; Jeon, S.W.; Kim, S.K.; Jeon, Y.H. Effects of Bispectral Index Monitoring as an Adjunct to Nurse-Administered Propofol Combined Sedation during Colonoscopy: A Randomized Clinical Trial. Korean J. Intern. Med. 2016, 31, 260–266. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Wang, K. Bispectral Index Monitoring During Anesthesia Promotes Early Postoperative Recovery of Cognitive Function and Reduces Acute Delirium in Elderly Patients with Colon Carcinoma: A Prospective Controlled Study Using the Attention Network Test. Med. Sci. Monit. 2018, 24, 7785–7793. [Google Scholar] [CrossRef] [PubMed]
- Ekmekçi, P.; Erkan, G.; Kazbek, B.K.; Köksoy, U.C.; Doganay, G.; Tüzüner, F. Effect of Different Sedation Regimes on Cognitive Functions in Colonoscopy. Euroasian J. Hepatogastroenterol. 2017, 7, 158–162. [Google Scholar] [CrossRef] [PubMed]


| Patient-Related Factors | Other Factors |
|---|---|
|
|
| Indication | Initial Dose | Maintenance Infusion | Effect-Site Concentration (Ce) |
|---|---|---|---|
| General Population | 1–2.5 mg/kg IV | 25–100 µg/kg/min IV | |
| Elderly or Frail Patients | 0.5–1 mg/kg IV | 10–50 µg/kg/min IV | 2–2.5 µg/mL * |
| Adjunct with Opioids | 0.5–1.5 mg/kg | 10–75 µg/kg/min IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudziński, K.; Szułdrzyński, K.; Jankowski, M.; Adamczyk, K. Current and Emerging Sedation Practices for Colonoscopy: A Narrative Review of Pharmacological Agents, High-Risk Populations, and Safety Considerations. J. Clin. Med. 2025, 14, 6583. https://doi.org/10.3390/jcm14186583
Chudziński K, Szułdrzyński K, Jankowski M, Adamczyk K. Current and Emerging Sedation Practices for Colonoscopy: A Narrative Review of Pharmacological Agents, High-Risk Populations, and Safety Considerations. Journal of Clinical Medicine. 2025; 14(18):6583. https://doi.org/10.3390/jcm14186583
Chicago/Turabian StyleChudziński, Kamil, Konstanty Szułdrzyński, Miłosz Jankowski, and Kamil Adamczyk. 2025. "Current and Emerging Sedation Practices for Colonoscopy: A Narrative Review of Pharmacological Agents, High-Risk Populations, and Safety Considerations" Journal of Clinical Medicine 14, no. 18: 6583. https://doi.org/10.3390/jcm14186583
APA StyleChudziński, K., Szułdrzyński, K., Jankowski, M., & Adamczyk, K. (2025). Current and Emerging Sedation Practices for Colonoscopy: A Narrative Review of Pharmacological Agents, High-Risk Populations, and Safety Considerations. Journal of Clinical Medicine, 14(18), 6583. https://doi.org/10.3390/jcm14186583






