24-Hour Movement Behaviour and Health Awareness as Possible Predictors of Infertility-Related Quality of Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
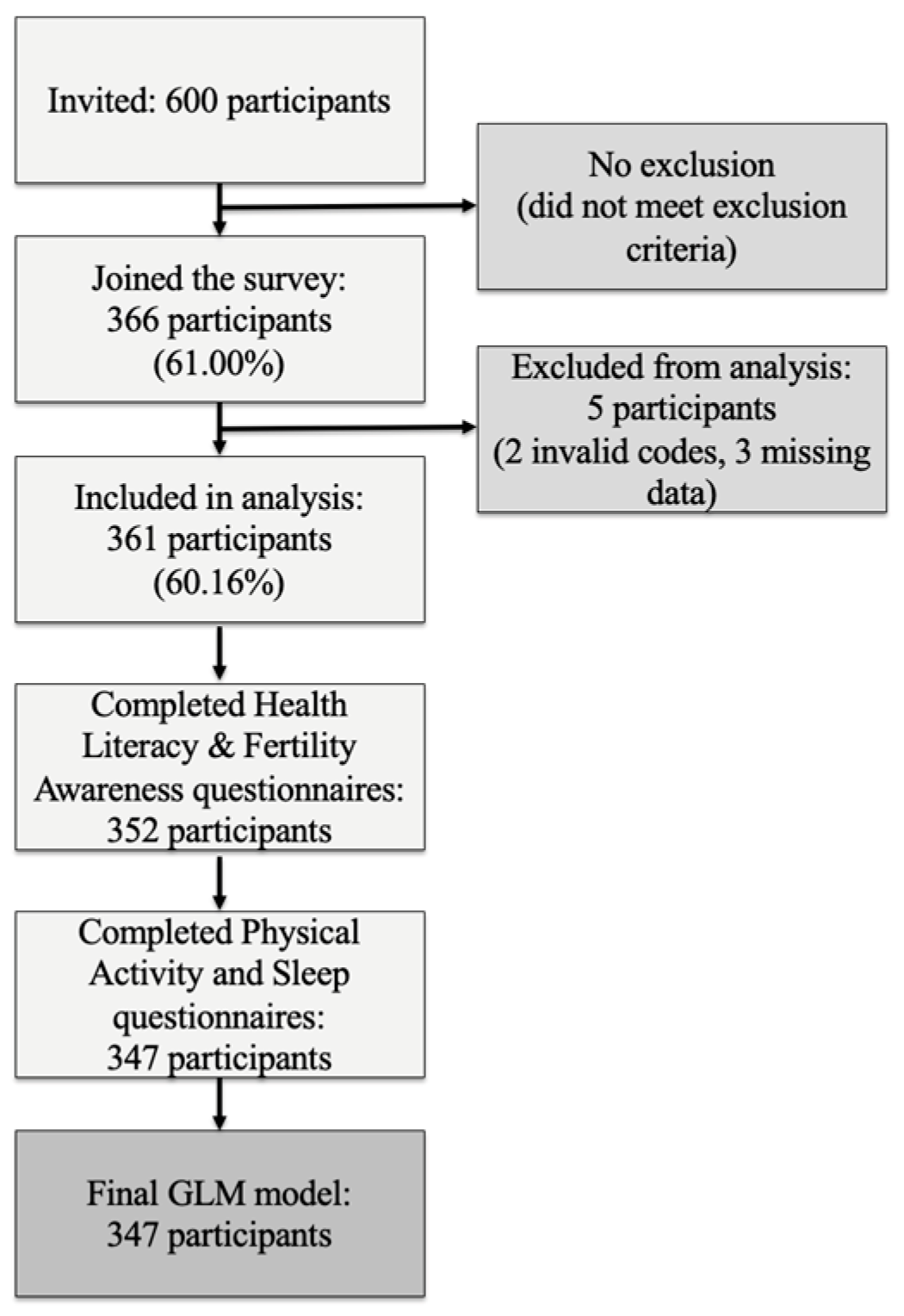
2.2. Recruitment and Eligibility
2.3. Data Collection Tools and Procedure
2.4. Statistical Analysis
3. Results
3.1. Characteristic of the Sample
3.2. Fertility Awareness and Health Literacy
3.3. 24-h Movement Behaviour Patterns
3.4. General and Infertility-Specific Quality of Life Assessment
3.5. Health Awareness and 24-h Movement Behaviour
3.6. General and Infertility-Specific Quality of Life and 24-h Movement Behaviour
3.7. Prediction of Infertility-Specific Quality of Life by 24-h Movement Behaviour and Health Awareness
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Athens Insomnia Scale |
| ART | Assisted Reproductive Technologies |
| BRIEF | Brief Health Literacy Screening Tool |
| EQUATOR | Enhancing the Quality and Transparency of Health Research |
| FAS | Fertility Awareness Survey |
| FertiQoL | Fertility Quality of Life Questionnaire |
| FPI | Fertility Problem Inventory |
| GBD | Global Burden of Disease Study |
| GLM | Generalised Linear Model |
| GPAQ-H | Global Physical Activity Questionnaire–Hungarian Version |
| IUI | Intrauterine Insemination |
| IVF | In Vitro Fertilisation |
| MBPI | Mind/Body Program for Infertility |
| MVPA | Moderate and Vigorous Physical Activity |
| RPA | Recreational Physical Activity |
| SB | Sedentary Behaviour |
| SD | Standard Deviation |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| WHOQOL-BREF | World Health Organisation Quality of Life-BREF |
| WPA | Work-related Physical Activity |
References
- Cox, C.M.; Thoma, M.E.; Tchangalova, N.; Mburu, G.; Bornstein, M.J.; Johnson, C.L.; Kiarie, J. Infertility prevalence and the methods of estimation from 1990 to 2021: A systematic review and meta-analysis. Hum. Reprod. Open 2022, 2022, hoac051. [Google Scholar] [CrossRef]
- WHO. Infertility Prevalence Estimates, 1990–2021; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Hwang, Y.J.; Lee, J.; Hwang, J.; Sim, H.; Kim, N.; Kim, T.S. Psychiatric considerations of infertility. Psychiatry Investig. 2024, 21, 1175–1182. [Google Scholar] [CrossRef]
- Azmoudeh, A.; Shahraki, Z.; Hoseini, F.-S.; Akbari-Asbagh, F.; Davari-Tanha, F.; Mortazavi, F. In vitro fertilization success and associated factors: A prospective cohort study. Int. J. Women’s Health Reprod. Sci. 2018, 6, 350–355. [Google Scholar]
- Di Nunzio, A.; Giarra, A.; Toscanesi, M.; Amoresano, A.; Piscopo, M.; Ceretti, E.; Zani, C.; Lorenzetti, S.; Trifuoggi, M.; Montano, L. Comparison between Macro and Trace Element Concentrations in Human Semen and Blood Serum in Highly Polluted Areas in Italy. Int. J. Environ. Res. Public Health 2022, 19, 11635. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, E.; Peñalver-Soler, R.; Almunia, N.; Pérez-Álvarez, M.; Luque, M.; Campillo, N.; Flores Monreal, A.; Arroyo-Manzanares, N.; Ruiz-Moreno, Y.; Jiménez, R. O-280 Unveiling the hidden danger: Detection and characterisation of microplastics in human follicular and seminal fluids. Hum. Reprod. 2025, 40, deaf097-280. [Google Scholar] [CrossRef]
- Doroftei, B.; Savuca, A.; Cretu, A.-M.; Maftei, R.; Anton, N.; Ilea, C.; Doroftei, M.; Puha, B. Microplastics and human fertility: A comprehensive review of their presence in human samples and reproductive implication. Ecotoxicol. Environ. Saf. 2025, 303, 118939. [Google Scholar] [CrossRef]
- Várnagy, Á.; Kőszegi, T.; Györgyi, E.; Sulyok, E.; Prémusz, V.; Bódis, J. Levels of total antioxidant capacity and 8-hydroxy-2'-deoxyguanosine of serum and follicular fluid in women undergoing in vitro fertilization: Focusing on endometriosis. Hum. Fertil. 2020, 23, 200–208. [Google Scholar] [CrossRef]
- Péntek, S.; Mauchart, P.; Várnagy, Á.; Kovács, K.; Bódis, J.; Sulyok, E. [Analysis of oxidative stress levels in IVF patients in relation to clinical characteristics]. Orvosi Hetil. 2025, 166, 1107–1116. [Google Scholar] [CrossRef]
- Prémusz, V.; Lendvai-Emmert, D.; Makai, A.; Amrein, K.; Chauhan, S.; Bódis, J.; Kovács, K.A.; Várnagy, Á. Pre-Treatment Physical Activity Could Positively Influence Pregnancy Rates in IVF despite the Induced Oxidative Stress: A Cohort Study on Salivary 8-Hydroxy-2'-deoxyguanosine. Antioxidants 2022, 11, 1586. [Google Scholar] [CrossRef]
- Cousineau, T.M.; Domar, A.D. Psychological impact of infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 293–308. [Google Scholar] [CrossRef]
- Chachamovich, J.R.; Chachamovich, E.; Ezer, H.; Fleck, M.P.; Knauth, D.; Passos, E.P. Investigating quality of life and health-related quality of life in infertility: A systematic review. J. Psychosom. Obstet. Gynecol. 2010, 31, 101–110. [Google Scholar] [CrossRef]
- Luk, B.H.K.; Loke, A.Y. The Impact of Infertility on the Psychological Well-Being, Marital Relationships, Sexual Relationships, and Quality of Life of Couples: A Systematic Review. J. Sex Marital. Ther. 2015, 41, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, E.; Szigeti, J.F.; Ujma, P.P.; Sexty, R.; Balog, P. Anxiety and depression among infertile women: A cross-sectional survey from Hungary. BMC Women’s Health 2017, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.X.; Ju, Y.X.; Gao, L.L.; Abdalla, H.M.; Masoud, S.S. Depression, anxiety and associated factors among infertile women in Zanzibar. J. Psychosom. Obstet. Gynecol. 2025, 46, 2522387. [Google Scholar] [CrossRef] [PubMed]
- Prémusz, V.; Makai, A.; Perjés, B.; Máté, O.; Hock, M.; Ács, P.; Koppán, M.; Bódis, J.; Várnagy, Á.; Lampek, K. Multicausal analysis on psychosocial and lifestyle factors among patients undergoing assisted reproductive therapy-with special regard to self-reported and objective measures of pre-treatment habitual physical activity. BMC Public Health 2021, 21, 1480. [Google Scholar] [CrossRef]
- Prémusz, V.; Ács, P.; Bódis, J.; Várnagy, Á.; Lászik, Á.; Makai, A. Introducing the Hungarian Version of the SCREENIVF Tool into the Clinical Routine Screening of Emotional Maladjustment. Int. J. Environ. Res. Public Health 2022, 19, 10147. [Google Scholar] [CrossRef]
- Szigeti, F.J.; Soltész, K.; Sipos, M.; Juhász, A.; Szöllősi, K.; Vesztergom, D.; Ujma, P.P.; Purebl, G. [The role of psychological screening and care in assisted reproduction]. Orvosi Hetil. 2024, 165, 455–463. [Google Scholar] [CrossRef]
- Szigeti, F.J.; Sexty, R.E.; Szabó, G.; Kazinczi, C.; Kéki, Z.; Sipos, M.; Ujma, P.P.; Purebl, G. The SCREENIVF Hungarian version is a valid and reliable measure accurately predicting possible depression in female infertility patients. Sci. Rep. 2024, 14, 12880. [Google Scholar] [CrossRef]
- Szigeti, F.J.; Kazinczi, C.; Szabó, G.; Sipos, M.; Ujma, P.P.; Purebl, G. The clinical effectiveness of the Mind/Body Program for Infertility on wellbeing and assisted reproduction outcomes: A randomized controlled trial in search for active ingredients. Hum. Reprod. 2024, 39, 1735–1751. [Google Scholar] [CrossRef]
- Szigeti, F.J.; Grevenstein, D.; Wischmann, T.; Lakatos, E.; Balog, P.; Sexty, R. Quality of life and related constructs in a group of infertile Hungarian women: A validation study of the FertiQoL. Hum. Fertil. 2022, 25, 456–469. [Google Scholar] [CrossRef]
- Szabo, G.; Szigeti, F.J.; Sipos, M.; Varbiro, S.; Gonda, X. [The role of psychological factors in the development and treatment of infertility]. Neuropsychopharmacol. Hung. 2023, 25, 123–130. [Google Scholar] [PubMed]
- Liang, Y.; Huang, J.; Zhao, Q.; Mo, H.; Su, Z.; Feng, S.; Li, S.; Ruan, X. Global, regional, and national prevalence and trends of infertility among individuals of reproductive age (15–49 years) from 1990 to 2021, with projections to 2040. Hum. Reprod. 2025, 40, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Verberg, M.F.G.; Eijkemans, M.J.C.; Heijnen, E.; Broekmans, F.J.; de Klerk, C.; Fauser, B.; Macklon, N.S. Why do couples drop-out from IVF treatment? A prospective cohort study. Hum. Reprod. 2008, 23, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Di Mattei, V.E.; Taranto, P.; Perego, G.; Desimone, S.; Rancoita, P.M.V.; Catarinella, A.; Cioffi, R.; Mangili, G.; Vanni, V.S.; Candiani, M. Identification of psychological profiles of cancer patients undergoing fertility preservation counseling. J. Clin. Med. 2023, 12, 4011. [Google Scholar] [CrossRef]
- Domar, A.D.; Gross, J.; Rooney, K.; Boivin, J. Exploratory randomized trial on the effect of a brief psychological intervention on emotions, quality of life, discontinuation, and pregnancy rates in in vitro fertilization patients. Fertil. Steril. 2015, 104, 440–451.e447. [Google Scholar] [CrossRef]
- Wdowiak, A.; Anusiewicz, A.; Bakalczuk, G.; Raczkiewicz, D.; Janczyk, P.; Makara-Studzińska, M. Assessment of Quality of Life in Infertility Treated Women in Poland. Int. J. Environ. Res. Public Health 2021, 18, 4275. [Google Scholar] [CrossRef]
- Rooney, K.L.; Domar, A.D. The impact of lifestyle behaviors on infertility treatment outcome. Curr. Opin. Obstet. Gynecol. 2014, 26, 181–185. [Google Scholar] [CrossRef]
- Domar, A.D.; Conboy, L.; Denardo-Roney, J.; Rooney, K.L. Lifestyle behaviors in women undergoing in vitro fertilization: A prospective study. Fertil. Steril. 2012, 97, 697–701.e691. [Google Scholar] [CrossRef]
- Kazemi, M.; Kim, J.Y.; Wan, C.; Xiong, J.D.; Michalak, J.; Xavier, I.B.; Ganga, K.; Tay, C.T.; Grieger, J.A.; Parry, S.A.; et al. Comparison of dietary and physical activity behaviors in women with and without polycystic ovary syndrome: A systematic review and meta-analysis of 39,471 women. Hum. Reprod. Update 2022, 28, 910–955. [Google Scholar] [CrossRef]
- Xie, F.; You, Y.; Guan, C.; Gu, Y.; Yao, F.; Xu, J. Association between physical activity and infertility: A comprehensive systematic review and meta-analysis. J. Transl. Med. 2022, 20, 237. [Google Scholar] [CrossRef]
- Zhang, H.; Hua, L.; Liu, D.; Su, X.; Chen, J.; Chen, J. Effects of physical activity on infertility in reproductive females. Reprod. Biol. Endocrinol. 2024, 22, 62. [Google Scholar] [CrossRef]
- Dale, R.; O’Rourke, T.; Nussbaumer-Streit, B.; Probst, T. 24-hour movement behaviours and mental health in non-clinical populations: A systematic review. PLoS ONE 2025, 20, e0325445. [Google Scholar] [CrossRef]
- Brinson, A.K.; da Silva, S.G.; Hesketh, K.R.; Evenson, K.R. Impact of physical activity and sedentary behavior on spontaneous female and male fertility: A systematic review. J. Phys. Act. Health 2023, 20, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Rich-Edwards, J.W.; Spiegelman, D.; Garland, M.; Hertzmark, E.; Hunter, D.J.; Colditz, G.A.; Willett, W.C.; Wand, H.; Manson, J.E. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology 2002, 13, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Dhair, A.; Abed, Y. The association of types, intensities and frequencies of physical activity with primary infertility among females in Gaza Strip, Palestine: A case-control study. PLoS ONE 2020, 15, e0241043. [Google Scholar] [CrossRef] [PubMed]
- Vitek, W.S.; Sun, F.; Cardozo, E.; Hoeger, K.M.; Hansen, K.R.; Santoro, N.; Zhang, H.; Legro, R.S. Moderate and increased physical activity is not detrimental to live birth rates among women with unexplained infertility and obesity. F S Rep. 2023, 4, 308–312. [Google Scholar] [CrossRef]
- Domar, A.D.; Rooney, K.L.; Milstein, M.; Conboy, L. Lifestyle habits of 12,800 IVF patients: Prevalence of negative lifestyle behaviors, and impact of region and insurance coverage. Hum. Fertil. 2015, 18, 253–257. [Google Scholar] [CrossRef]
- European Commission. Special Eurobarometer 525. Available online: https://europa.eu/eurobarometer/api/deliverable/download/file?deliverableId=83674 (accessed on 15 June 2025).
- Prémusz, V.; Makai, A.; Gács, B.; Simon-Ugron, Á.; Nagy, Á.; Ács, P.; Lampek, K.; Várnagy, Á. Relationship between pre-treatment habitual physical activity and success of assisted reproduction. Stud. Univ. Babes-Bolyai Educ. Artis Gymnast. 2018, 63, 47–58. [Google Scholar] [CrossRef]
- Prémusz, V.; Makai, A.; Gács, B.; Nagy, Á.; Perjés, B.; Ács, P.; Lampek, K.; Várnagy, Á. Preliminary study on pre-treatment physical activity and quality of life in infertility. Exerc. Qual. Life J. 2019, 11, 5–17. [Google Scholar] [CrossRef]
- Máté, G.; Balló, A.; Szántó, Á.; Kopa, Z.; Török, A. [Assessment of lifestyle habits of women and men participating in infertility treatment]. Orvosi Hetil. 2024, 165, 1423–1432. [Google Scholar] [CrossRef]
- WHO. Global Status Report on Physical Activity 2022; World Health Organisation: Geneva, Switzerland, 2023. [Google Scholar]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Correction to Lancet Glob Health 2018; 6: e1077-86. Lancet Glob. Health 2019, 7, e36. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef] [PubMed]
- Hulteen, R.M.; Smith, J.J.; Morgan, P.J.; Barnett, L.M.; Hallal, P.C.; Colyvas, K.; Lubans, D.R. Global participation in sport and leisure-time physical activities: A systematic review and meta-analysis. Prev. Med. 2017, 95, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Bize, R.; Johnson, J.A.; Plotnikoff, R.C. Physical activity level and health-related quality of life in the general adult population: A systematic review. Prev. Med. 2007, 45, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Kilfoyle, K.A.; Vitko, M.; O’Conor, R.; Bailey, S.C. Health literacy and women’s reproductive health: A systematic review. J. Women’s Health 2016, 25, 1237–1255. [Google Scholar] [CrossRef]
- Brathwaite, S.; Alabi, O.; Simpson, L.; Massarweh, N. Exploring Health Literacy and Vascular Access Decision Making: A Scoping Review. J. Clin. Med. 2024, 13, 3734. [Google Scholar] [CrossRef]
- Bae, S.H.; Lee, J.J.; Son, S.Y.; Kim, H.Y.; Ju, M.K. A Cross-Sectional Analysis of Health Literacy and Compliance to Treatment in Organ Transplant Recipients. J. Clin. Med. 2023, 12, 977. [Google Scholar] [CrossRef]
- Mashamba-Thompson, T.P. Diagnostics Literacy Advocacy Model for Vulnerable Populations. Diagnostics 2022, 12, 716. [Google Scholar] [CrossRef]
- Rutherford, J.; Taylor, A.; Holman, R.; MacDonald, J.; Jarrett, D.; Bigrigg, A. Low literacy: A hidden problem in family planning clinics. BMJ Sex. Reprod. Health 2006, 32, 235–240. [Google Scholar] [CrossRef]
- Zalewska, O.; Wszołek, K.; Pięt, M.; Wilczak, M.; Chmaj-Wierzchowska, K. Women’s awareness of reproductive health. Medicina 2024, 60, 158. [Google Scholar] [CrossRef]
- Gossett, D.R.; Nayak, S.; Bhatt, S.; Bailey, S.C. What do healthy women know about the consequences of delayed childbearing? J. Health Commun. 2013, 18 (Suppl. S1), 118–128. [Google Scholar] [CrossRef] [PubMed]
- Mátyás, G.; Vincze, F.; Bíró, É. [Validation of health literacy questionnaires in Hungarian adult sample]. Orvosi Hetil. 2021, 162, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Daniluk, J.C.; Koert, E.; Cheung, A. Childless women’s knowledge of fertility and assisted human reproduction: Identifying the gaps. Fertil. Steril. 2012, 97, 420–426. [Google Scholar] [CrossRef]
- Ács, P.; Betlehem, J.; Oláh, A.; Bergier, B.; Morvay-Sey, K.; Makai, A.; Prémusz, V. Cross-cultural adaptation and validation of the Global Physical Activity Questionnaire among healthy Hungarian adults. BMC Public Health 2020, 20, 1056. [Google Scholar] [CrossRef]
- Perczel-Forintos, D.; Kiss, Z.; Ajtay, G. Kérdőívek, Becslőskálák a Klinikai Pszichológiában. [Queries and Estimation Scales in Clinical Psychology]; Országos Pszichiátriai és Neurológiai Intézet: Budapest, Hungary, 2018. [Google Scholar]
- Paulik, E.; Belec, B.; Molnár, R.; Müller, A.; Belicza, E.; Kullmann, L.; Nagymajtényi, L. [Applicability of the abbreviated version of the World Health Organization’s quality of life questionnaire in Hungary]. Orvosi Hetil. 2007, 148, 155–160. [Google Scholar] [CrossRef]
- Cserepes, R.E.; Korosi, T.; Bugan, A. [Characteristics of infertility-specific quality of life in Hungarian couples]. Orvosi Hetil. 2014, 155, 783–788. [Google Scholar] [CrossRef]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef]
- Gignac, G.E.; Szodorai, E.T. Effect size guidelines for individual differences researchers. Personal. Individ. Differ. 2016, 102, 74–78. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Ferguson, C.J. An effect size primer: A guide for clinicians and researchers. Prof. Psychol. Res. Pract. 2009, 40, 532. [Google Scholar] [CrossRef]
- Oberg, A.L.; Mahoney, D.W. Linear mixed effects models. In Topics in Biostatistics; Humana Press: Totowa, NJ, USA, 2007; pp. 213–234. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Buja, A.; Rabensteiner, A.; Sperotto, M.; Grotto, G.; Bertoncello, C.; Cocchio, S.; Baldovin, T.; Contu, P.; Lorini, C.; Baldo, V. Health literacy and physical activity: A systematic review. J. Phys. Act. Health 2020, 17, 1259–1274. [Google Scholar] [CrossRef]
- Ho, T.G.; Hosseinzadeh, H.; Rahman, B.; Sheikh, M. Health literacy and health-promoting behaviours among Australian-Singaporean communities living in Sydney metropolitan area. Proc. Singap. Healthc. 2018, 27, 125–131. [Google Scholar] [CrossRef]
- Friis, K.; Lasgaard, M.; Rowlands, G.; Osborne, R.H.; Maindal, H.T. Health literacy mediates the relationship between educational attainment and health behavior: A Danish population-based study. J. Health Commun. 2016, 21, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Chawłowska, E.; Lipiak, A.; Krzysztoszek, J.; Krupa, B.; Staszewski, R. Reproductive health literacy and fertility awareness among Polish female students. Front. Public Health 2020, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Boedt, T.; Matthys, C.; Lie Fong, S.; De Neubourg, D.; Vereeck, S.; Seghers, J.; Van der Gucht, K.; Weyn, B.; Geerts, D.; Spiessens, C. Systematic development of a mobile preconception lifestyle programme for couples undergoing IVF: The PreLiFe-programme. Hum. Reprod. 2021, 36, 2493–2505. [Google Scholar] [CrossRef]
- Diener, E. Subjective well-being: The science of happiness and a proposal for a national index. Am. Psychol. 2000, 55, 34. [Google Scholar] [CrossRef]
- Antinozzi, C.; Di Luigi, L.; Sireno, L.; Caporossi, D.; Dimauro, I.; Sgrò, P. Protective Role of Physical Activity and Antioxidant Systems During Spermatogenesis. Biomolecules 2025, 15, 478. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress: Relationship with exercise and training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean diet as an antioxidant: The impact on metabolic health and overall wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Mastrominas, M.; Yiannakouris, N. Adherence to the Mediterranean diet and IVF success rate among non-obese women attempting fertility. Hum. Reprod. 2018, 33, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Zabaleta, M.E. Mediterranean diet: Woman fertility and pregnancy. Mediterr. J. Nutr. Metab. 2020, 13, 101–111. [Google Scholar] [CrossRef]
- Moutzouroulia, A.; Asimakopoulou, Z.; Tzavara, C.; Asimakopoulos, K.; Adonakis, G.; Kaponis, A. The impact of infertility on the mental health of women undergoing in vitro fertilization treatment. Sex. Reprod. Healthc. 2025, 43, 101072. [Google Scholar] [CrossRef]
- Skirton, H.; Goldsmith, L.; Jackson, L.; Tibben, A. Quality in genetic counselling for presymptomatic testing--clinical guidelines for practice across the range of genetic conditions. Eur. J. Hum. Genet. 2013, 21, 256–260. [Google Scholar] [CrossRef]
- Hansen, K.; Mische Lawson, L.; Wilpers, A. Prenatal Psychosocial Distress Screening for Individuals Experiencing Pregnancies Complicated by Fetal Anomalies. J. Pers. Med. 2025, 15, 322. [Google Scholar] [CrossRef]
- Muteri, T.A. Psychological Impact of False-Positive Results in Obstetric Screening: A Systematic Review. Clin. Exp. Obstet. Gynecol. 2025, 52, 26696. [Google Scholar] [CrossRef]
- Udry-Jørgensen, L.; Darwiche, J.; Germond, M.; Wunder, D.; Vial, Y. Anxiety, depression, and attachment before and after the first-trimester screening for Down syndrome: Comparing couples who undergo ART with those who conceive spontaneously. Prenat. Diagn. 2015, 35, 1287–1293. [Google Scholar] [CrossRef][Green Version]
| Sociodemographic and Anthropometric Characteristics | ||
|---|---|---|
| n | % | |
| Type of residence | ||
| Capital city | 86 | 23.8% |
| County seat | 52 | 14.4% |
| City | 128 | 35.5% |
| Village | 95 | 26.3% |
| Level of education | ||
| Primary | 12 | 3.3% |
| Secondary | 157 | 43.5% |
| Tertiary | 192 | 53.2% |
| Household income | ||
| Below average | 30 | 8.3% |
| Average | 239 | 66.2% |
| Above average | 92 | 25.5% |
| Age | Mean | SD |
| Years | 34.68 | 5.03 |
| Anthropometrics | Mean | SD |
| Height (cm) | 165.66 | 6.29 |
| Weight (kg) | 68.91 | 14.39 |
| BMI (kg/m2) | 25.12 | 5.15 |
| Clinical and Reproductive Health Characteristics | ||
| Childbearing history | Mean | SD |
| In relationship (years) | 7.99 | 4.69 |
| Attempting to conceive (month) | 40.17 | 30.09 |
| Treatment cycles (n) | 2.34 | 1.55 |
| Own children | n | % |
| 0 | 285 | 78.9 |
| 1 | 52 | 14.5 |
| 2 | 24 | 6.6 |
| Cause of infertility | n | % |
| Female factor | 123 | 34.1% |
| Male factor | 57 | 15.8% |
| Combined factor | 69 | 19.1% |
| Unknown | 97 | 26.9% |
| Diagnostic process ongoing | 15 | 4.2% |
| Type of Fertility Treatment | ||
| No treatment yet | 23 | 6.4% |
| Tubal flushing | 17 | 4.7% |
| Hormonal therapy | 26 | 7.2% |
| IUI | 39 | 10.8% |
| IVF/ICSI/FET | 256 | 70.9% |
| Scales | Responses | n | % |
|---|---|---|---|
| Fertility Knowledge | Does not have knowledge | 18 | 5.1% |
| Some knowledge | 60 | 17.0% | |
| Acceptable knowledge | 181 | 51.4% | |
| Reliable knowledge | 93 | 26.4% | |
| ART Knowledge | Does not have knowledge | 20 | 5.7% |
| Some knowledge | 67 | 19.0% | |
| Acceptable knowledge | 177 | 50.3% | |
| Reliable knowledge | 88 | 25.0% | |
| ART Attitude | Strongly opposes | 4 | 1.1% |
| Opposes | 54 | 15.3% | |
| Neither supports nor opposes | 167 | 47.4% | |
| Supports | 124 | 35.2% | |
| Strongly supports | 3 | 0.9% | |
| FAS Total Score | Max 20 points | Mean | 9.69 |
| SD | 2.54 | ||
| BRIEF Total Score | Max 20 points | Mean | 9.22 |
| SD | 2.87 |
| WHOQOL-BREF | Mean | SD | |
|---|---|---|---|
| SRH | 73.71 | 15.13 | |
| QoL | 65.45 | 18.60 | |
| WHOQOL-BREF Domains | Physical | 79.36 | 12.75 |
| Psychological | 67.85 | 16.46 | |
| Social | 70.57 | 20.22 | |
| Environmental | 73.16 | 14.87 | |
| FertiQoL | Mean | SD | |
| SRH | 69.99 | 18.66 | |
| QoL | 64.69 | 24.53 | |
| FertiQoL Domains | Emotional | 60.64 | 22.86 |
| Mind–Body | 58.42 | 23.70 | |
| Relational | 77.61 | 16.93 | |
| Social | 64.88 | 22.00 | |
| Core Scale | 65.39 | 17.47 | |
| Environment (n = 342) | 44.97 | 12.74 | |
| Tolerability (n = 342) | 69.04 | 20.47 | |
| Treatment Scale (n = 342) | 54.60 | 9.89 | |
| FertiQoL Total | 62.31 | 13.75 | |
| 24-HMB | BRIEF | FAS | |
|---|---|---|---|
| W MVPA | r | −0.133 * | −0.020 |
| p | 0.012 | 0.709 | |
| R MVPA | r | 0.066 | 0.156 ** |
| p | 0.212 | 0.003 | |
| Total MVPA | r | −0.103 * | 0.068 |
| p | 0.050 | 0.207 | |
| SB | r | 0.072 | 0.062 |
| p | 0.174 | 0.244 | |
| Sleep time | r | 0.034 | 0.013 |
| p | 0.525 | 0.805 | |
| AIS | r | −0.095 | 0.071 |
| p | 0.073 | 0.186 | |
| B | SE | β | t | p | η2p | |
|---|---|---|---|---|---|---|
| Model (Intercept) | 67.354 | 5.107 | 13.188 | ≤0.001 | ||
| Sleep Quality (AIS) | −1.29 | 0.209 | −0.469 | −6.149 | ≤0.001 | 0.100 |
| Sedentary Behaviour (GPAQ) | −0.008 | 0.003 | −0.079 | −2.652 | 0.008 | 0.020 |
| Health Literacy (BRIEF) | 0.63 | 0.261 | 0.285 | 2.420 | 0.016 | 0.017 |
| Fertility Awareness (FAS) | −0.67 | 0.277 | −0.310 | −2.420 | 0.016 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prémusz, V.; Kovács, R.; Skriba, E.; Szmatona, G.; Tándor, Z.; Makai, A.; Ács, P.; Kovács, K.; Várnagy, Á.; Veres-Balajti, I. 24-Hour Movement Behaviour and Health Awareness as Possible Predictors of Infertility-Related Quality of Life. J. Clin. Med. 2025, 14, 6552. https://doi.org/10.3390/jcm14186552
Prémusz V, Kovács R, Skriba E, Szmatona G, Tándor Z, Makai A, Ács P, Kovács K, Várnagy Á, Veres-Balajti I. 24-Hour Movement Behaviour and Health Awareness as Possible Predictors of Infertility-Related Quality of Life. Journal of Clinical Medicine. 2025; 14(18):6552. https://doi.org/10.3390/jcm14186552
Chicago/Turabian StylePrémusz, Viktória, Réka Kovács, Eszter Skriba, Gábor Szmatona, Zoltán Tándor, Alexandra Makai, Pongrác Ács, Kálmán Kovács, Ákos Várnagy, and Ilona Veres-Balajti. 2025. "24-Hour Movement Behaviour and Health Awareness as Possible Predictors of Infertility-Related Quality of Life" Journal of Clinical Medicine 14, no. 18: 6552. https://doi.org/10.3390/jcm14186552
APA StylePrémusz, V., Kovács, R., Skriba, E., Szmatona, G., Tándor, Z., Makai, A., Ács, P., Kovács, K., Várnagy, Á., & Veres-Balajti, I. (2025). 24-Hour Movement Behaviour and Health Awareness as Possible Predictors of Infertility-Related Quality of Life. Journal of Clinical Medicine, 14(18), 6552. https://doi.org/10.3390/jcm14186552







