Serious Adverse Drug Reactions to COVID-19 Vaccines in the Pediatric Population: A Retrospective, Cross-Sectional Study Utilizing the Eudravigilance Database for the European Economic Area
Abstract
1. Introduction
2. Aim
3. Methods and Materials
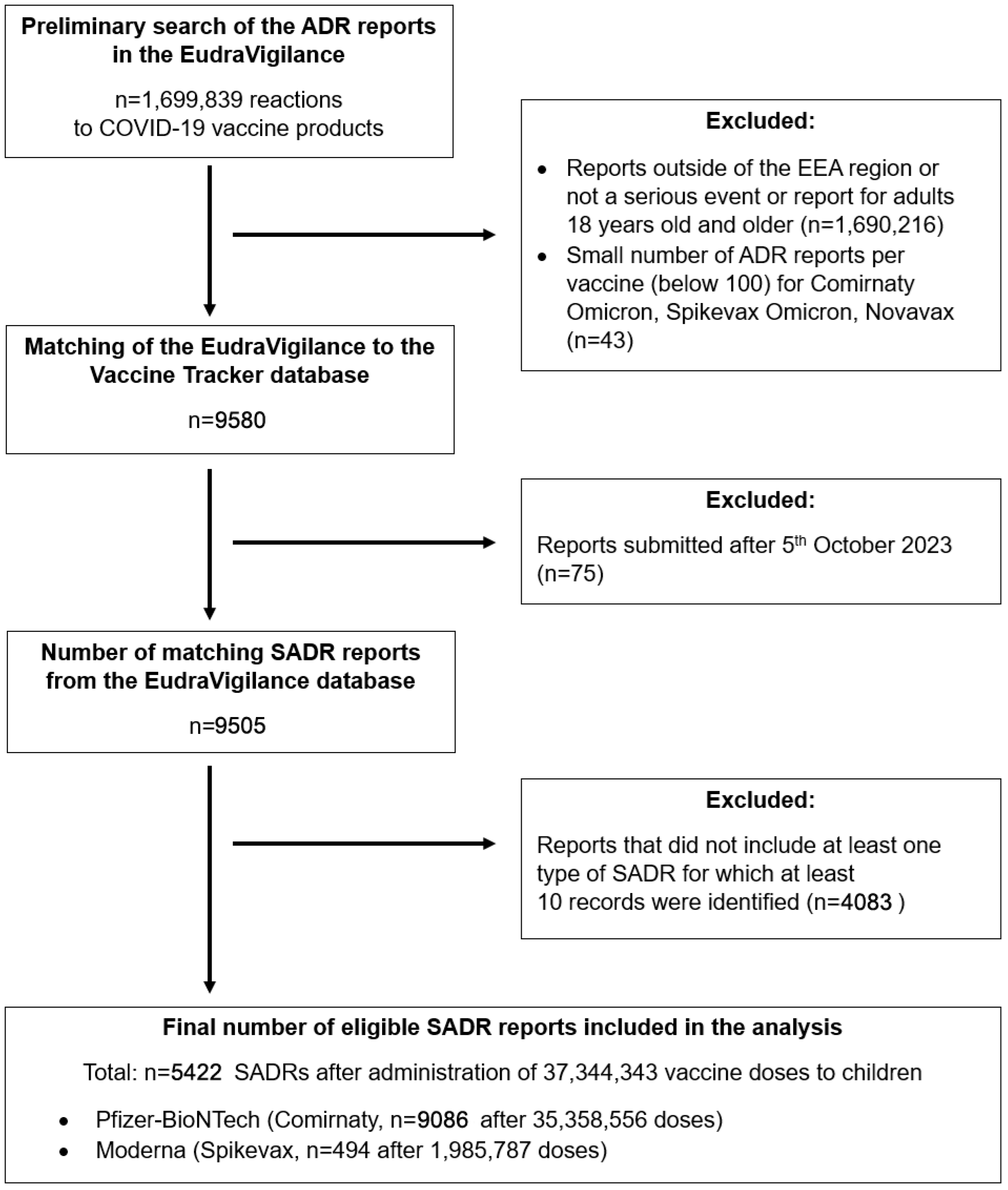
3.1. Data Acquisition—EudraVigilance Database
3.2. Data Acquisition—ECDC Vaccine Tracker Database
3.3. Data Filtering and Processing
3.4. Normalization Per ADR Report
3.5. Trial-Adjusted Scaling
3.6. Statistical Analysis
4. Results
4.1. General Characteristics
4.2. Estimated Real-World Reporting Rates of SADRs
4.3. Estimated Real-World Reporting Rates of SADR Categories
5. Discussion
5.1. Comparison to EMA’s Data
5.2. Comparison to the VAERS Database
5.3. Comparison to Vaccine Registration Trials
5.4. Estimated Real-World Reporting Rates of SADR Categories
5.5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 Pandemic and Research Gaps: Understanding SARS-CoV-2 Interaction with the ACE2 Receptor and Implications for Therapy. Theranostics 2020, 10, 7448. [Google Scholar] [CrossRef]
- Swenson, K.E.; Swenson, E.R. Pathophysiology of Acute Respiratory Distress Syndrome and COVID-19 Lung Injury. Crit. Care Clin. 2021, 37, 749. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601. [Google Scholar] [CrossRef]
- Griffin, D.O. Postacute Sequelae of COVID (PASC or Long COVID): An Evidenced-Based Approach. Open Forum Infect. Dis. 2024, 11, ofae462. [Google Scholar] [CrossRef]
- Hilser, J.R.; Spencer, N.J.; Afshari, K.; Gilliland, F.D.; Hu, H.; Deb, A.; Lusis, A.J.; Wilson Tang, W.H.; Hartiala, J.A.; Hazen, S.L.; et al. COVID-19 Is a Coronary Artery Disease Risk Equivalent and Exhibits a Genetic Interaction With ABO Blood Type. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.A.; Metaxaki, M.; Sithole, N.; Landín, P.; Martín, P.; Salinas-Botrán, A. Cardiovascular Disease and COVID-19: A Systematic Review. IJC Heart Vasc. 2024, 54, 101482. [Google Scholar] [CrossRef] [PubMed]
- Vosko, I.; Zirlik, A.; Bugger, H. Impact of COVID-19 on Cardiovascular Disease. Viruses 2023, 15, 508. [Google Scholar] [CrossRef] [PubMed]
- Fitero, A.; Negrut, N.; Popa, A.; John, H.T.; Ferician, A.C.; Manole, F.; Marian, P. Integrated Analysis of Remdesivir and Paxlovid in COVID-19 Patients: A Personalized Approach to High-Risk Individuals for Severe Evolution. J. Clin. Med. 2024, 13, 6670. [Google Scholar] [CrossRef]
- Wolfe, C.R.; Tomashek, K.M.; Patterson, T.F.; Gomez, C.A.; Marconi, V.C.; Jain, M.K.; Yang, O.O.; Paules, C.I.; Palacios, G.M.R.; Grossberg, R.; et al. Baricitinib versus Dexamethasone for Adults Hospitalised with COVID-19 (ACTT-4): A Randomised, Double-Blind, Double Placebo-Controlled Trial. Lancet Respir. Med. 2022, 10, 888. [Google Scholar] [CrossRef]
- Pérez-Alba, E.; Nuzzolo-Shihadeh, L.; Aguirre-García, G.M.; Espinosa-Mora, J.; Lecona-Garcia, J.D.; Flores-Pérez, R.O.; Mendoza-Garza, M.; Camacho-Ortiz, A. Baricitinib plus Dexamethasone Compared to Dexamethasone for the Treatment of Severe COVID-19 Pneumonia: A Retrospective Analysis. J. Microbiol. Immunol. Infect. 2021, 54, 787. [Google Scholar] [CrossRef]
- Basoulis, D.; Tsakanikas, A.; Gkoufa, A.; Bitsani, A.; Karamanakos, G.; Mastrogianni, E.; Georgakopoulou, V.E.; Makrodimitri, S.; Voutsinas, P.M.; Lamprou, P.; et al. Effectiveness of Oral Nirmatrelvir/Ritonavir vs. Intravenous Three-Day Remdesivir in Preventing Progression to Severe COVID-19: A Single-Center, Prospective, Comparative, Real-Life Study. Viruses 2023, 15, 1515. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, E.; Caffarelli, C.; Santamaria, F.; Corsello, G. Year 2022: Exploring COVID-19 Pandemic in Children. Ital. J. Pediatr. 2023, 49, 1–5. [Google Scholar] [CrossRef]
- Luxi, N.; Giovanazzi, A.; Capuano, A.; Crisafulli, S.; Cutroneo, P.M.; Fantini, M.P.; Ferrajolo, C.; Moretti, U.; Poluzzi, E.; Raschi, E.; et al. COVID-19 Vaccination in Pregnancy, Paediatrics, Immunocompromised Patients, and Persons with History of Allergy or Prior SARS-CoV-2 Infection: Overview of Current Recommendations and Pre- and Post-Marketing Evidence for Vaccine Efficacy and Safety. Drug Saf. 2021, 44, 1247. [Google Scholar] [CrossRef]
- Staiano, A.; Agostiniani, R.; Bozzola, E.; Russo, R.; Corsello, G. COVID 19 Vaccine in the Pediatric Age: The Recommendation of the Italian Pediatric Society. Ital. J. Pediatr. 2022, 48, 46. [Google Scholar] [CrossRef] [PubMed]
- Zinzi, A.; Gaio, M.; Liguori, V.; Ruggiero, R.; Tesorone, M.; Rossi, F.; Rafaniello, C.; Capuano, A. Safety Monitoring of MRNA COVID-19 Vaccines in Children Aged 5 to 11 Years by Using EudraVigilance Pharmacovigilance Database: The CoVaxChild Study. Vaccines 2023, 11, 401. [Google Scholar] [CrossRef]
- Nazar, W.; Romantowski, J.; Niedoszytko, M.; Daniłowicz-Szymanowicz, L. Cardiac Adverse Drug Reactions to COVID-19 Vaccines. A Cross-Sectional Study Based on the Europe-Wide Data. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 599–607. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Frenck, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Doherty, M.; Buchy, P.; Standaert, B.; Giaquinto, C.; Prado-Cohrs, D. Vaccine Impact: Benefits for Human Health. Vaccine 2016, 34, 6707–6714. [Google Scholar] [CrossRef] [PubMed]
- Ahmadizar, F.; Luxi, N.; Raethke, M.; Schmikli, S.; Riefolo, F.; Saraswati, P.W.; Bucsa, C.; Osman, A.; Liddiard, M.; Maques, F.B.; et al. Safety of COVID-19 Vaccines Among the Paediatric Population: Analysis of the European Surveillance Systems and Pivotal Clinical Trials. Drug Saf. 2023, 46, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, É.; Star, K.; Jonville-Béra, A.P.; Durrieu, G. Pharmacovigilance in Pediatrics. Therapie 2018, 73, 171–180. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). EudraVigilance. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/pharmacovigilance-research-development/eudravigilance (accessed on 9 February 2025).
- European Medicines Agency (EMA). EMA Recommends Approval of Comirnaty and Spikevax COVID-19 Vaccines for Children from 6 Months of Age. Available online: https://www.ema.europa.eu/en/news/ema-recommends-approval-comirnaty-spikevax-covid-19-vaccines-children-6-months-age (accessed on 9 February 2025).
- European Medicines Agency (EMA). EMA Recommends Authorisation of Nuvaxovid for Adolescents Aged 12 to 17. Available online: https://www.ema.europa.eu/en/news/ema-recommends-authorisation-nuvaxovid-adolescents-aged-12-17 (accessed on 9 February 2025).
- CHMP Summary of Product Characteristics—Nuvaxovid. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/nuvaxovid (accessed on 9 February 2025).
- Summary of Product Characteristics—Spikevax. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/spikevax (accessed on 9 February 2025).
- Summary of Product Characteristics—Comirnaty. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty (accessed on 9 February 2025).
- Elzagallaai, A.A.; Greff, M.J.E.; Rieder, M.J. Adverse Drug Reactions in Children: The Double-Edged Sword of Therapeutics. Clin. Pharmacol. Ther. 2017, 101, 725–735. [Google Scholar] [CrossRef]
- Nikolopoulou, G.B.; Maltezou, H.C. COVID-19 in Children: Where Do We Stand? Arch. Med. Res. 2022, 53, 1–8. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Hatziantoniou, S.; Theodoridou, K.; Vasileiou, K.; Anastassopoulou, C.; Tsakris, A. Anaphylaxis Rates Following MRNA COVID-19 Vaccination in Children and Adolescents: Analysis of Data Reported to EudraVigilance. Vaccine 2023, 41, 2382. [Google Scholar] [CrossRef]
- WHO. SAGE Roadmap for Prioritizing Uses of COVID-19 Vaccines: An Approach to Optimize the Global Impact of COVID-19 Vaccines, Based on Public Health Goals, Global and National Equity, and Vaccine Access and Coverage Scenarios. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2023.1 (accessed on 9 February 2025).
- Matsumoto, N.; Shimizu, J.; Yokoyama, Y.; Tsukahara, H.; Yorifuji, T. Adverse Reactions in Young Children Receiving the Coronavirus Disease 2019 Vaccine. Pediatr. Int. 2023, 65, e15696. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Wang, Y.; Yazici, D.; Azkur, D.; Ogulur, I.; Azkur, A.K.; Yang, Z.; Chen, X.; Zhang, A.; et al. Recent Developments in the Immunopathology of COVID-19. Allergy 2023, 78, 369–388. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Characteristics and Outcomes of US Children and Adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) Compared with Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef]
- Mascolo, A.; di Mauro, G.; Fraenza, F.; Gaio, M.; Zinzi, A.; Pentella, C.; Rossi, F.; Capuano, A.; Sportiello, L. Maternal, Fetal and Neonatal Outcomes among Pregnant Women Receiving COVID-19 Vaccination: The Preg-Co-Vax Study. Front. Immunol. 2022, 13, 965171. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. EudraVigilance: Electronic Reporting. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-and-development/pharmacovigilance-research-and-development/eudravigilance/eudravigilance-electronic-reporting (accessed on 3 February 2024).
- Brown, E.G.; Wood, L.; Wood, S. The Medical Dictionary for Regulatory Activities (MedDRA). Drug Saf. 1999, 20, 109–117. [Google Scholar] [CrossRef]
- Hazell, L.; Shakir, S.A.W. Under-Reporting of Adverse Drug Reactions: A Systematic Review. Drug Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2021, 32, NEJMoa2116298. [Google Scholar] [CrossRef]
- Muñoz, F.M.; Sher, L.D.; Sabharwal, C.; Gurtman, A.; Xu, X.; Kitchin, N.; Lockhart, S.; Riesenberg, R.; Sexter, J.M.; Czajka, H.; et al. Evaluation of BNT162b2 COVID-19 Vaccine in Children Younger than 5 Years of Age. N. Engl. J. Med. 2023, 388, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Creech, C.B.; Anderson, E.; Berthaud, V.; Yildirim, I.; Atz, A.M.; Melendez Baez, I.; Finkelstein, D.; Pickrell, P.; Kirstein, J.; Yut, C.; et al. Evaluation of MRNA-1273 COVID-19 Vaccine in Children 6 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Creech, C.B.; Berthaud, V.; Piramzadian, A.; Johnson, K.A.; Zervos, M.; Garner, F.; Griffin, C.; Palanpurwala, K.; Turner, M.; et al. Evaluation of MRNA-1273 Vaccine in Children 6 Months to 5 Years of Age. N. Engl. J. Med. 2022, 387, 1673–1687. [Google Scholar] [CrossRef]
- Ali, K.; Berman, G.; Zhou, H.; Deng, W.; Faughnan, V.; Coronado-Voges, M.; Ding, B.; Dooley, J.; Girard, B.; Hillebrand, W.; et al. Evaluation of MRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/glossary-terms/summary-product-characteristics (accessed on 9 February 2025).
- European Medicines Agency (EMA). COVID-19 Vaccine Spikevax Approved for Children Aged 12 to 17 in EU. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccine-spikevax-approved-children-aged-12-17-eu (accessed on 9 February 2025).
- European Medicines Agency (EMA). First COVID-19 Vaccine Approved for Children Aged 12 to 15 in EU. Available online: https://www.ema.europa.eu/en/news/first-covid-19-vaccine-approved-children-aged-12-15-eu (accessed on 9 February 2025).
- Hause, A.M.; Baggs, J.; Marquez, P.; Abara, W.E.; Olubajo, B.; Myers, T.R.; Su, J.R.; Thompson, D.; Gee, J.; Shimabukuro, T.T.; et al. Safety Monitoring of COVID-19 Vaccine Booster Doses Among Persons Aged 12–17 Years—United States, December 9, 2021–February 20, 2022. MMWR Morb. Mortal. Wkly. Rep. 2025, 71, 347–351. [Google Scholar] [CrossRef]
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Su, J.R.; Hugueley, B.; Thompson, D.; Gee, J.; Shimabukuro, T.T.; Shay, D.K. Safety Monitoring of Pfizer-BioNTech COVID-19 Vaccine Booster Doses Among Children Aged 5–11 Years—United States, May 17–July 31, 2022. MMWR Morb. Mortal. Wkly. Rep. 2025, 71, 1047–1051. [Google Scholar] [CrossRef]
- Hause, A.M.; Gee, J.; Baggs, J.; Abara, W.E.; Marquez, P.; Thompson, D.; Su, J.R.; Licata, C.; Rosenblum, H.G.; Myers, T.R.; et al. COVID-19 Vaccine Safety in Adolescents Aged 12–17 Years—United States, December 14, 2020–July 16, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 70, 1053–1058. [Google Scholar] [CrossRef]
- Hause, A.M.; Shay, D.K.; Klein, N.P.; Abara, W.E.; Baggs, J.; Cortese, M.M.; Fireman, B.; Gee, J.; Glanz, J.M.; Goddard, K.; et al. Safety of COVID-19 Vaccination in United States Children Ages 5 to 11 Years. Pediatrics 2022, 150, e2022057313. [Google Scholar] [CrossRef] [PubMed]
- Nazar, W.; Romantowski, J.; Nazar, G.; Niedoszytko, M.; Braun-Dullaeus, R.; Daniłowicz-Szymanowicz, L. Serious Adverse Drug Reactions Associated with Anti-SARS-CoV-2 Vaccines and Their Reporting Trends in the EudraVigilance Database. Sci. Rep. 2025, 15, 18582. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, Z. Cross-Sectional Studies: Strengths, Weaknesses, and Recommendations. Chest 2020, 158, S65–S71. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. COVID-19 Vaccine Tracker. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#distribution-tab (accessed on 3 February 2024).
| Demographic Characteristics | Comirnaty | Spikevax |
|---|---|---|
| Count | 5018 | 404 |
| Primary Source Qualification: Healthcare Professional, n (%) | 2531.0 (50.4) | 234.0 (57.9) |
| Primary Source Qualification: Non Healthcare Professional, n (%) | 2487.0 (49.6) | 170.0 (42.1) |
| Patient Age Group: 12–17 Years, n (%) | 4360.0 (86.9) | 380.0 (94.1) |
| Patient Age Group: 0–1 Month, n (%) | 56.0 (1.1) | 14.0 (3.5) |
| Patient Age Group: 2 Months–2 Years, n (%) | 64.0 (1.3) | 4.0 (1.0) |
| Patient Age Group: 3–11 Years, n (%) | 538.0 (10.7) | 6.0 (1.5) |
| Patient Sex: Male, n (%) | 2483.0 (49.5) | 204.0 (50.5) |
| Patient Sex: Female, n (%) | 2435.0 (48.5) | 197.0 (48.8) |
| Patient Sex: Not Specified, n (%) | 100.0 (2.0) | 3.0 (0.7) |
| Variable | Total Reported ADRs (n) | Comirnaty—Trial-Adjusted Rate (Per Million ADR Reports, 95% CI) | Spikevax—Trial-Adjusted Rate (Per Million ADR Reports, 95% CI) |
|---|---|---|---|
| All adverse drug reactions | 11,802 | 5792 (4634–6950) | 5671 (4536–6805) |
| Headache | 915 | 437 (331–550) | 592 (483–707) |
| Myocarditis | 672 | 322 (244–405) | 419 (342–501) |
| Syncope | 542 | 265 (201–333) | 279 (228–334) |
| Dizziness | 442 | 217 (165–274) | 206 (168–246) |
| Dyspnoea | 413 | 199 (151–251) | 239 (195–286) |
| Nausea | 377 | 179 (136–226) | 253 (206–302) |
| Vomiting | 367 | 178 (135–224) | 206 (168–246) |
| Loss of consciousness | 248 | 120 (91–151) | 140 (114–167) |
| Abdominal pain | 243 | 120 (91–151) | 106 (87–127) |
| Myalgia | 235 | 111 (84–140) | 166 (136–199) |
| Rash | 214 | 110 (83–138) | 40 (33–48) |
| Seizure | 213 | 110 (83–138) | 40 (33–48) |
| Tachycardia | 200 | 101 (77–127) | 60 (49–72) |
| Pain in extremity | 186 | 89 (68–112) | 113 (92–135) |
| Myopericarditis | 178 | 79 (60–99) | 193 (157–231) |
| Pericarditis | 178 | 87 (66–109) | 93 (76–111) |
| Arthralgia | 175 | 81 (61–102) | 146 (119–175) |
| Urticaria | 156 | 77 (58–97) | 73 (60–87) |
| Paraesthesia | 149 | 74 (56–93) | 60 (49–72) |
| Diarrhea | 140 | 72 (55–91) | 27 (22–32) |
| Lymphadenopathy | 137 | 67 (51–85) | 66 (54–79) |
| Palpitations | 131 | 63 (48–80) | 73 (60–87) |
| Arrhythmia | 109 | 57 (43–71) | 13 (11–16) |
| Hypoaesthesia | 107 | 53 (41–67) | 40 (33–48) |
| Cough | 99 | 48 (37–61) | 53 (43–64) |
| Category of Adverse Drug Reactions | Total Reported ADRs (n) | Comirnaty—Trial-Adjusted Rate (Per Million ADR Reports, 95% CI) | Spikevax—Trial-Adjusted Rate (Per Million ADR Reports, 95% CI) |
|---|---|---|---|
| Neuropsychiatric disorders category | 2532 | 1239 (939–1559) | 1263 (1031–1510) |
| Cardiovascular disorders category | 1753 | 853 (647–1073) | 938 (765–1121) |
| Gastroenterological disorders category | 1047 | 514 (389–646) | 505 (413–604) |
| Musculoskeletal and connective tissue disorders category | 716 | 345 (262–435) | 419 (342–501) |
| Respiratory, thoracic and mediastinal disorders category | 705 | 343 (260–431) | 379 (309–453) |
| Skin and subcutaneous tissue disorders category | 639 | 315 (239–397) | 286 (233–342) |
| Ear and eye disorders category | 358 | 176 (134–222) | 166 (136–199) |
| Hematooncological disorders category | 288 | 144 (109–181) | 106 (87–127) |
| Obstetrical and gynecological disorders category | 272 | 137 (104–172) | 86 (71–103) |
| Immune system disorders category | 211 | 105 (79–132) | 86 (71–103) |
| Renal and urinary disorders category | 70 | 32 (24–40) | 66 (54–79) |
| Congenital, familial and genetic disorders category | 13 | 5 (4–6) | 27 (22–32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazar, G.; Olszlegier, J.; Kamińska, A.; Plata-Nazar, K.; Nazar, W. Serious Adverse Drug Reactions to COVID-19 Vaccines in the Pediatric Population: A Retrospective, Cross-Sectional Study Utilizing the Eudravigilance Database for the European Economic Area. J. Clin. Med. 2025, 14, 6542. https://doi.org/10.3390/jcm14186542
Nazar G, Olszlegier J, Kamińska A, Plata-Nazar K, Nazar W. Serious Adverse Drug Reactions to COVID-19 Vaccines in the Pediatric Population: A Retrospective, Cross-Sectional Study Utilizing the Eudravigilance Database for the European Economic Area. Journal of Clinical Medicine. 2025; 14(18):6542. https://doi.org/10.3390/jcm14186542
Chicago/Turabian StyleNazar, Grzegorz, Julia Olszlegier, Aleksandra Kamińska, Katarzyna Plata-Nazar, and Wojciech Nazar. 2025. "Serious Adverse Drug Reactions to COVID-19 Vaccines in the Pediatric Population: A Retrospective, Cross-Sectional Study Utilizing the Eudravigilance Database for the European Economic Area" Journal of Clinical Medicine 14, no. 18: 6542. https://doi.org/10.3390/jcm14186542
APA StyleNazar, G., Olszlegier, J., Kamińska, A., Plata-Nazar, K., & Nazar, W. (2025). Serious Adverse Drug Reactions to COVID-19 Vaccines in the Pediatric Population: A Retrospective, Cross-Sectional Study Utilizing the Eudravigilance Database for the European Economic Area. Journal of Clinical Medicine, 14(18), 6542. https://doi.org/10.3390/jcm14186542








