The Prognostic Roles of Systemic Inflammatory Markers Before Abiraterone or Enzalutamide Therapy in Metastatic Castration-Resistant Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Centers
2.2. Patient Selection
- (1)
- Histologically confirmed prostate adenocarcinoma;
- (2)
- Castration-resistant disease, defined by biochemical, radiological, or clinical progression despite castrate levels of testosterone (<50 ng/dL);
- (3)
- Initiation of at least one cycle of ABI or ENZA;
- (4)
- Availability of baseline clinical variables, PSA data, and pre-treatment complete blood count parameters.
2.3. Data Collection
2.4. Laboratory and Radiological Assessments, Variable Definitions, and ROC Analysis
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, H.; Roobol, M.J.; Chapple, C.R.; Catto, J.W.; N’Dow, J.; Sønksen, J.; Stenzl, A.; Wirth, M. Prostate-specific antigen testing as part of a risk-adapted early detection strategy for prostate cancer: European Association of Urology position and recommendations for 2021. Eur. Urol. 2021, 80, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Aliberti, A.; Bada, M.; Rapisarda, S.; Natoli, C.; Schips, L.; Cindolo, L. Adherence to hormonal deprivation therapy in prostate cancer in clinical practice: A retrospective, single-center study. Minerva Urol. Nefrol. 2019, 71, 181–184. [Google Scholar] [CrossRef]
- Le, T.K.; Duong, Q.H.; Baylot, V.; Fargette, C.; Baboudjian, M.; Colleaux, L.; Taïeb, D.; Rocchi, P. Castration-Resistant Prostate Cancer: From Uncovered Resistance Mechanisms to Current Treatments. Cancers 2023, 15, 5047. [Google Scholar] [CrossRef]
- ŞEN, F. Kastrasyon Dirençli Metastatik Prostat Kanserli Hastaya Yaklaşım. Turk. Klin. Med. Oncol.-Spec. Top. 2015, 8, 39–50. [Google Scholar]
- Quistini, A.; Chierigo, F.; Fallara, G.; Depalma, M.; Tozzi, M.; Maggi, M.; Jannello, L.M.I.; Pellegrino, F.; Mantica, G.; Terracciano, D.; et al. Androgen Receptor Signalling in Prostate Cancer: Mechanisms of Resistance to Endocrine Therapies. Res. Rep. Urol. 2025, 17, 211–223. [Google Scholar] [CrossRef]
- Poon, D.M.C.; Cheung, W.S.K.; Chiu, P.K.F.; Chung, D.H.S.; Kung, J.B.T.; Lam, D.C.M.; Leung, A.K.C.; Ng, A.C.F.; O’Sullivan, J.M.; Teoh, J.Y.C.; et al. Treatment of metastatic castration-resistant prostate cancer: Review of current evidence and synthesis of expert opinions on radioligand therapy. Front. Oncol. 2025, 15, 1530580. [Google Scholar] [CrossRef]
- He, L.; Fang, H.; Chen, C.; Wu, Y.; Wang, Y.; Ge, H.; Wang, L.; Wan, Y.; He, H. Metastatic castration-resistant prostate cancer: Academic insights and perspectives through bibliometric analysis. Medicine 2020, 99, e19760. [Google Scholar] [CrossRef]
- George, D.J.; Ramaswamy, K.; Yang, H.; Liu, Q.; Zhang, A.; Greatsinger, A.; Ivanova, J.; Thompson, B.; Emir, B.; Hong, A.; et al. Real-world overall survival with abiraterone acetate versus enzalutamide in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2024, 27, 756–764. [Google Scholar] [CrossRef]
- Shore, N.D.; Ionescu-Ittu, R.; Laliberté, F.; Yang, L.; Lejeune, D.; Yu, L.; Duh, M.S.; Mahendran, M.; Kim, J.; Ghate, S.R. Beyond Frontline Therapy with Abiraterone and Enzalutamide in Metastatic Castration-Resistant Prostate Cancer: A Real-World US Study. Clin. Genitourin. Cancer 2021, 19, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; Mulders, P.F.A.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Bono, J.S.d.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B.; Saad, F.; et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; Wit, R.d.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Fiorentino, V.; Pepe, L.; Pizzimenti, C.; Zuccalà, V.; Pepe, P.; Cianci, V.; Mondello, C.; Tuccari, G.; Fadda, G.; Giuffrè, G.; et al. PD-L1 expression in prostate cancer and Gleason Grade Group: Is there any relationship? Findings from a multi-institutional cohort. Pathol.-Res. Pract. 2025, 269, 155916. [Google Scholar] [CrossRef]
- Kiełb, P.; Kowalczyk, K.; Gurwin, A.; Nowak, Ł.; Krajewski, W.; Sosnowski, R.; Szydełko, T.; Małkiewicz, B. Novel Histopathological Biomarkers in Prostate Cancer: Implications and Perspectives. Biomedicines 2023, 11, 1552. [Google Scholar] [CrossRef]
- Fiorentino, V.; Martini, M.; Dell’Aquila, M.; Musarra, T.; Orticelli, E.; Larocca, L.M.; Rossi, E.; Totaro, A.; Pinto, F.; Lenci, N.; et al. Histopathological Ratios to Predict Gleason Score Agreement between Biopsy and Radical Prostatectomy. Diagnostics 2021, 11, 10. [Google Scholar] [CrossRef]
- Pepe, P.; Pepe, L.; Fiorentino, V.; Curduman, M.; Pennisi, M.; Fraggetta, F. PSMA PET/CT Accuracy in Diagnosing Prostate Cancer Nodes Metastases. In Vivo 2024, 38, 2880–2885. [Google Scholar] [CrossRef]
- Vlajnic, T.; Bubendorf, L. Molecular pathology of prostate cancer: A practical approach. Pathology 2021, 53, 36–43. [Google Scholar] [CrossRef]
- Kumarasamy, C.; Tiwary, V.; Sunil, K.; Suresh, D.; Shetty, S.; Muthukaliannan, G.K.; Baxi, S.; Jayaraj, R. Prognostic Utility of Platelet–Lymphocyte Ratio, Neutrophil–Lymphocyte Ratio and Monocyte–Lymphocyte Ratio in Head and Neck Cancers: A Detailed PRISMA Compliant Systematic Review and Meta-Analysis. Cancers 2021, 13, 4166. [Google Scholar] [CrossRef] [PubMed]
- Şahin, E.; Kefeli, U.; Zorlu, Ş.; Seyyar, M.; Ozkorkmaz Akdag, M.; Can Sanci, P.; Karakayali, A.; Ucuncu Kefeli, A.; Bakkal Temi, Y.; Cabuk, D.; et al. Prognostic role of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index, and pan-immune-inflammation value in metastatic castration-resistant prostate cancer patients who underwent 177Lu–PSMA-617. Medicine 2023, 102, e35843. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Xiong, H.; Feng, Y.; Liao, G.; Tong, T.; Pang, J. Revealing the prognostic landscape of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with abiraterone or enzalutamide: A meta-analysis. Prostate Cancer Prostatic Dis. 2020, 23, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef]
- Zhou, M.; Liang, J.; Hui, J.; Xu, J. Inflammation-related indicators have a potential to increase overall quality of the prostate cancer management: A narrative review. Transl. Androl. Urol. 2023, 12, 809–822. [Google Scholar] [CrossRef]
- Peng, H.; Luo, X. Prognostic significance of elevated pretreatment systemic inflammatory markers for patients with prostate cancer: A meta-analysis. Cancer Cell Int. 2019, 19, 70. [Google Scholar] [CrossRef]
- Wu, H.; Gong, M.; Yuan, R. Relationship between neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) in peripheral blood and prognosis after castration therapy for prostate cancer. Indian J. Cancer 2024, 61, 193–199. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef]
- Eisenhauera, E.; Therasseb, P.; Bogaertsc, J.; Schwartzd, L.; Sargente, D.; Fordf, R.; Danceyg, J.; Arbuckh, S.; Gwytheri, S.; Mooneyg, M. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Salciccia, S.; Frisenda, M.; Bevilacqua, G.; Viscuso, P.; Casale, P.; De Berardinis, E.; Di Pierro, G.B.; Cattarino, S.; Giorgino, G.; Rosati, D.; et al. Prognostic role of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with non-metastatic and metastatic prostate cancer: A meta-analysis and systematic review. Asian J. Urol. 2024, 11, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Ryan Charles, J.; Smith Matthew, R.; de Bono Johann, S.; Molina, A.; Logothetis Christopher, J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats Josep, M.; Ng, S.; et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R., Jr.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- de Wit, R.; de Bono, J.; Sternberg Cora, N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.-C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef]
- Pisano, C.; Tucci, M.; RF, D.I.S.; Turco, F.; Samuelly, A.; Bungaro, M.; Vignani, F.; Tarenghi, F.; Scagliotti, G.V.; Maio, M.D.I.; et al. Prognostic role of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with metastatic castration resistant prostate cancer treated with abiraterone or enzalutamide. Minerva Urol. Nephrol. 2021, 73, 803–814. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; He, Y.; Chen, X.; Liu, N.; Ding, Z.; Li, J. Prognostic role of platelet to lymphocyte ratio in prostate cancer: A meta-analysis. Medicine 2018, 97, e12504. [Google Scholar] [CrossRef]
- de Wit, R.; Wülfing, C.; Castellano, D.; Kramer, G.; Eymard, J.C.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Bamias, A.; Carles, J.; et al. Baseline neutrophil-to-lymphocyte ratio as a predictive and prognostic biomarker in patients with metastatic castration-resistant prostate cancer treated with cabazitaxel versus abiraterone or enzalutamide in the CARD study. ESMO Open 2021, 6, 100241. [Google Scholar] [CrossRef]
- Liu, D.; Czigany, Z.; Heij, L.R.; Bouwense, S.A.W.; van Dam, R.; Lang, S.A.; Ulmer, T.F.; Neumann, U.P.; Bednarsch, J. The Value of Platelet-to-Lymphocyte Ratio as a Prognostic Marker in Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 438. [Google Scholar] [CrossRef]
- Fukuokaya, W.; Kimura, T.; Urabe, F.; Kimura, S.; Tashiro, K.; Tsuzuki, S.; Koike, Y.; Sasaki, H.; Miki, K.; Egawa, S. Blood platelet volume predicts treatment-specific outcomes of metastatic castration-resistant prostate cancer. Int. J. Clin. Oncol. 2020, 25, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Taban, H.; Erman, M.; Guven, D.C.; Aktas, B.Y.; Yilmaz, F.; Yaşar, S.; Yildirim, H.C.; Aslan, F.; Aksoy, S. Prognostic Nutritional Index and a Blood-Based Prognostic Tool in Prostate Cancer Treated with Abiraterone, Enzalutamide or Cabazitaxel. Medicina 2025, 61, 1105. [Google Scholar] [CrossRef] [PubMed]


| Variable | Value |
|---|---|
| Age at diagnosis (years) | 69 (40–90) |
| PSA at diagnosis (median, ng/mL) | 82 (4–214,404) |
| PSA before ABI/ENZA (median, ng/mL) | 2.84 (0.84–14.84) |
| Gleason score | |
| 4 + 5 | 44 (41.5%) |
| 4 + 4 | 22 (20.8%) |
| 5 + 5 | 13 (12.3%) |
| 4 + 3 | 10 (9.4%) |
| 3 + 4 | 7 (6.6%) |
| Treatment in castration-sensitive phase | |
| ADT | 56 (52.8%) |
| Docetaxel + ADT | 50 (47.2%) |
| First-line treatment in CRPC phase | |
| Enzalutamide | 51 (48.1%) |
| Abiraterone | 32 (30.2%) |
| Docetaxel | 18 (17.0%) |
| Cabazitaxel | 3 (2.8%) |
| Lutetium | 2 (1.9%) |
| Prior docetaxel before ABI/ENZA | |
| Yes | 67 (63.2%) |
| No | 39 (36.8%) |
| ECOG PS | |
| 0 | 21 (19.8%) |
| 1 | 82 (77.4%) |
| 2 | 3 (2.8%) |
| Number of prior treatments before ABI/ENZA | |
| 1 line | 83 (78.3%) |
| 2 lines | 23 (21.7%) |
| Treatment response | |
| Yes | 75 (70.8%) |
| No | 31 (29.2%) |
| Clinical response | |
| Complete response | 16 (15.1%) |
| Partial response | 59 (55.7%) |
| Stable disease | 13 (12.3%) |
| Progression | 18 (17.0%) |
| PSA response | |
| Yes | 75 (70.8%) |
| No | 31 (29.2%) |
| NLR | |
| ≤2.83 | 51 (48.1%) |
| >2.83 | 55 (51.9%) |
| PLR | |
| ≤156 | 52 (49.1%) |
| >156 | 54 (50.9%) |
| MPV before ABI/ENZA (median, fL) | 9.7 (9.2–10.4) |
| MPV after ABI/ENZA (median, fL) | 9.7 (9.2–10.4) |
| PDW before ABI/ENZA (median, fL) | 11.2 (10.5–12.1) |
| PDW after ABI/ENZA (median, fL) | 11.3 (10.6–12.0) |
| P-LCR before ABI/ENZA, % | 156.1 (IQR 120–195) |
| P-LCR after ABI/ENZA, % | 156.1 (IQR 120–196) |
| Variable | Median PFS (Months) (95% CI) | p-Value (PFS) | Median OS (Months) (95% CI) | p-Value (OS) |
|---|---|---|---|---|
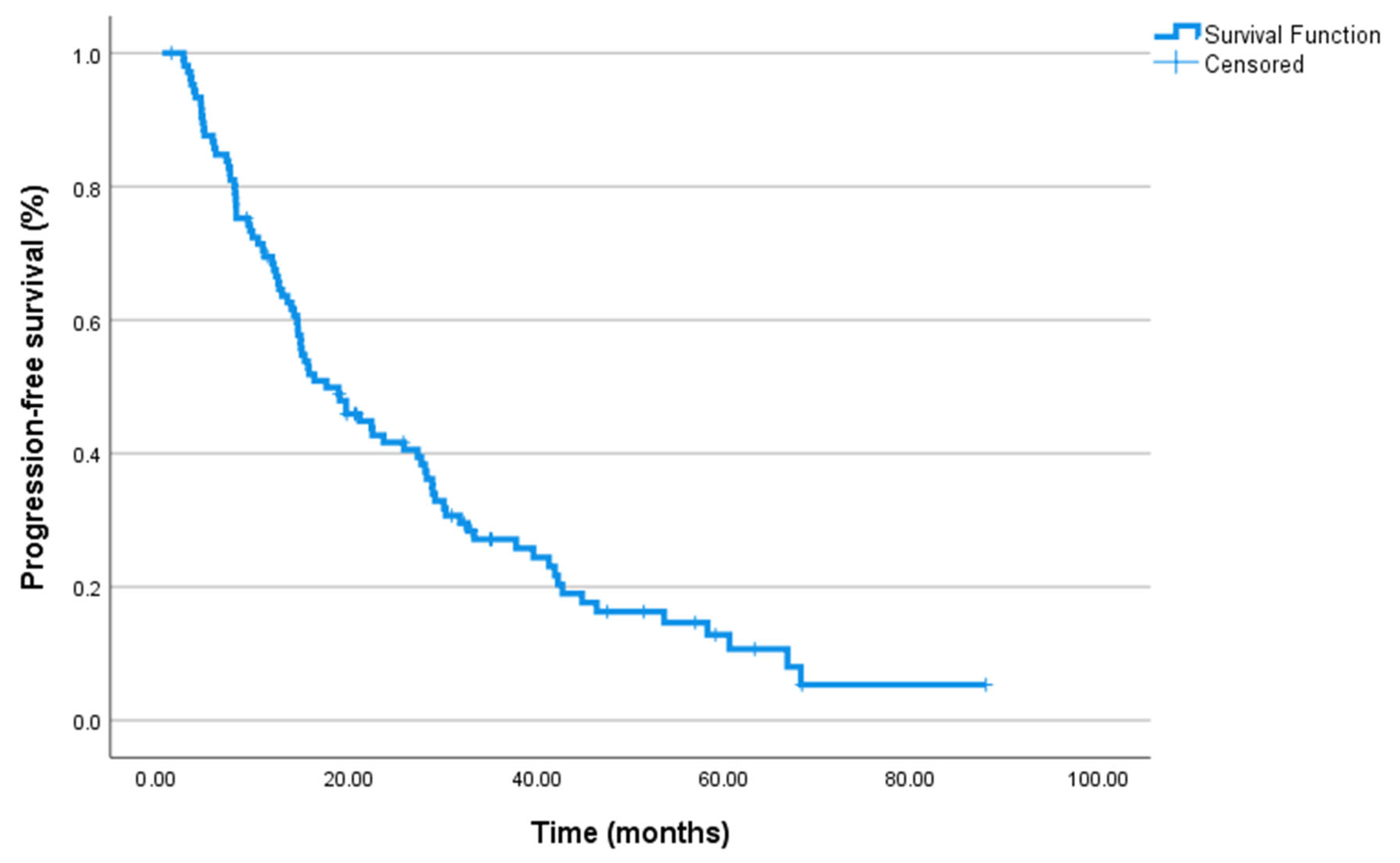
| Overall cohort | 17.5 (11.99–23.07) | – | 24.4 (18.6–30.2) | – |
| First-line treatment | 0.618 | 0.884 | ||
| ADT only | 19.6 (12.6–26.7) | 22.0 (17.3–26.7) | ||
| Docetaxel + ADT | 14.8 (9.0–20.6) | 27.6 (17.8–37.4) | ||
| Prior docetaxel use | 0.896 | 0.744 | ||
| Not administered | 19.6 (7.4–31.8) | 22.03 (16.93–27.14) | ||
| Administered | 15.6 (10.5–20.6) | 24.97 (18.45–31.49) | ||
| ECOG PS | 0.963 | 0.498 | ||
| ECOG PS 0 | 18.8 (1.3–36.4) | 37.03 (13.44–60.62) | ||
| ECOG PS 1 | 17.5 (11.6–23.5) | 22.03 (17.11–26.96) | ||
| ECOG PS 2 | 22.4 (9.7–35.2) | 0.24 (0.00–48.57) | ||
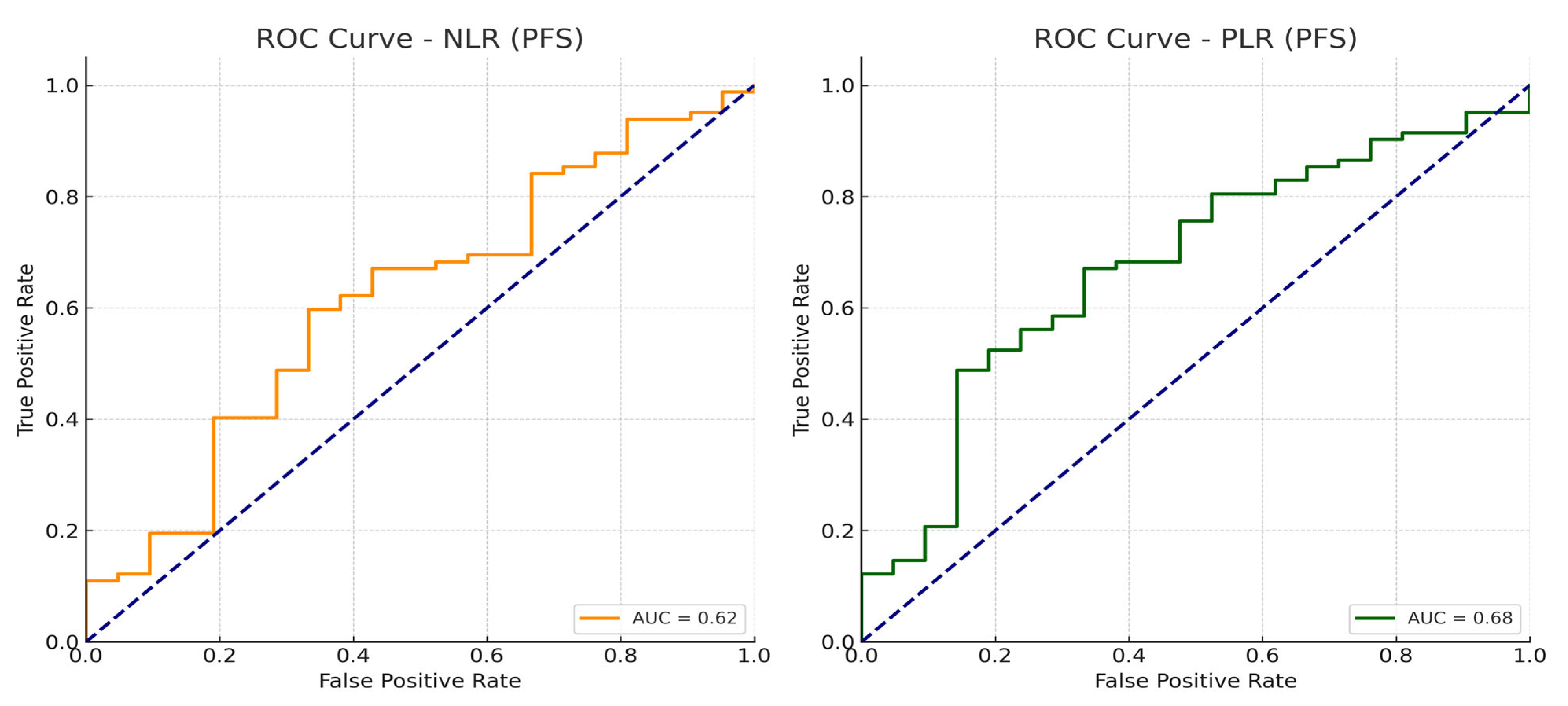
| NLR | 0.022 | 0.004 | ||
| NLR ≤ 2.83 | 27.67 (19.79–35.54) | 37.1 (18.72–55.48) | ||
| NLR > 2.83 | 14.07 (9.36–18.77) | 18.83 (13.52–24.15) | ||
| PLR | 0.004 | <0.001 | ||
| PLR ≤ 156 | 28.87 (25.88–31.86) | 46.2 (33.5–58.9) | ||
| PLR > 156 | 13.80 (9.32–18.28) | 16.7 (10.9–22.5) | ||
| Treatment response status | <0.001 | |||
| Response | 28.23 (24.67–31.80) | - | - | |
| No response | 7.83 (6.92–8.74) | - | - | |
| PSA response status | <0.001 | |||
| Response | 28.2 (24.7–31.8) | - | - | |
| No response | 7.8 (6.9–8.7) | - | - | |
| Line of systemic therapy | 0.637 | |||
| ABI/ENZA as first line | - | 25.7 (16.8–34.5) | ||
| ABI/ENZA in second line or later | - | 31.6(12.3–35.5) | ||
| MPV ≤ 9.7 vs. >9.7 fL | 17.0 (12.0–22.0) vs. 17.5 (11.5–23.5) | 0.74 | 23.0 (17.0–29.0) vs. 24.0 (18.0–30.0) | 0.62 |
| PDW low vs. high | 16.8 (11.2–22.4) vs. 18.0 (12.3–23.7) | 0.84 | 22.5 (17.0–28.0) vs. 23.5 (18.5–29.0) | 0.77 |
| P-LCR low vs. high | 16.9 (11.5–22.3) vs. 17.8 (12.7–23.9) | 0.46 | 23.1 (17.5–28.7) vs. 24.2 (18.9–29.5) | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muğlu, H.; Sünger, E.; Şeker Can, L.; Hamdard, J.; Açıkgöz, Ö.; Yıldız, Ö.; Ölmez, Ö.F.; Şeker, M.; Bilici, A. The Prognostic Roles of Systemic Inflammatory Markers Before Abiraterone or Enzalutamide Therapy in Metastatic Castration-Resistant Prostate Cancer. J. Clin. Med. 2025, 14, 6536. https://doi.org/10.3390/jcm14186536
Muğlu H, Sünger E, Şeker Can L, Hamdard J, Açıkgöz Ö, Yıldız Ö, Ölmez ÖF, Şeker M, Bilici A. The Prognostic Roles of Systemic Inflammatory Markers Before Abiraterone or Enzalutamide Therapy in Metastatic Castration-Resistant Prostate Cancer. Journal of Clinical Medicine. 2025; 14(18):6536. https://doi.org/10.3390/jcm14186536
Chicago/Turabian StyleMuğlu, Harun, Erdem Sünger, Lamia Şeker Can, Jamshid Hamdard, Özgür Açıkgöz, Özcan Yıldız, Ömer Fatih Ölmez, Mesut Şeker, and Ahmet Bilici. 2025. "The Prognostic Roles of Systemic Inflammatory Markers Before Abiraterone or Enzalutamide Therapy in Metastatic Castration-Resistant Prostate Cancer" Journal of Clinical Medicine 14, no. 18: 6536. https://doi.org/10.3390/jcm14186536
APA StyleMuğlu, H., Sünger, E., Şeker Can, L., Hamdard, J., Açıkgöz, Ö., Yıldız, Ö., Ölmez, Ö. F., Şeker, M., & Bilici, A. (2025). The Prognostic Roles of Systemic Inflammatory Markers Before Abiraterone or Enzalutamide Therapy in Metastatic Castration-Resistant Prostate Cancer. Journal of Clinical Medicine, 14(18), 6536. https://doi.org/10.3390/jcm14186536






