A Decade of Innovation: Short-Term Outcomes of 150 Robotic Liver Resections
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
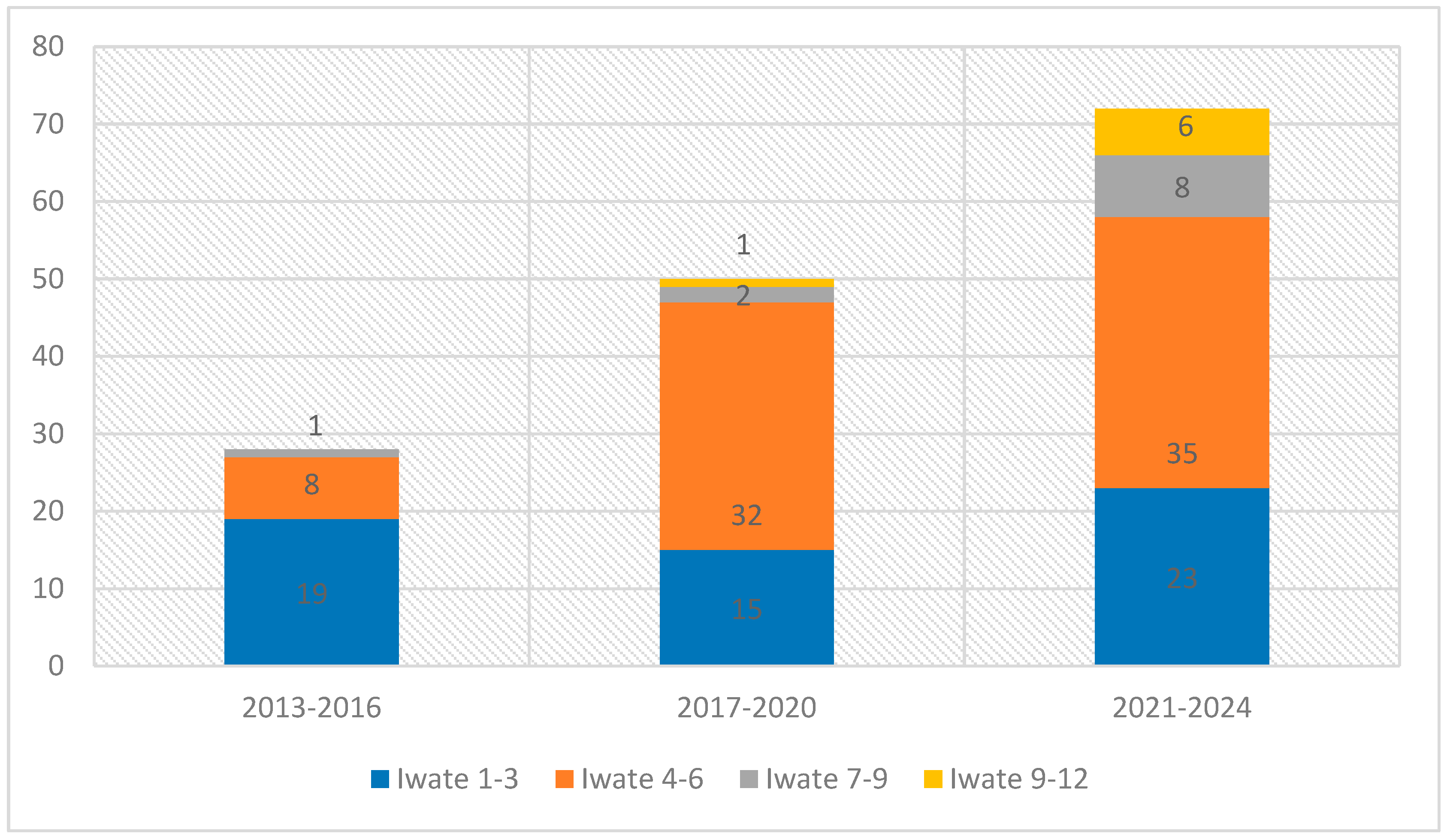
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Alpha-fetoprotein |
| ALC | Alcoholic Liver Cirrhosis |
| ALPSS | Associating Liver Partition and Portal vein ligation for Staged hepatectomy |
| ASA | American Society of Anaesthesiologists |
| CA19-9 | Carbohydrate Antigen 19-9 |
| CCA | Cholangiocarcinoma |
| CEA | Carcinoembryonic Antigen |
| CRLM | Colorectal liver metastases |
| GBC | Gallbladder cancer |
| ERAS | Enhanced Recovery After Surgery (ERAS) |
| GIST | Gastrointestinal stromal tumor |
| HCC | Hepatocellular carcinoma |
| ICG | Indocyanine Green |
| LLR | Laparoscopic liver resection |
| LOS | length of stay |
| MELD | Model for end-stage liver disease |
| MILS | Minimally invasive liver surgery |
| NASH | Non alcolic steat hepatitis |
| NCRLM | Non colorectal liver metastases |
| PDAC | Pancreatic ductal adenocarcinoma |
| RLR | Robotic liver resection |
References
- Reich, H.; McGlynn, F.; DeCaprio, J.; Budin, R. Laparoscopic excision of benign liver lesions. Obstet. Gynecol. 1991, 78, 956–958. [Google Scholar]
- Himpens, J.; Leman, G.; Cadiere, G.B. Telesurgical laparoscopic cholecystectomy. Surg. Endosc. 1998, 12, 1091. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Bianco, F.M.; Daskalaki, D.; Gonzalez-Ciccarelli, L.F.; Kim, J.; Benedetti, E. Robotic liver surgery: Technical aspects and review of the literature. HepatoBiliary Surg. Nutr. 2016, 5, 311–321. [Google Scholar] [CrossRef]
- Liu, R.; Hilal, M.A.; Wakabayashi, G.; Han, H.S.; Palanivelu, C.; Boggi, U.; Hackert, T.; Kim, H.J.; Wang, X.Y.; Hu, M.G.; et al. International experts consensus guidelines on robotic liver resection in 2023. World J. Gastroenterol. 2023, 29, 4815–4830. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Coratti, A.; Sbrana, F.; Addeo, P.; Bianco, F.M.; Buchs, N.C.; Annechiarico, M.; Benedetti, E. Robotic liver surgery: Results for 70 resections. Surgery 2011, 149, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.C.H.; Yang, G.P.C.; Tang, C.N. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: Short-term outcome. Am. J. Surg. 2013, 205, 697–702. [Google Scholar] [CrossRef]
- Brolese, A.; Rigoni, M.; Pasquale, A.; Viel, G.; Brolese, M.; Ciarleglio, F.A. The role of robotic surgery for the treatment of hilar cholangiocarcinoma: A systematic review. Front. Oncol. 2022, 12, 1001838. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Hara, T.; Matsushima, H.; Soyama, A.; Eguchi, S. Essential updates 2022/2023: A review of current topics in robotic hepatectomy. Ann. Gastroenterol. Surg. 2024, 8, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Sijberden, J.P.; Hoogteijling, T.J.; Aghayan, D.; Ratti, F.; Tan, E.K.; Morrison-Jones, V.; Lanari, J.; Haentjens, L.; Wei, K.; Tzedakis, S.; et al. Robotic Versus Laparoscopic Liver Resection in Various Settings: An International Multicenter Propensity Score Matched Study of 10.075 Patients. Ann. Surg. 2024, 280, 108–117. [Google Scholar] [CrossRef]
- Liu, R.; Wakabayashi, G.; Kim, H.J.; Choi, G.H.; Yiengpruksawan, A.; Fong, Y.; He, J.; Boggi, U.; Troisi, R.I.; Efanov, M.; et al. International consensus statement on robotic hepatectomy surgery in 2018. World J. Gastroenterol. 2019, 25, 1432–1444. [Google Scholar] [CrossRef]
- Tanaka, S.; Kawaguchi, Y.; Kubo, S.; Kanazawa, A.; Takeda, Y.; Hirokawa, F.; Nitta, H.; Nakajima, T.; Kaizu, T.; Kaibori, M.; et al. Validation of index-based IWATE criteria as an improved difficulty scoring system for laparoscopic liver resection. Surgery 2019, 165, 731–740. [Google Scholar] [CrossRef]
- Beard, R.E.; Khan, S.; Troisi, R.I.; Montalti, R.; Vanlander, A.; Fong, Y.; Kingham, T.P.; Boerner, T.; Berber, E.; Kahramangil, B.; et al. Long-Term and Oncologic Outcomes of Robotic Versus Laparoscopic Liver Resection for Metastatic Colorectal Cancer: A Multicenter, Propensity Score Matching Analysis. World J. Surg. 2020, 44, 887–895. [Google Scholar] [CrossRef]
- Owens, W.D.; Felts, J.A.; Spitznagel, E.L. ASA physical status classifications: A study of consistency of ratings. Anesthesiology 1978, 49, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.A. The comprehensive complication index: A novel continuous scale to measure surgical morbidity. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, B.; Marichez, A.; Adam, J.P.; Laurent, C. Use of a Urinary Catheter for the Intracorporeal Pringle Maneuver During Laparoscopic Liver Resection: Detailed Surgical Technique with Video. Indian J. Surg. 2022, 84, 406–408. [Google Scholar] [CrossRef]
- Pasquale, A.; Marinelli, L.; Ciarleglio, F.A.; Campora, M.; Salimian, N.; Viel, G.; Brolese, A. Robotic resection of a single adenoid cystic tumor liver metastasis using ICG fluorescence. A case report and literature review. Front. Surg. 2023, 10, 1162639. [Google Scholar] [CrossRef]
- Hilal, M.A.; Aldrighetti, L.; Dagher, I.; Edwin, B.; Troisi, R.I.; Alikhanov, R.; Aroori, S.; Belli, G.; Besselink, M.; Briceno, J.; et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann. Surg. 2018, 268, 11–18. [Google Scholar] [CrossRef]
- Berber, E.; Akyildiz, H.Y.; Aucejo, F.; Gunasekaran, G.; Chalikonda, S.; Fung, J. Robotic versus laparoscopic resection of liver tumours. Hpb 2010, 12, 583–586. [Google Scholar] [CrossRef]
- Görgec, B.; Zwart, M.; Nota, C.L.; Bijlstra, O.D.; Bosscha, K.; De Boer, M.T.; De Wilde, R.F.; Draaisma, W.A.; Gerhards, M.F.; Liem, M.S.; et al. Implementation and Outcome of Robotic Liver Surgery in the Netherlands: A Nationwide Analysis. Ann. Surg. 2023, 277, E1269–E1277. [Google Scholar] [CrossRef]
- Brolese, A.; Rigoni, M.; Vitale, A.; de Pretis, G.; Avancini, I.; Pravadelli, C.; Frisinghelli, M.; Rozzanigo, U.; Luppi, G.; Dionisi, F.; et al. Role of laparoscopic and robotic liver resection compared to open surgery in elderly hepatocellular carcinoma patients: A systematic review and metaanalysis. Hepatoma Res. 2020, 6, 1–15. [Google Scholar] [CrossRef]
- Goh, B.K.P.; Lee, S.Y.; Teo, J.Y.; Kam, J.H.; Jeyaraj, P.R.; Cheow, P.C.; Chow, P.K.H.; Ooi, L.L.P.J.; Chung, A.Y.F.; Chan, C.Y. Changing trends and outcomes associated with the adoption of minimally invasive hepatectomy: A contemporary single-institution experience with 400 consecutive resections. Surg. Endosc. 2018, 32, 4658–4665. [Google Scholar] [CrossRef]
- Balzano, E.; Bernardi, L.; Roesel, R.; Vagelli, F.; Ghinolfi, D.; Tincani, G.; Catalano, G.; Melandro, F.; Petrusic, A.; Popeskou, S.G.; et al. Robotic versus laparoscopic liver resections: Propensity-matched comparison of two-center experience. Surg. Endosc. 2023, 37, 8123–8132. [Google Scholar] [CrossRef]
- Reddy, K.; Gharde, P.; Tayade, H.; Patil, M.; Reddy, L.S.; Surya, D. Advancements in Robotic Surgery: A Comprehensive Overview of Current Utilizations and Upcoming Frontiers. Cureus 2023, 15, e50415. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Wakabayashi, G.; Nitta, H.; Ito, N.; Hasegawa, Y.; Takahara, T. Systematic review of robotic liver resection. Surg. Endosc. 2013, 27, 732–739. [Google Scholar] [CrossRef]
- Pilz da Cunha, G.; Sijberden, J.P.; Gobardhan, P.; Lips, D.J.; Terkivatan, T.; Marsman, H.A.; Patijn, G.A.; Leclercq, W.K.G.; Bosscha, K.; Mieog, J.S.D.; et al. Risk factors and outcomes of conversions in robotic and laparoscopic liver resections: A nationwide analysis. Surgery 2024, 178, 108820. [Google Scholar] [CrossRef]
- Bozkurt, E.; Sijberden, J.P.; Hilal, M.A. What Is the Current Role and What Are the Prospects of the Robotic Approach in Liver Surgery? Cancers 2022, 14, 4268. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, Q.; Xu, Y.; Wang, W. Comparative clinical outcomes of robotassisted liver resection versus laparoscopic liver resection: A meta-analysis. PLoS ONE 2020, 15, e0240593. [Google Scholar] [CrossRef]
- Knitter, S.; Feldbrügge, L.; Nevermann, N.; Globke, B.; Galindo, S.A.O.; Winklmann, T.; Krenzien, F.; Haber, P.K.; Malinka, T.; Lurje, G.; et al. Robotic versus laparoscopic versus open major hepatectomy—An analysis of costs and postoperative outcomes in a single-center setting. Langenbeck’s Arch. Surg. 2023, 408, 214. [Google Scholar] [CrossRef]
- Spiegelberg, J.; Iken, T.; Diener, M.K.; Fichtner-Feigl, S. Robotic-Assisted Surgery for Primary Hepatobiliary Tumors—Possibilities and Limitations. Cancers 2022, 14, 265. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, F.; Petrowsky, H.; Magistri, P.; Halazun, K.J. Robotic liver resection: Hurdles and beyond. Int. J. Surg. 2020, 82, 155–162. [Google Scholar] [CrossRef]
- Zhu, P.; Liao, W.; Ding, Z.Y.; Chen, L.; Zhang, W.G.; Zhang, B.X.; Chen, X.P. Learning Curve in Robot-Assisted Laparoscopic Liver Resection. J. Gastrointest. Surg. 2019, 23, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Aldrighetti, L.; Ratti, F.; Cillo, U.; Ferrero, A.; Ettorre, G.M.; Guglielmi, A.; Giuliante, F.; Calise, F. Diffusion, outcomes and implementation of minimally invasive liver surgery: A snapshot from the I Go MILS (Italian Group of Minimally Invasive Liver Surgery) Registry. Updates Surg. 2017, 69, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Chen, Y.; Yang, K.; Ma, D.; Gong, X.; Shen, B.; Peng, C. ScienceDirect Clinical efficacy of robot-assisted versus laparoscopic liver resection: A meta analysis. Asian J. Surg. 2019, 42, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Durán, M.; Briceño, J.; Padial, A.; Anelli, F.M.; Sánchez-Hidalgo, J.M.; Ayllón, M.D.; Calleja-Lozano, R.; García-Gaitan, C. Short-term outcomes of robotic liver resection: An initial singleinstitution experience. World J. Hepatol. 2022, 14, 224–233. [Google Scholar] [CrossRef]



| Variable | Value |
|---|---|
| Age > 70 | 60 (40%) |
| Sex | |
| Male | 100 (66%) |
| BMI (kg/m2) | 26 ± 4 (range 21–49) |
| ASA score | |
| I–II | 102 (68%) |
| III–IV | 48 (32%) |
| Previous abdominal surgery | 57 (38%) |
| Comorbidities | |
| Diabetes | 22 (15%) |
| Hypertension | 54 (36%) |
| Cardiovascular disease | 37 (25%) |
| Pulmonary disease | 15 (10%) |
| Cirrhosis | 74 (49.3%) |
| Tumor indication | |
| Hepatocellular carcinoma (HCC) | 72 (48%) |
| Colorectal liver metastases (CRLM) | 31 (21%) |
| Cholangiocarcinoma (CCA) | 6 (4%) |
| Gallbladder carcinoma (GBC) | 2 (1%) |
| Non-CRLM metastases (NCRLM) | 13 (9%) |
| Benign disease | 26 (17%) |
| Lesion characteristics | |
| Lesion size (mm) | 34 ± 25 (range 10–150) |
| Number of lesions | |
| Single | 124 (83%) |
| Multiple | 26 (17%) |
| Preoperative chemotherapy | 40 (26%) |
| R0 resection achieved | 123 (82%) |
| Tumor markers | |
| AFP (n = 72) | Median 5.9 (range 0–15,384) |
| CEA (n = 32) | Median 1.5 (range 0–28.5) |
| CA 19.9 (n = 33) | Median 18.7 (range 0–592) |
| Type of resection | |
| Wedge resection | 93 (62%) |
| Anatomical segmentectomy | 23 (15.5%) |
| Bisegmentectomy | 21 (14%) |
| Left hepatectomy | 8 (5%) |
| Right hepatectomy | 4 (3%) |
| Central hepatectomy | 1 (0.5%) |
| Iwate difficulty score | |
| Low (1–3) | 57 (38%) |
| Intermediate (4–6) | 75 (50%) |
| Advanced (7–9) | 11 (7%) |
| Expert (10–12) | 7 (5%) |
| Variable | Value |
|---|---|
| Etiology | |
| Alcohol-related cirrhosis (ALC) | 31 (42%) |
| Non-alcoholic steatohepatitis (NASH) | 18 (24%) |
| Hepatitis C virus (HCV) | 16 (22%) |
| Hepatitis B virus (HBV) | 5 (7%) |
| Other causes | 4 (5%) |
| Portal hypertension | 27 (36%) |
| Child–Pugh class | |
| A | 67 (91%) |
| B | 7 (9%) |
| Platelet count (×103/µL) | 188 ± 80 (range 80–415) |
| MELD score | 9 (range 6–18) |
| Secondary Lesions | Value |
|---|---|
| CRLM | 31 (70%) |
| GIST | 3 (7%) |
| NET | 2 (4.5%) |
| Melanoma liver metastasis | 3 (7%) |
| Ampullary Adenocarcinoma, (AAC) | 1 (2.3%) |
| Adenoidocistic carcinoma, (AdCC) | 1 (2.3%) |
| Thymoma | 1 (2.3%) |
| Endometrial cancer | 1 (2.3%) |
| PDAC | 1 (2.3%) |
| Variable | Value |
|---|---|
| Operative outcomes | |
| Operative time (min) | 250 ± 119 (range 77–840) |
| Console time (min) | 184 (range 5–700) |
| Conversion to open surgery | 18 (12%) |
| Associated procedures | 11 (8%) |
| Drain placement | 82 (55%) |
| Pringle maneuver | 5 (3%) |
| Blood loss (mL) | 159 ± 228 (range 0–2000) |
| Blood loss < 100 mL | 99 (66%) |
| Blood transfusion | 8 (5%) |
| ICG fluorescence used | 122 (81%) |
| Postoperative outcomes | |
| Length of hospital stay (days) | 7 (mean); median 5 (range 2–46) |
| Overall morbidity | 36 (24%) |
| Clavien–Dindo grade ≥ III | 15 (10%) |
| Hemorrhage | 2 (1.3%) |
| Biliary leakage | 9 (6%) |
| Reoperation | 8 (5%) |
| Ascites | 5 (3.3%) |
| Liver failure | 1 (0.5%) |
| Readmission within 30 days | 4 (3%) |
| Mortality | 2 (1.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquale, A.; Ciarleglio, F.A.; Marinelli, L.; Viel, G.; Valcanover, S.; Salimian, N.; Marcucci, S.; Brolese, M.; Beltempo, P.; Brolese, A. A Decade of Innovation: Short-Term Outcomes of 150 Robotic Liver Resections. J. Clin. Med. 2025, 14, 6530. https://doi.org/10.3390/jcm14186530
Pasquale A, Ciarleglio FA, Marinelli L, Viel G, Valcanover S, Salimian N, Marcucci S, Brolese M, Beltempo P, Brolese A. A Decade of Innovation: Short-Term Outcomes of 150 Robotic Liver Resections. Journal of Clinical Medicine. 2025; 14(18):6530. https://doi.org/10.3390/jcm14186530
Chicago/Turabian StylePasquale, Alessio, Francesco A. Ciarleglio, Laura Marinelli, Giovanni Viel, Stefano Valcanover, Nick Salimian, Stefano Marcucci, Marco Brolese, Paolo Beltempo, and Alberto Brolese. 2025. "A Decade of Innovation: Short-Term Outcomes of 150 Robotic Liver Resections" Journal of Clinical Medicine 14, no. 18: 6530. https://doi.org/10.3390/jcm14186530
APA StylePasquale, A., Ciarleglio, F. A., Marinelli, L., Viel, G., Valcanover, S., Salimian, N., Marcucci, S., Brolese, M., Beltempo, P., & Brolese, A. (2025). A Decade of Innovation: Short-Term Outcomes of 150 Robotic Liver Resections. Journal of Clinical Medicine, 14(18), 6530. https://doi.org/10.3390/jcm14186530






