The Influence of Optimal Sleep Onset Time and Duration on Risk of Stroke: A Community-Based, Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Cross-Sectional Study Design and Participants
2.2. Measurements
2.3. Data Analysis
2.4. Language Editing
3. Results
3.1. Baseline Characteristics
3.2. Sleep Onset Time and the Risk of Stroke
3.3. Sleep Duration and the Risk of Stroke
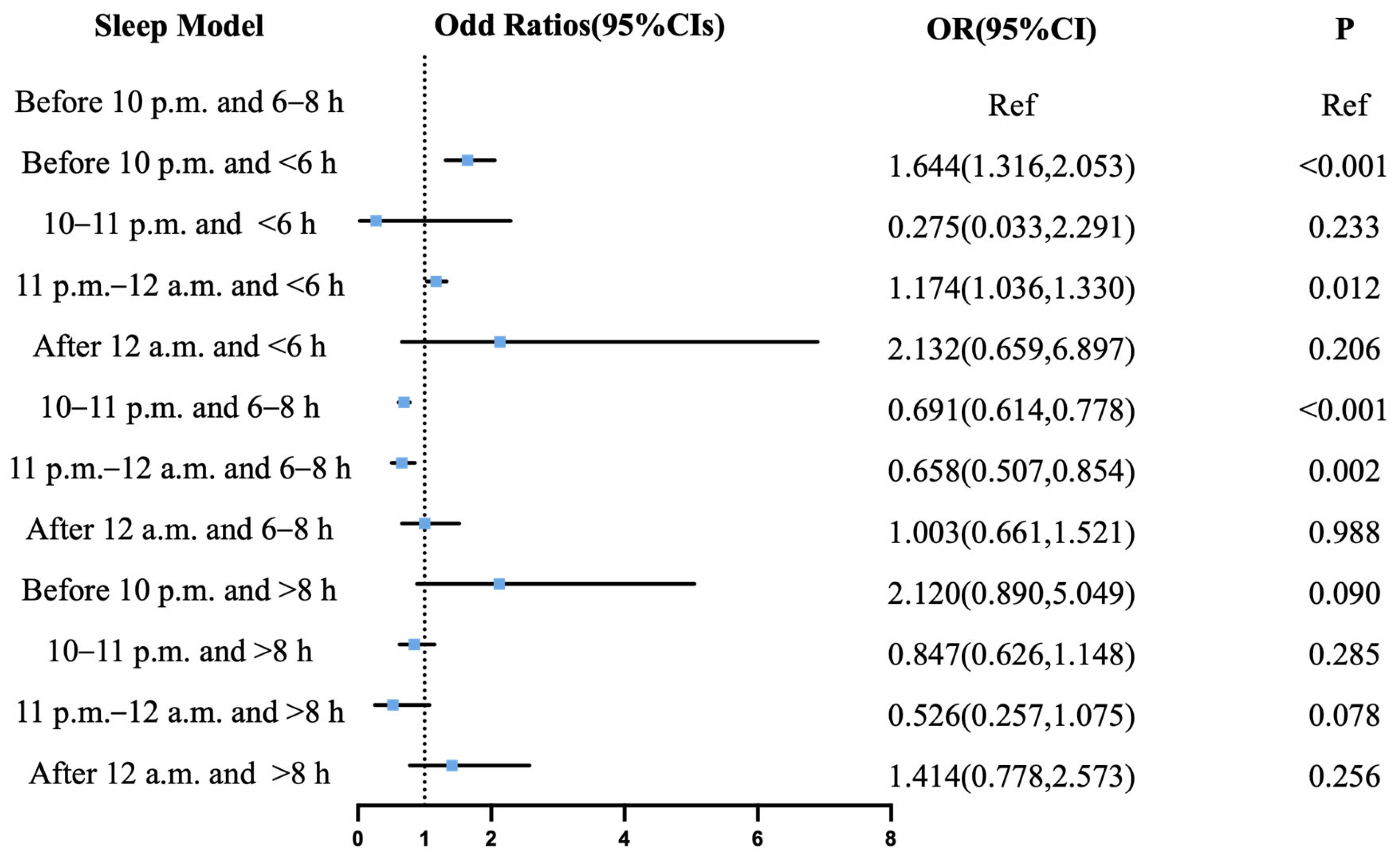
3.4. Joint Effects of Sleep Onset and Duration
3.5. Subgroup Analysis
3.6. Sensitivity Analysis
4. Discussion
Strengths and Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOT | Sleep onset time |
| IS | Ischemic stroke |
| ICH | Intracerebral hemorrhage |
References
- Morin, C.M.; Jarrin, D.C. Epidemiology of Insomnia: Prevalence, Course, Risk Factors, and Public Health Burden. Sleep Med. Clin. 2022, 17, 173–191. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Liu, J.; Meng, Y.; Li, J.; Zhou, P.; Xu, M.; Yan, Q.; Li, Q.; Yin, X.; et al. Prevalence of sleep disturbances and associated factors among Chinese residents: A web-based empirical survey of 2019. J. Glob. Health 2023, 13, 04071. [Google Scholar] [CrossRef]
- Xiaoli, Y.; Xiaobing, L.; Xiaoxia, A.; Liang, Z.; Xianmei, Z.; Quanfu, Y.; Zongfen, L.; Liya, Y. A Survey on Sleep Quality of the People Aged over 18 in Liaoning Province. Health Educ. Health Promot. 2013, 8, 433–435. [Google Scholar] [CrossRef]
- Li, J.; Vitiello, M.V.; Gooneratne, N.S. Sleep in Normal Aging. Sleep Med. Clin. 2018, 13, 161–171. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Miller, M.A. Sleep and Cardio-Metabolic Disease. Curr. Cardiol. Rep. 2017, 19, 110. [Google Scholar] [CrossRef]
- Wu, M.P.; Lin, H.J.; Weng, S.F.; Ho, C.H.; Wang, J.J.; Hsu, Y.W. Insomnia subtypes and the subsequent risks of stroke: Report from a nationally representative cohort. Stroke 2014, 45, 1349–1354. [Google Scholar] [CrossRef]
- Gottlieb, E.; Landau, E.; Baxter, H.; Werden, E.; Howard, M.E.; Brodtmann, A. The bidirectional impact of sleep and circadian rhythm dysfunction in human ischaemic stroke: A systematic review. Sleep Med. Rev. 2019, 45, 54–69. [Google Scholar] [CrossRef]
- von Ruesten, A.; Weikert, C.; Fietze, I.; Boeing, H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PLoS ONE 2012, 7, e30972. [Google Scholar] [CrossRef]
- Kakizaki, M.; Kuriyama, S.; Nakaya, N.; Sone, T.; Nagai, M.; Sugawara, Y.; Hozawa, A.; Fukudo, S.; Tsuji, I. Long sleep duration and cause-specific mortality according to physical function and self-rated health: The Ohsaki Cohort Study. J. Sleep Res. 2013, 22, 209–216. [Google Scholar] [CrossRef]
- Leng, Y.; Cappuccio, F.P.; Wainwright, N.W.; Surtees, P.G.; Luben, R.; Brayne, C.; Khaw, K.T. Sleep duration and risk of fatal and nonfatal stroke: A prospective study and meta-analysis. Neurology 2015, 84, 1072–1079. [Google Scholar] [CrossRef]
- Jean-Louis, G.; Williams, N.J.; Sarpong, D.; Pandey, A.; Youngstedt, S.; Zizi, F.; Ogedegbe, G. Associations between inadequate sleep and obesity in the US adult population: Analysis of the national health interview survey (1977–2009). BMC Public Health 2014, 14, 290. [Google Scholar] [CrossRef]
- Buxton, O.M.; Marcelli, E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc. Sci. Med. 2010, 71, 1027–1036. [Google Scholar] [CrossRef]
- Gangwisch, J.E.; Heymsfield, S.B.; Boden-Albala, B.; Buijs, R.M.; Kreier, F.; Pickering, T.G.; Rundle, A.G.; Zammit, G.K.; Malaspina, D. Short sleep duration as a risk factor for hypertension: Analyses of the first National Health and Nutrition Examination Survey. Hypertension 2006, 47, 833–839. [Google Scholar] [CrossRef]
- Hannerz, H.; Albertsen, K.; Nielsen, M.L.; Tuchsen, F.; Burr, H. Occupational factors and 5-year weight change among men in a danish national cohort. Health Psychol. 2004, 23, 283–288. [Google Scholar] [CrossRef]
- Ellingsen, T.; Bener, A.; Gehani, A.A. Study of shift work and risk of coronary events. J. R. Soc. Promot. Health 2007, 127, 265–267. [Google Scholar] [CrossRef]
- Suwazono, Y.; Dochi, M.; Sakata, K.; Okubo, Y.; Oishi, M.; Tanaka, K.; Kobayashi, E.; Kido, T.; Nogawa, K. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity 2008, 16, 1887–1893. [Google Scholar] [CrossRef]
- Suwazono, Y.; Dochi, M.; Oishi, M.; Tanaka, K.; Kobayashi, E.; Sakata, K. Shiftwork and impaired glucose metabolism: A 14-year cohort study on 7104 male workers. Chronobiol. Int. 2009, 26, 926–941. [Google Scholar] [CrossRef]
- Kawachi, I.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Manson, J.E.; Speizer, F.E.; Hennekens, C.H. Prospective study of shift work and risk of coronary heart disease in women. Circulation 1995, 92, 3178–3182. [Google Scholar] [CrossRef]
- Bigert, C.; Kader, M.; Andersson, T.; Selander, J.; Bodin, T.; Gustavsson, P.; Härmä, M.; Ljungman, P.; Albin, M. Night and shift work and incidence of cerebrovascular disease—A prospective cohort study of healthcare employees in Stockholm. Scand. J. Work Environ. Health 2022, 48, 31–40. [Google Scholar] [CrossRef]
- Vitale, I.; Pietrocola, F.; Guilbaud, E.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostini, M.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; et al. Apoptotic cell death in disease-Current understanding of the NCCD 2023. Cell Death Differ. 2023, 30, 1097–1154. [Google Scholar] [CrossRef]
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.D.; Kramer, A.; Maier, B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412. [Google Scholar] [CrossRef]
- Hashiramoto, A.; Yamane, T.; Tsumiyama, K.; Yoshida, K.; Komai, K.; Yamada, H.; Yamazaki, F.; Doi, M.; Okamura, H.; Shiozawa, S. Mammalian clock gene Cryptochrome regulates arthritis via proinflammatory cytokine TNF-alpha. J. Immunol. 2010, 184, 1560–1565. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Wang, S.; Zou, X.; Tang, L.; Chen, L.; Ma, J.; Li, Y.; Yao, T.; Zhang, X.; et al. Prevalence and 10-Year Risk of Intracerebral Hemorrhage in Central China Using Estimates From the 1 Million Cross-Sectional Study. Neurology 2025, 104, e213545. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Weitzman, E.; Moore-Ede, M.C.; Zimmerman, J.C.; Knauer, R.S. Human sleep: Its duration and organization depend on its circadian phase. Science 1980, 210, 1264–1267. [Google Scholar] [CrossRef]
- Wyatt, J.K.; Ritz-De Cecco, A.; Czeisler, C.A.; Dijk, D.J. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am. J. Physiol. 1999, 277, R1152–R1163. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Mistretta, J.; Hu, K.; Scheer, F. Circadian Misalignment Increases C-Reactive Protein and Blood Pressure in Chronic Shift Workers. J. Biol. Rhythm. 2017, 32, 154–164. [Google Scholar] [CrossRef]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- He, Q.; Sun, H.; Wu, X.; Zhang, P.; Dai, H.; Ai, C.; Shi, J. Sleep duration and risk of stroke: A dose-response meta-analysis of prospective cohort studies. Sleep Med. 2017, 32, 66–74. [Google Scholar] [CrossRef]
- Huang, Y.M.; Xia, W.; Ge, Y.J.; Hou, J.H.; Tan, L.; Xu, W.; Tan, C.C. Sleep duration and risk of cardio-cerebrovascular disease: A dose-response meta-analysis of cohort studies comprising 3.8 million participants. Front. Cardiovasc. Med. 2022, 9, 907990. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Cao, S.; Yin, X.; Gong, Y.; Gan, Y.; Zhou, Y.; Lu, Z. Sleep duration and risk of stroke events and stroke mortality: A systematic review and meta-analysis of prospective cohort studies. Int. J. Cardiol. 2016, 223, 870–876. [Google Scholar] [CrossRef]
- Cheng, Y.; Ding, Y.; Elmadhoun, A.; Ji, X.; Geng, X. The link between sleep duration and stroke risk. Brain Circ. 2025, 11, 1–8. [Google Scholar] [CrossRef]
- Titova, O.E.; Michaëlsson, K.; Larsson, S.C. Correction to: Sleep Duration and Stroke: Prospective Cohort Study and Mendelian Randomization Analysis. Stroke 2020, 51, e347. [Google Scholar] [CrossRef]
- Eguchi, K.; Hoshide, S.; Ishikawa, S.; Shimada, K.; Kario, K. Short sleep duration is an independent predictor of stroke events in elderly hypertensive patients. J. Am. Soc. Hypertens. 2010, 4, 255–262. [Google Scholar] [CrossRef]
- Kröller-Schön, S.; Daiber, A.; Steven, S.; Oelze, M.; Frenis, K.; Kalinovic, S.; Heimann, A.; Schmidt, F.P.; Pinto, A.; Kvandova, M.; et al. Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Eur. Heart J. 2018, 39, 3528–3539. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, L.; Wang, A.; Wang, Y.; Wang, S.; Ning, G.; Mu, Y. Association of sleep duration with stroke, myocardial infarction, and tumors in a Chinese population with metabolic syndrome: A retrospective study. Lipids Health Dis. 2020, 19, 155. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, H.; Jiang, Y.R.; Sun, W.Q.; Song, Y.J.; Liu, S.J.; Jiang, F. Relationship between Duration of Sleep and Hypertension in Adults: A Meta-Analysis. J. Clin. Sleep Med. 2015, 11, 1047–1056. [Google Scholar] [CrossRef]
- Sunbul, M.; Kanar, B.G.; Durmus, E.; Kivrak, T.; Sari, I. Acute sleep deprivation is associated with increased arterial stiffness in healthy young adults. Sleep Breath. 2014, 18, 215–220. [Google Scholar] [CrossRef]
- Sauvet, F.; Leftheriotis, G.; Gomez-Merino, D.; Langrume, C.; Drogou, C.; Van Beers, P.; Bourrilhon, C.; Florence, G.; Chennaoui, M. Effect of acute sleep deprivation on vascular function in healthy subjects. J. Appl. Physiol. 1985, 108, 68–75. [Google Scholar] [CrossRef]
- Dettoni, J.L.; Consolim-Colombo, F.M.; Drager, L.F.; Rubira, M.C.; Souza, S.B.; Irigoyen, M.C.; Mostarda, C.; Borile, S.; Krieger, E.M.; Moreno, H., Jr.; et al. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J. Appl. Physiol. 1985, 113, 232–236. [Google Scholar] [CrossRef]
- Sauvet, F.; Drogou, C.; Bougard, C.; Arnal, P.J.; Dispersyn, G.; Bourrilhon, C.; Rabat, A.; Van Beers, P.; Gomez-Merino, D.; Faraut, B.; et al. Vascular response to 1 week of sleep restriction in healthy subjects. A metabolic response? Int. J. Cardiol. 2015, 190, 246–255. [Google Scholar] [CrossRef]
- Nedeltcheva, A.V.; Kessler, L.; Imperial, J.; Penev, P.D. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J. Clin. Endocrinol. Metab. 2009, 94, 3242–3250. [Google Scholar] [CrossRef]
- Gangwisch, J.E. A review of evidence for the link between sleep duration and hypertension. Am. J. Hypertens. 2014, 27, 1235–1242. [Google Scholar] [CrossRef]
- Makarem, N.; Shechter, A.; Carnethon, M.R.; Mullington, J.M.; Hall, M.H.; Abdalla, M. Sleep Duration and Blood Pressure: Recent Advances and Future Directions. Curr. Hypertens. Rep. 2019, 21, 33. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2019, 135, e96–e121. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Duffy, J.F.; Shanahan, T.L.; Brown, E.N.; Mitchell, J.F.; Rimmer, D.W.; Ronda, J.M.; Silva, E.J.; Allan, J.S.; Emens, J.S.; et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 1999, 284, 2177–2181. [Google Scholar] [CrossRef] [PubMed]



| Variable | Controls (n = 13,458) | Ischemic Stroke (n = 8168) | P1 a | Intracerebral Hemorrhage (n = 3172) | P2 b |
|---|---|---|---|---|---|
| Sex | <0.001 | 0.273 | |||
| Female | 5924 (44) | 3354 (41) | 1183 (37) | ||
| Male | 7534 (56) | 4814 (59) | 1989 (63) | ||
| Age | 67 (55, 74) | 72 (65, 78) | <0.001 | 69 (59, 76) | <0.001 |
| BMI | 22.68 (21.01, 24.44) | 23.03 (21.22, 24.98) | <0.001 | 22.94 (21.19, 24.97) | <0.001 |
| Ethnic groups | <0.001 | <0.001 | |||
| Han | 11,159 (83) | 6476 (79) | 2652 (84) | ||
| Other minorities | 2299 (17) | 1692 (21) | 520 (16) | ||
| Education | <0.001 | <0.001 | |||
| None or lower than junior high school | 6871 (51) | 5350 (65) | 1888 (60) | ||
| Junior high school qualification | 4690 (35) | 2203 (27) | 1019 (32) | ||
| Senior high school qualification and higher | 1897 (14) | 615 (8) | 265 (8) | ||
| Smoking statues | <0.001 | 0.789 | |||
| Non-smoker | 9946 (74) | 5815 (71) | 2209 (70) | ||
| Smoker | 3512 (26) | 2353 (29) | 963 (30) | ||
| Alcohol abuse | 0.006 | 0.185 | |||
| Non-alcohol abuser | 11,410 (85) | 6810 (83) | 2517 (79) | ||
| Alcohol abuser | 2048 (15) | 1358 (17) | 655 (21) | ||
| History of hypertension | <0.001 | 0.006 | |||
| No | 7139 (53) | 1915 (23) | 637 (20) | ||
| Yes | 2660 (20) | 4046 (50) | 1536 (48) | ||
| Unknown | 3659 (27) | 2207 (27) | 999 (31) | ||
| History of diabetes | <0.001 | <0.001 | |||
| No | 7943 (59) | 4283 (52) | 1603 (51) | ||
| Yes | 591 (4) | 1019 (12) | 217 (7) | ||
| Unknown | 4924 (37) | 2866 (35) | 1352 (43) | ||
| History of atrial fibrillation | <0.001 | 0.028 | |||
| No | 11,289 (84) | 5805 (71) | 2193 (69) | ||
| Yes | 202 (2) | 400 (5) | 145 (5) | ||
| Unknown | 1967 (15) | 1963 (24) | 834 (26) | ||
| History of coronary heart disease | |||||
| No | 11,226 (83) | 5533 (68) | <0.001 | 2218 (70) | 0.015 |
| Yes | 498 (4) | 990 (12) | 262 (8) | ||
| Unknown | 1734 (13) | 1645 (20) | 692 (22) | ||
| History of hyperlipidemia | <0.001 | <0.001 | |||
| No | 5738 (43) | 2573 (32) | 894 (28) | ||
| Yes | 947 (7) | 1604 (20) | 532 (17) | ||
| Unknown | 6773 (50) | 3991 (48) | 1746 (55) | ||
| Snore | <0.001 | 0.016 | |||
| No | 9175 (68) | 4083 (50) | 1472 (46) | ||
| Yes | 4283 (32) | 4085 (50) | 1700 (54) |
| Unadjusted Model | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | OR (95%CI) | p Value | |
| Sleep onset time | ||||||
| Ischemic stroke | ||||||
| Before 10 p.m. | Ref | Ref | Ref | Ref | Ref | Ref |
| 10–11 p.m. | 0.623 (0.582,0.667) | <0.001 | 0.751 (0.700,0.807) | <0.001 | 0.764 (0.707,0.826) | <0.001 |
| 11 p.m.–12 a.m. | 0.443 (0.380,0.518) | <0.001 | 0.774 (0.655,0.915) | 0.003 | 0.677 (0.563,0.815) | <0.001 |
| After 12 a.m. | 1.045 (0.756,1.444) | 0.792 | 1.499 (1.054,2.131) | 0.024 | 1.297 (0.879,1.916) | 0.190 |
| Intracerebral hemorrhage | ||||||
| Before 10 p.m. | Ref | Ref | Ref | Ref | Ref | Ref |
| 10–11 p.m. | 0.637 (0.578,0.702) | <0.001 | 0.684 (0.619,0.756) | <0.001 | 0.682 (0.612,0.760) | <0.001 |
| 11 p.m.–12 a.m. | 0.567 (0.461,0.698) | <0.001 | 0.759 (0.611,0.943) | 0.013 | 0.658 (0.520,0.832) | <0.001 |
| After 12 a.m. | 1.063 (0.680,1.661) | 0.788 | 1.287 (0.811,2.043) | 0.284 | 1.247 (0.752,2.068) | 0.393 |
| Sleep duration | ||||||
| Ischemic stroke | ||||||
| <6 h | Ref | Ref | Ref | Ref | Ref | Ref |
| 6~8 h | 0.348 (0.308,0.393) | <0.001 | 0.425 (0.376,0.482) | <0.001 | 0.494 (0.432,0.565) | <0.001 |
| >8 h | 0.471 (0.412,0.539) | <0.001 | 0.546 (0.476,0.627) | <0.001 | 0.604 (0.520,0.701) | <0.001 |
| Intracerebral hemorrhage | ||||||
| <6 h | Ref | Ref | Ref | Ref | Ref | Ref |
| 6~8 h | 0.475 (0.401,0.563) | <0.001 | 0.524 (0.441,0.622) | <0.001 | 0.607 (0.502,0.733) | <0.001 |
| >8 h | 0.595 (0.493,0.718) | <0.001 | 0.646 (0.534,0.781) | <0.001 | 0.733 (0.595,0.904) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, Y.; Zhang, J.; Tang, L.; Zhang, Y.; Wang, S.; Zou, X.; Chen, L.; Li, Y.; Zeng, Y.; et al. The Influence of Optimal Sleep Onset Time and Duration on Risk of Stroke: A Community-Based, Cross-Sectional Study. J. Clin. Med. 2025, 14, 6529. https://doi.org/10.3390/jcm14186529
Ma J, Wang Y, Zhang J, Tang L, Zhang Y, Wang S, Zou X, Chen L, Li Y, Zeng Y, et al. The Influence of Optimal Sleep Onset Time and Duration on Risk of Stroke: A Community-Based, Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(18):6529. https://doi.org/10.3390/jcm14186529
Chicago/Turabian StyleMa, Junyi, Yang Wang, Ji Zhang, Li Tang, Yupeng Zhang, Sai Wang, Xuelun Zou, Lei Chen, Ye Li, Yi Zeng, and et al. 2025. "The Influence of Optimal Sleep Onset Time and Duration on Risk of Stroke: A Community-Based, Cross-Sectional Study" Journal of Clinical Medicine 14, no. 18: 6529. https://doi.org/10.3390/jcm14186529
APA StyleMa, J., Wang, Y., Zhang, J., Tang, L., Zhang, Y., Wang, S., Zou, X., Chen, L., Li, Y., Zeng, Y., Wang, D., & Zhang, L. (2025). The Influence of Optimal Sleep Onset Time and Duration on Risk of Stroke: A Community-Based, Cross-Sectional Study. Journal of Clinical Medicine, 14(18), 6529. https://doi.org/10.3390/jcm14186529






