Current Clinical Trial Landscape of Gastroenteropancreatic Neuroendocrine Tumors: A New Era of Landmark Trials
Abstract
1. Introduction
2. Pathophysiology
3. Classification
4. Clinical Presentation
5. Treatment
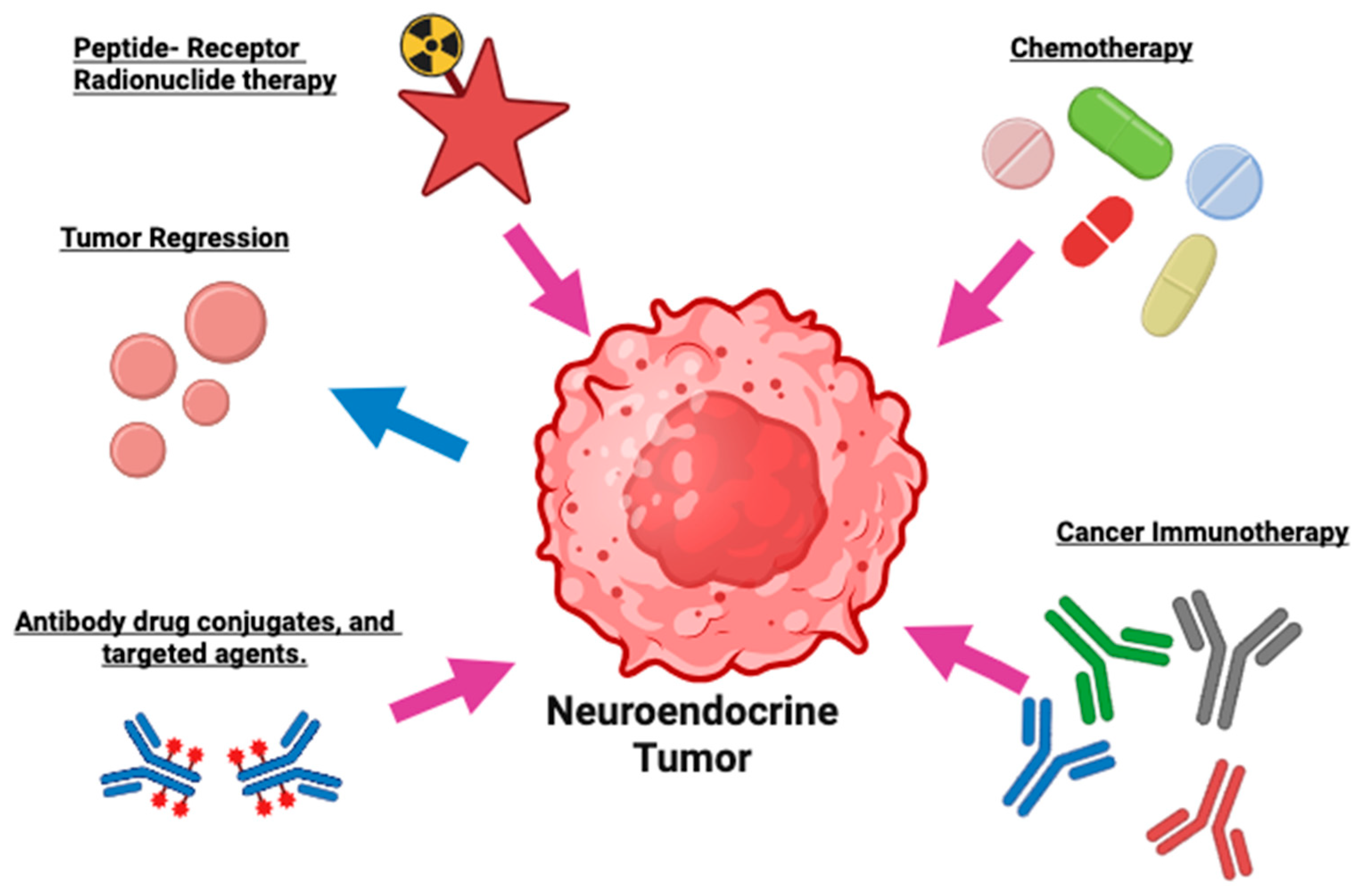
6. Targeted Treatment in GEP-NETs
6.1. Radioligand Treatment/Peptide Receptor Radionuclide Therapy (PRRT)
6.2. Novel Radioligand Agents
6.3. Comparative Trials
6.4. Radioligand Combination Trials
- DNA Repair Enzyme Inhibitors: Combining (177)Lu-DOTATATE with DNA repair enzyme inhibitors enhances the therapeutic effect by increasing DNA damage in neuroendocrine tumor cells [44]. This synergy results in more effective tumor cell kill and potentially improved patient outcomes. These inhibitors prevent the repair of radiation-induced DNA breaks, amplifying (177)Lu-DOTATATE efficacy in treating neuroendocrine tumors. Peposertib is a deoxyribonucleic acid protein kinase (DNA-PK) inhibitor which can enhance the DNA damage caused by (177)Lu-DOTATATE. DNA-PK is a DNA repair enzyme which when upregulated enhances repair of double stranded DNA breaks causing resistance to radiation induced DNA damage. The combination of peposertib and (177)Lu-DOTATATE is currently being studied in an investigator initiated, multi-center phase I clinical trial in patients with well differentiated GEP-NETs after failure of at least one prior line of systemic treatment. Patients with prior RLT will be excluded. The study plans to enroll 29 patients. No current results are available [53].
- II.
- Immune Check Point Inhibitors: Immune check point inhibitors (ICIs) work by blocking the interaction of program death (PD) proteins and program death ligands 1 (PD-L1) expressed on immune and cancer cells, respectively, thus removing the inhibition of the immune system to kill cancer cells. Limited animal study data is available for the combination of PRRT and ICIs citing no safety concerns. A phase I study showed safety and favorable toxicity in lung NETs. Currently, a phase II trial evaluates the combination of 177-Lu DOTATE with nivolumab (anti-PD1) in adult patients with grade 3 advanced neuroendocrine tumors and neuroendocrine carcinomas. The trial is ongoing, and no results are available (NCT04525638) [56].
| Trial ID | Drug | Cancer Type | Phase | Status |
|---|---|---|---|---|
| NCT05477576 | [225Ac]DOTATATE (RYZ101) [47] | Advanced, well-differentiated GEP-NETs | III | Active |
| NCT05153772 | [212Pb]DOTAMTATE [49] | unresectable or metastatic somatostatin receptor-expressing GEP-NETs | II | Active |
| NCT05773274 | Lutetium (Lu-177) Dotatate [50] | Advanced, well-differentiated GEP-NETs | II | Active |
| NCT03049189 | Lu-Edotreotide [51] | SSTR+- G1/G2 advanced/metastatic GEP-NETs | III | Active |
| NCT04919226 | Lu-Edotreotide [52] | G2/G3 advanced/metastaticGEP-NETs | III | Active |
| NCT05724108 | triapine plus [177]Lu DOTATATE [54] | SSTR-GEP-NETs | II | Active |
| NCT04086485 | Olaparib [55] | GEP-NETs | I/II | Active |
| NCT04525638 | Nivolumab [56] | NET and Neuroendocrine carcinomas | II | Unknown |
| NCT06041516 | Antibody Drug conjugate ADCT-701 [57] | NET and Neuroendocrine carcinomas | I | Active |
| NCT06943755 | Zanzalintinib | Advanced or Metastatic NETs | II/III | Actice |
| NCT05040360 | Capecitabine and Temozolomide | High-risk pNET | II | Active |
7. Tyrosine Kinase Inhibitors (TKIs)
8. Chemotherapy
9. Miscellaneous
10. Discussion/Future Directions
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hofland, J.; Kaltsas, G.; De Herder, W. Advances in the diagnosis and management of well-differentiated neuroendocrine neoplasms. Endocr. Rev. 2020, 41, 371–403. [Google Scholar] [CrossRef]
- Clift, A.; Kidd, M.; Bodei, L.; Toumpanakis, C.; Baum, R.; Oberg, K.; Modlin, I.; Frilling, A. Neuroendocrine neoplasms of the small bowel and pancreas. Neuroendocrinology 2020, 110, 444–476. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing but NET: A review of neuroendocrine tumors and carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Carvão, J.; Dinis-Ribeiro, M.; Pimentel-Nunes, P.; Libânio, D. Neuroendocrine tumors of the gastrointestinal tract: A focused review and practical approach for gastroenterologists. GE Port. J. Gastroenterol. 2021, 28, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Modica, R.; Liccardi, A.; Minotta, R.; Cannavale, G.; Benevento, E.; Colao, A. Current understanding of pathogenetic mechanisms in neuroendocrine neoplasms. Expert Rev. Endocrinol. Metab. 2024, 19, 49–61. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA A Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef]
- Cives, M.; Pelle’, E.; Strosberg, J. Emerging treatment options for gastroenteropancreatic neuroendocrine tumors. J. Clin. Med. 2020, 9, 3655. [Google Scholar] [CrossRef]
- Banck, M.S.; Kanwar, R.; Kulkarni, A.A.; Boora, G.K.; Metge, F.; Kipp, B.R.; Zhang, L.; Thorland, E.C.; Minn, K.T.; Tentu, R.; et al. The genomic landscape of small intestine neuroendocrine tumors. J. Clin. Investig. 2013, 123, 2502–2508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, R.; Chen, S.; He, Y.; Li, Y.; Mu, S.; Jin, A. Machine learning based predictive model and genetic mutation landscape for high-grade colorectal neuroendocrine carcinoma: A SEER database analysis with external validation. Front. Oncol. 2025, 15, 1509170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef] [PubMed]
- Florio, T. Molecular mechanisms of the antiproliferative activity of somatostatin receptors (SSTRs) in neuroendocrine tumors. Front. Biosci. 2008, 13, 822–840. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, K. Functioning and nonfunctioning pNENs. Curr. Opin. Endocr. Metab. Res. 2021, 18, 284–290. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Poultsides, G.A. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J. Gastroenterol. WJG 2015, 21, 9512–9525. [Google Scholar] [CrossRef] [PubMed]
- Somnay, K.; Surpur, S.; Saini, P.; Gibson, C.; Luo, J. Early definitive diagnosis and management of incidental neuroendocrine tumors found on gastrointestinal endoscopy. Curēus 2023, 15, e44718. [Google Scholar] [CrossRef]
- Ahmed, M. Gastrointestinal neuroendocrine tumors in 2020. World J. Gastrointest. Oncol. 2020, 12, 791–807. [Google Scholar] [CrossRef]
- Huang, P.; Tsai, K.; Liang, C.; Tai, W.; Rau, K.; Wu, K.; Huang, C.; Chuah, S. Prognostic factors of patients with gastroenteropancreatic neuroendocrine neoplasms. Kaohsiung J. Med. Sci. 2018, 34, 650–656. [Google Scholar] [CrossRef]
- Hermans, B.C.M.; de Vos-Geelen, J.; Derks, J.L.; Latten, L.; Liem, I.H.; van der Zwan, J.M.; Speel, E.M.; Dercksen, M.W.; Dingemans, A.C. Unique metastatic patterns in neuroendocrine neoplasms of different primary origin. Neuroendocrinology 2021, 111, 1111–1120. [Google Scholar] [CrossRef]
- Keck, K.J.; Maxwell, J.E.; Menda, Y.; Bellizzi, A.; Dillon, J.; O’Dorisio, T.M.; Howe, J.R. Identification of primary tumors in patients presenting with metastatic gastroenteropancreatic neuroendocrine tumors. Surgery 2017, 161, 272–279. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sonbol, M.B.; Mazza, G.L.; Mi, L.; Oliver, T.; Starr, J.; Gudmundsdottir, H.; Cleary, S.P.; Hobday, T.; Halfdanarson, T.R. Survival and incidence patterns of pancreatic neuroendocrine tumors over the last two decades: A SEER database analysis. Oncologist 2022, 27, 573–578. [Google Scholar] [CrossRef]
- Strosberg, J.; Gardner, N.; Kvols, L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the Mid-Gut. Neuroendocrinology 2009, 89, 471–476. [Google Scholar] [CrossRef]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.-F.; et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Theodoropoulou, M.; Stalla, G.K. Somatostatin receptors: From signaling to clinical practice. Front. Neuroendocrinol. 2013, 34, 228–252. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.; Arnold, R. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): Results of long-term survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.T.; Dasari, A.; Liyanage, N.; Cox, D.; Lowenthal, S.P.; Wolin, E.M. Tumor response in the CLARINET study of lanreotide depot vs. placebo in patients with metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs). J. Clin. Oncol. 2016, 34, 434. [Google Scholar] [CrossRef]
- Yao, J.C.; Pavel, M.; Lombard-Bohas, C.; Van Cutsem, E.; Voi, M.; Brandt, U.; He, W.; Chen, D.; Capdevila, J.; De Vries, E.G.E.; et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: Overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 Study. J. Clin. Oncol. 2016, 34, 3906–3913. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H.; et al. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients with Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 2023, 41, 1359–1369. [Google Scholar] [CrossRef]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet. 2024, 403, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Wiedenmann, B.; Auernhammer, C.; Bartenstein, P.; Bartsch, D.K.; Begum, N.; Fottner, C.; Führer, D.; Gebauer, B.; Goretzki, P.E. Practice guideline neuroendocrine tumors—AWMF-Reg. 021-27. Ann. Oncol. 2021, 32, 222–235. [Google Scholar]
- Del Rivero, J.; Perez, K.; Kennedy, E.B.; Mittra, E.S.; Vijayvergia, N.; Arshad, J.; Basu, S.; Chauhan, A.; Dasari, A.N.; Bellizzi, A.M.; et al. Systemic therapy for tumor control in metastatic well-differentiated gastroenteropancreatic neuroendocrine tumors: ASCO Guideline. J. Clin. Oncol. 2023, 41, 5049–5067. [Google Scholar] [CrossRef] [PubMed]
- Puccini, A.; Poorman, K.; Salem, M.E.; Soldato, D.; Seeber, A.; Goldberg, R.M.; Shields, A.F.; Xiu, J.; Battaglin, F.; Berger, M.D.; et al. Comprehensive genomic profiling of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs). Clin. Cancer Res. 2020, 26, 5943–5951. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Del Rivero, J.; Ramirez, R.A.; Soares, H.P.; Li, D. Treatment sequencing strategies in advanced neuroendocrine tumors: A review. Cancers 2022, 14, 5248. [Google Scholar] [CrossRef]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. ENETS Consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef]
- Akirov, A.; Larouche, V.; Alshehri, S.; Asa, S.L.; Ezzat, S. Treatment options for pancreatic neuroendocrine tumors. Cancers 2019, 11, 828. [Google Scholar] [CrossRef]
- Hofland, J.; Zandee, W.T.; de Herder, W.W. Role of Biomarker Tests for Diagnosis of Neuroendocrine Tumours. Nat. Rev. Endocrinol. 2018, 14, 656–669. [Google Scholar] [CrossRef]
- Lindholm, D.P.; Oberg, K. Biomarkers and Molecular Imaging in Gastroenteropancreatic Neuroendocrine Tumors. Horm. Metab. Res. 2011, 43, 832–837. [Google Scholar] [CrossRef]
- Modlin, I.; Kidd, M.; Falconi, M.; Filosso, P.; Frilling, A.; Malczewska, A.; Toumpanakis, C.; Valk, G.; Pacak, K.; Bodei, L.; et al. A Multigenomic Liquid Biopsy Biomarker for Neuroendocrine Tumor Disease Outperforms CgA and Has Surgical and Clinical Utility. Ann. Oncol. 2021, 32, 1425–1433. [Google Scholar] [CrossRef]
- Tsoli, M.; Koumarianou, A.; Angelousi, A.; Kaltsas, G. Established and Novel Circulating Neuroendocrine Tumor Biomarkers for Diagnostic, Predictive and Prognostic Use. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101785. [Google Scholar] [CrossRef] [PubMed]
- Komarnicki, P.; Musiałkiewicz, J.; Stańska, A.; Maciejewski, A.; Gut, P.; Mastorakos, G.; Ruchała, M. Circulating Neuroendocrine Tumor Biomarkers: Past, Present and Future. J. Clin. Med. 2022, 11, 5542. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; Hofland, L.J.; Moreno, M.A.G.; Castaño, J.P.; de Herder, W.W.; Feelders, R.A. Neuroendocrine Neoplasms: Current and Potential Diagnostic, Predictive and Prognostic Markers. Endocr. Relat. Cancer 2019, 26, R157–R179. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177 Lu-dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Sabet, A.; Ezziddin, K.; Pape, U.F.; Ahmadzadehfar, H.; Mayer, K.; Pöppel, T.; Guhlke, S.; Biersack, H.J.; Ezziddin, S. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J. Nucl. Med. 2013, 54, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Ulaner, G.A.; Halperin, D.M.; Strosberg, J.R.; Mehr, S.H.; Li, D.; Soares, H.P.; Anthony, L.B.; Kotiah, S.D.; Jacene, H.; et al. ACTION-1 phase Ib/3 trial of RYZ101 in somatostatin receptor subtype 2–expressing (SSTR2+) gastroenteropancreatic neuroendocrine tumors (GEP-NET) progressing after 177 Lu somatostatin analogue (SSA) therapy: Initial safety analysis. J. Clin. Oncol. 2023, 41, 4132. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Naqvi, S.; Cohn, A.L.; Delpassand, E.; Wagner, V.J.; Torgue, J.; Woloski, R.; Manuel, A.; Maluccio, M.A. Safety, tolerability and efficacy of 212 Pb-Dotamtate as a targeted alpha therapy for subjects with unresectable or metastatic somatostatin receptor-expressing gastroenteropancreatic neuroendocrine tumors (SSTR+ GEP-NETs): A phase 2 study. J. Clin. Oncol. 2024, 42, 4020. [Google Scholar] [CrossRef]
- Chauhan, A.; O’Callaghan, C.; Myrehaug, S.; Bodei, L.; Kunz, P.; Dasari, A.; Strosberg, J.; Alexander, S.; Cheung, W.; Singh, S. NET RETREAT: A Phase II Study of 177Lutetium-Dotatate Retreatment vs. Everolimus in Metastatic/Unresectable Midgut NET. Endocr. Abstr. 2023, 98, T11. [Google Scholar] [CrossRef]
- Pavel, M.E.; Rinke, A.; Baum, R.P. 1335—TiP—COMPETE trial: Peptide receptor radionuclide therapy (PRRT) with 177Lu-edotreotide vs. everolimus in progressive GEP-NET. Ann. Oncol. 2018, 29, VIII478. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Reidy, D.; Vijayvergia, N.; Halperin, D.; Goldstein, G.; Kong, G.; Michael, M.; Leyden, S.; Grozinsky-Glasberg, S.; Sorbye, H.; et al. Compose: Pivotal Phase III Trial of 177LuEdotreotide Versus Best Standard of Care in Well-differentiated Aggressive Grade 2 and Grade 3 Gastroenteropancreatic Neuroendocrine Tumors. Available online: https://nanets.net/abstracts-archive/2021-1/1606-nanets-2021-abstracts-c30-compose-pivotal-phase-iii-trial-of-177lu-edotreotide-versus-best-standard-of-care-in-well-differentiated-aggressive-grade-2-and-grade-3-gastroenteropancreatic/file (accessed on 1 November 2024).
- Chauhan, A.; Kolesar, J.; Yan, D.; Li, D.; Khurana, A.; Carson, W.E.; Arnold, S.M.; Gore, S.; Rubinstein, L.; Kohn, E.C.; et al. ETCTN 10450: A phase I trial of peposertib and lutetium 177 DOTATATE in well-differentiated somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs). J. Clin. Oncol. 2023, 41, TPS658. [Google Scholar] [CrossRef]
- Chauhan, A.; Kunos, C.; El Khouli, R.; Kolesar, J.; Weiss, H.; Carson, W.E.; Evers, M.B.; Kidd, M.S.; Beumer, J.H.; Arnold, S.M.; et al. Etctn 10388: A phase I trial of triapine and lutetium Lu 177 dotatate in well-differentiated somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs). J. Clin. Oncol. 2020, 38, TPS4660. [Google Scholar] [CrossRef]
- Hallqvist, A.; Brynjarsdóttir, E.; Krantz, T.; Sjögren, M.; Svensson, J.; Bernhardt, P. 177 Lu-DOTATATE in Combination with PARP Inhibitor Olaparib Is Feasible in Patients with Somatostatin-Positive Tumors: Results from the LuPARP Phase I Trial. J. Nucl. Med. 2025, 66, 707–712. [Google Scholar] [CrossRef]
- di Santo, G.; Santo, G.; Sviridenko, A.; Virgolini, I. Peptide receptor radionuclide therapy combinations for neuroendocrine tumours in ongoing clinical trials: Status 2023. Theranostics 2024, 14, 940–953. [Google Scholar] [CrossRef]
- Del Rivero, J.; Glod, J.; Magee, T.; Cooper, K.; Rivero, A.; Lanfranconi, T.; Patel, R.; Figg, W.D.; Pommier, Y.; Widemann, B.C.; et al. A first-in-human phase I trial with antibody drug conjugate ADCT-701 in neuroendocrine tumors and carcinomas. J. Clin. Oncol. 2025, 43, TPS672. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513, Erratum in N. Engl. J. Med. 2011, 364, 1082. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Chan, J.A.; Geyer, S.; Meyerhardt, J.A. Cabozantinib in Advanced Neuroendocrine Tumors. N. Engl. J. Med. 2025, 392, 1869–1870. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Zemla, T.J.; Geyer, S.M.; Knopp, M.V.; Behr, S.; Pulsipher, S.; Acoba, J.D.; Shergill, A.; Wolin, E.M.; Halfdanarson, T.R.; et al. Efficacy and safety of cabozantinib for advanced gastrointestinal (GI) neuroendocrine tumors (NET) after progression on prior therapy: Subgroup analysis of the phase 3 CABINET trial (Alliance A021602). J. Clin. Oncol. 2025, 43, 666. [Google Scholar] [CrossRef]
- Soares, H.P.; Guthrie, K.A.; Ahmad, S.A.; Washington, M.K.; Ramnaraign, B.H.; Raj, P.N.; Seigel, C.; Bellasea, S.; Chiorean, E.G.; Dasari, A.; et al. Randomized phase II trial of postoperative adjuvant capecitabine and temozolomide versus observation in high-risk pancreatic neuroendocrine tumors: SWOG 2104. J. Clin. Oncol. 2022, 40, TPS515. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Novel therapeutics for patients with well-differentiated gastroenteropancreatic neuroendocrine tumors. Ther. Adv. Med. Oncol. 2021, 13, 17588359211018047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halperin, D.M.; Johnson, M.L.; Chan, J.A.; Hart, L.L.; Cook, N.; Patel, V.M.; Schlechter, B.L.; Cave, J.; Dowlati, A.; Blaszkowsky, L.C.; et al. The safety and efficacy of PEN-221 somatostatin analog (SSA)-DM1 conjugate in patients (PTS) with advanced GI mid-gut neuroendocrine tumor (NET): Phase 2 results. J. Clin Oncol. 2021, 39, 4110. [Google Scholar] [CrossRef]
- Davis, C.H.; Laird, A.M.; Libutti, S.K. Resistant gastroenteropancreatic neuroendocrine tumors: A definition and guideline to medical and surgical management. Proc. Bayl. Univ. Med. Cent. 2024, 37, 104–110. [Google Scholar] [CrossRef]
- Modica, R.; Liccardi, A.; Minotta, R.; Cannavale, G.; Benevento, E.; Colao, A. Therapeutic strategies for patients with neuroendocrine neoplasms: Current perspectives. Expert Rev. Endocrinol. Metabol. 2022, 17, 389–403. [Google Scholar] [CrossRef]
- Fine, R.L.; Gulati, A.P.; Krantz, B.A.; Moss, R.A.; Schreibman, S.; Tsushima, D.A.; Mowatt, K.B.; Dinnen, R.D.; Mao, Y.; Stevens, P.D.; et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer 2014, 120, 1511–1517. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peshin, S.; Modi, S.; Babakhanlou, R.; Arshad, J. Current Clinical Trial Landscape of Gastroenteropancreatic Neuroendocrine Tumors: A New Era of Landmark Trials. J. Clin. Med. 2025, 14, 6522. https://doi.org/10.3390/jcm14186522
Peshin S, Modi S, Babakhanlou R, Arshad J. Current Clinical Trial Landscape of Gastroenteropancreatic Neuroendocrine Tumors: A New Era of Landmark Trials. Journal of Clinical Medicine. 2025; 14(18):6522. https://doi.org/10.3390/jcm14186522
Chicago/Turabian StylePeshin, Supriya, Shivani Modi, Rodrick Babakhanlou, and Junaid Arshad. 2025. "Current Clinical Trial Landscape of Gastroenteropancreatic Neuroendocrine Tumors: A New Era of Landmark Trials" Journal of Clinical Medicine 14, no. 18: 6522. https://doi.org/10.3390/jcm14186522
APA StylePeshin, S., Modi, S., Babakhanlou, R., & Arshad, J. (2025). Current Clinical Trial Landscape of Gastroenteropancreatic Neuroendocrine Tumors: A New Era of Landmark Trials. Journal of Clinical Medicine, 14(18), 6522. https://doi.org/10.3390/jcm14186522





