Acetazolamide per os in Decompensated Chronic Heart Failure: Randomized Multicenter Trial ORION-A
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
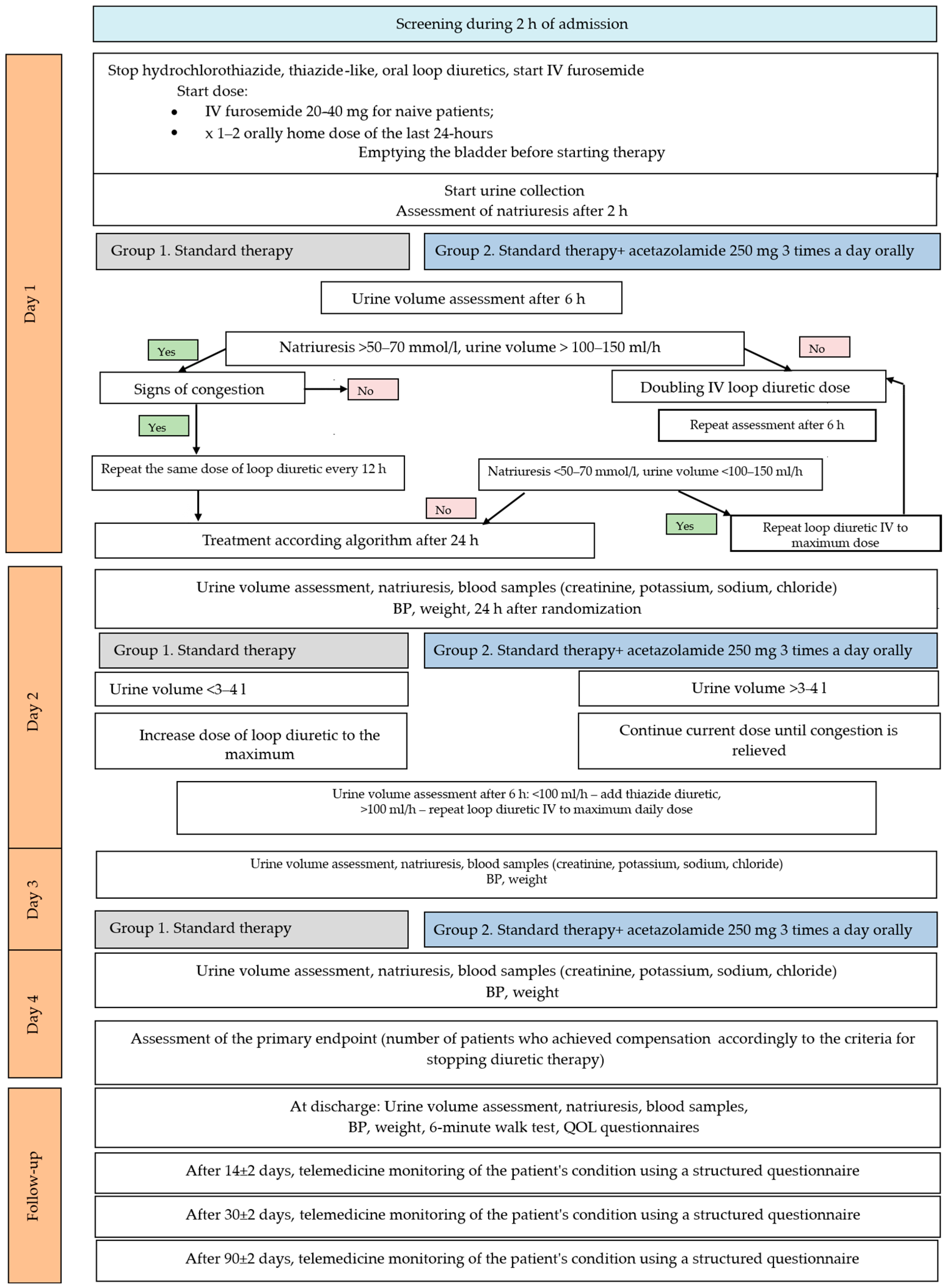
2.2. Study Design
2.3. Study Intervention
2.4. Urinary Collections and Blood Samples
2.5. Study Endpoints
2.6. Safety Monitoring
2.7. Statistical Analysis
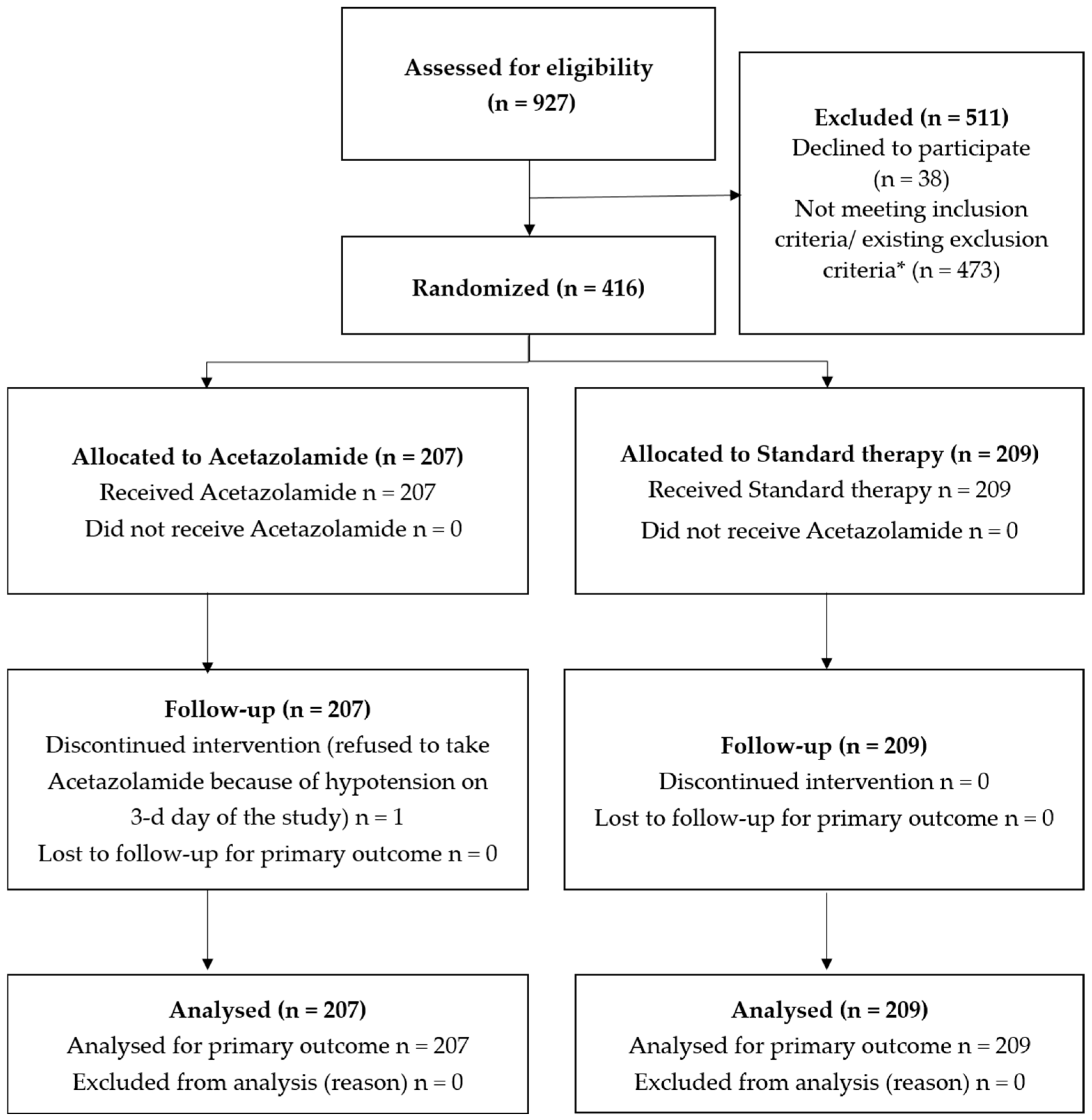
3. Results
3.1. Study Population
3.2. Primary Endpoints
3.3. Secondary Endpoints
3.4. Safety and Adverse Events
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ARB | Angiotensin receptor blocker |
| ARNI | Angiotensin receptor–neprilysin inhibitor |
| BNP | Brain natriuretic peptide |
| COPD | Chronic obstructive pulmonary disease |
| CRT | Cardiac resynchronization therapy |
| eGFR | Estimated glomerular filtration rate |
| GDMT | Guideline-directed medical therapy |
| HF | Heart failure |
| ICD | Implantable cardioverter-defibrillator |
| HFmrEF | Heart failure with mildly reduced ejection fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| LVEF | Left ventricular ejection fraction |
| MRA | Mineralocorticoid receptor antagonist |
| NT-proBNP | N-terminal pro–B-type natriuretic peptide |
| SBP | Systolic blood pressure |
| SGLT2 | Sodium–glucose cotransporter 2 |
Appendix A
| Investigators’ Names | Affiliations |
|---|---|
| Abdilazizova E.A. | Interstate Educational Organization of Higher Education Kyrgyz-Russian Slavic University named after the first President of the Russian Federation B.N. Yeltsin, Bishkek, Kyrgyz Republic |
| Amirova Yu.I., Vdovina S.V., Gabidullova D.A., Emelyanova N.G., Kornyakova N.I., Ponomar I.A., Sinkevich O.V., Sheshunova E.M. | Samara Regional Clinical Cardiology Dispensary named after V.P. Polyakov, Samara, Russia |
| Budkina M.L. | Privolzhsky Research Medical University, Nizhny Novgorod, Russia |
| Demeshchenko E.A. | Volgograd Regional Clinical Cardiology Center, Volgograd, Russia |
| Ivantsov E.N. | Kazan State Medical University, Kazan, Russia |
| Lazareva D.V. | Ryazan regional clinical cardiology dispensary, Ryazan, Russia |
| Nurgaziewa D.S. | Regional Clinical Hospital, Saratov, Russia |
| Pereverzeva K.G., Pravkina E.A. | Ryazan State Medical University, Ryazan, Russia |
| Podusov A.S., Troshina N.V., Shafigulin A.R. | Central Medical and Sanitary Unit named after Honored Doctor of Russia V.A. Egorov, Ulyanovsk, Russia |
| Fedorishina O.V. | Russian Medical Academy of Continuing Professional Education, Irkutsk, Russia |
| Frolova E.S. | Altai regional cardiological dispensary, Barnaul, Russia |
| Shlyakova A.A. | City Clinical Hospital No. 13 of the Avtozavodsky District of Nizhny Novgorod, Nizhny Novgorod, Russia |
| Yagudina R.N. | Irkutsk City Clinical Hospital No. 3, Irkutsk, Russia |
References
- Cooper, L.B.; Lippmann, S.J.; DiBello, J.R.; Gorsh, B.; Curtis, L.H.; Sikirica, V.; Hernandez, A.F.; Sprecher, D.L.; Laskey, W.K.; Saini, R.; et al. The Burden of Congestion in Patients Hospitalized With Acute Decompensated Heart Failure. Am. J. Cardiol. 2019, 124, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Girerd, N.; Seronde, M.F.; Coiro, S.; Chouihed, T.; Bilbault, P.; Braun, F.; Kenizou, D.; Maillier, B.; Nazeyrollas, P.; Roul, G.; et al. Integrative Assessment of Congestion in Heart Failure Throughout the Patient Journey. JACC Heart Fail. 2018, 6, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Kumric, M.; Kurir, T.T.; Bozic, J.; Slujo, A.B.; Glavas, D.; Miric, D.; Lozo, M.; Zanchi, J.; Borovac, J.A. Pathophysiology of Congestion in Heart Failure: A Contemporary Review. Card. Fail. Rev. 2024, 10, e13. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Martens, P.; Ameloot, K.; Haemels, V.; Penders, J.; Dupont, M.; Tang, W.H.W.; Droogné, W.; Mullens, W. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur. J. Heart Fail. 2019, 21, 1415–1422. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Rubanenko, O.A.; Rubanenko, A.O.; Villevalde, S.V.; Duplyakov, D.V. Efficacy and safety of acetazolamide in patients with NYHA class II–IV decompensated heart failure: Protocol of an open-label prospective randomized multicenter study (ORION-A). Russ. J. Cardiol. 2023, 28, 5477. [Google Scholar] [CrossRef]
- Hopewell, S.; Chan, A.W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 statement: Updated guideline for reporting randomised trials. BMJ 2025, 389, e081123. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Model List of Essential Medicines—23rd List, 2023. In The Selection and Use of Essential Medicines 2023: Executive Summary of the Report of the 24th WHO Expert Committee on the Selection and Use of Essential Medicines, 24–28 April 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Malik, B.A.; Nnodebe, I.; Fayaz, A.; Inayat, H.; Murtaza, S.F.; Umer, M.; Zaidi, S.A.T.; Amin, A. Effect of Acetazolamide as Add-On Diuretic Therapy in Patients with Heart Failure: A Meta-Analysis. Cureus 2023, 15, e37792. [Google Scholar] [CrossRef] [PubMed]
- Imiela, T.; Budaj, A. Acetazolamide as Add-on Diuretic Therapy in Exacerbations of Chronic Heart Failure: A Pilot Study. Clin. Drug Investig. 2017, 37, 1175–1181. [Google Scholar] [CrossRef]
- Kosiorek, A.; Urban, S.; Detyna, J.; Biegus, J.; Hurkacz, M.; Zymliński, R. Diuretic, natriuretic, and chloride-regaining effects of oral acetazolamide as an add-on therapy for acute heart failure with volume overload: A single-center, prospective, randomized study. Pol. Arch. Intern. Med. 2023, 133, 16526. [Google Scholar] [CrossRef] [PubMed]
- Addison, J.D.; Peterson, E.J.; Meyenburg, L. Intravenous or Oral Acetazolamide for Treatment of Diuretic-Induced Alkalosis in Patients with Heart Failure. Ann. Pharmacother. 2023, 57, 1241–1247. [Google Scholar] [CrossRef]
- Kumari, N.; Roy, P.; Roy, S.; Wang, C.; Das, S.; Pandey, N.; Mondal, S.K.; Bose, A.; Sun, C.C.; Ghosh, A. Development of direct compression Acetazolamide tablet with improved bioavailability in healthy human volunteers enabled by cocrystallization with p-Aminobenzoic acid. Int. J. Pharm. 2024, 652, 123793. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2024, 26, 5–17. [Google Scholar] [CrossRef]
- Mullens, W.; Schulze, P.C.; Westphal, J.; Bogoviku, J.; Bauersachs, J. Great debate: In patients with decompensated heart failure, acetazolamide in addition to loop diuretics is the first choice. Eur. Heart J. 2023, 44, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Ter Maaten, J.M.; Beldhuis, I.E.; van der Meer, P.; Krikken, J.A.; Postmus, D.; Coster, J.E.; Nieuwland, W.; van Veldhuisen, D.J.; Voors, A.A.; Damman, K. Natriuresis-guided diuretic therapy in acute heart failure: A pragmatic randomized trial. Nat. Med. 2023, 29, 2625–2632. [Google Scholar] [CrossRef]
| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Characteristic | Standard Therapy Acetazolamide (−) n = 209 | Standard Therapy Acetazolamide (+) n = 207 | p |
|---|---|---|---|
| Clinical and demographic parameters | |||
| Age, years | 68 (61–74) | 67 (61–73) | 0.732 |
| Male sex, n (%) | 128 (61.2%) | 133 (64.3%) | 0.526 |
| Race/ethnicity | 0.865 | ||
| White, n (%) | 160 (76.6%) | 157 (75.8%) | |
| Asian, n (%) | 50 (24.2%) | 49 (23.4%) | |
| Smoking, n (%) | 0.788 | ||
| Current | 39 (18.7%) | 42 (20.3%) | |
| No | 136 (65.1%) | 134 (64.7%) | |
| Ex-smokers | 31 (14.8%) | 26 (12.6%) | |
| Unknown | 3 (1.4%) | 5 (2.4%) | |
| Weight, kg | 88 (77.0–98.2) | 90 (78.0–103.0) | 0.214 |
| Body mass index, kg/m2 | 30.1(27.6–34.6) | 31.1 (27.2–35.7) | 0.372 |
| Functional class NYHA, n (%) | 0.732 | ||
| II | 37 (17.7%) | 29 (14.0%) | |
| III | 108 (51.7%) | 108 (52.2%) | |
| IV | 64 (30.6%) | 70 (33.8%) | |
| LVEF, % | 40.0 (30.0–51.0) | 40.0 (30.0–53.5) | 0.753 |
| HFpEF, n (%) | 58 (27.8%) | 64 (30.9%) | 0.674 |
| HFmrEF, n (%) | 40 (19.1%) | 34 (16.4%) | |
| HFrEF, n (%) | 111 (53.1%) | 109 (52.7%) | |
| Components of congestion score, n (%) | |||
| Edema | 170 (81.3%) | 175 (84.5%) | 0.386 |
| Pleural effusion | 65 (31.1%) | 73 (35.2%) | 0.426 |
| Ascites | 52 (24.9%) | 55 (26.6%) | 0.736 |
| Systolic blood pressure, mm Hg | 121 (111–144) | 122 (113–132) | 0.374 |
| Diastolic blood pressure, mm Hg | 82 (73–94) | 84 (73.0–82.5) | 0.229 |
| Laboratory parameters | |||
| NT-proBNP, pg/mL | 1534.0 (612.8–3592.5) | 1445.5 (713.0–3139.5) | 0.702 |
| BNP, pg/mL | 580 (186–1557) | 692 (195–1777) | 0.385 |
| eGFR, mL/min/1.73 m2 | 54 (43–68) | 60 (47–71) | 0.021 |
| Hemoglobin, g/dL | 13.4 (12.0–14.6) | 13.0 (11.8–14.4) | 0.345 |
| Sodium, mmol/L | 140 (138–142) | 141 (138–143) | 0.102 |
| Potassium, mmol/L | 4.39 (3.90–4.80) | 4.40 (4.00–4.75) | 0.812 |
| Natriuresis, mmol/L | 110.00 (76.28–128.50) | 115.65 (91.23–144.86) | 0.098 |
| Diuresis, mL | 2100 (1800–2500) | 2100 (1600–2500) | 0.867 |
| Comorbidities, n (%) | |||
| Hypertension | 196 (93.8%) | 186 (89.9%) | 0.144 |
| Stroke history | 28 (13.4%) | 17 (8.2%) | 0.089 |
| Ischemic heart disease | 161 (77.0%) | 140 (67.6%) | 0.032 |
| Atrial fibrillation/flutter | 126 (60.3%) | 116 (56%) | 0.380 |
| Diabetes mellitus | 32 (15.3%) | 27 (13%) | 0.507 |
| Chronic kidney disease | |||
| C3 | 116 (55.5%) | 95 (45.9%) | 0.050 |
| C4 | 12 (5.7%) | 9 (4.3%) | 0.671 |
| COPD | 44 (21.1%) | 38 (18.4%) | 0.490 |
| Treatment *, n (%) | |||
| ACE inhibitor | 101 (48.3%) | 104 (50.2%) | 0.696 |
| ARB | 30 (14.4%) | 21 (10.1%) | 0.191 |
| ARNI | 47 (22.5%) | 51 (25.6%) | 0.439 |
| Beta-blockers | 196 (93.8%) | 186 (89.9%) | 0.372 |
| Mineralocorticoid receptor antagonist | 187 (89.5%) | 183 (88.4%) | 0.728 |
| SGLT2 inhibitors | 153 (73.2%) | 158 (76.3%) | 0.463 |
| Quadruple therapy | |||
| Overall | 121 (57.9%) | 115 (55.6%) | 0.630 |
| HFpEF | 20 (34.5%) | 22 (34.4%) | 0.990 |
| HFmrEF | 20 (50.0%) | 15 (44.1%) | 0.613 |
| HFrEF | 81 (73.0%) | 78 (71.6%) | 0.815 |
| Dose of furosemide *, mg | 40 (40–60) | 40 (40–60) | 0.143 |
| Dose of furosemide, mg (day 2) | 60 (40–80) | 80 (40–100) | 0.403 |
| Dose of furosemide, mg (day 3) | 80 (40–80) | 80 (40–100) | 0.450 |
| CRT **, n (%) | 1 (0.5%) | 3 (1.4%) | 0.371 |
| ICD **, n (%) | 3 (1.4%) | 5 (2.4%) | 0.501 |
| Parameter | Standard Therapy Acetazolamide (−) n = 209 | Standard Therapy Acetazolamide (+) n = 207 | p |
|---|---|---|---|
| Increase in the volume of urine excreted in the first 72 h of hospitalization (from the time of randomization), mL | 5825 (4826–6926) | 6060 (5225–7335) | 0.028 |
| Weight loss within 72 h, kg (delta) | 3.00 (2.00–5.00) | 3.00 (2.00–5.10) | 0.897 |
| Weight loss during hospitalization, kg (delta) | 4.00 (3.00–7.25) | 4.00 (2.45–8.05) | 0.816 |
| Natriuresis (mmol/L): | |||
| Day 2 | 101.25 (73.96–119.80) | 113.40 (90.40–140.80) | 0.004 |
| Day 3 | 95.41 (68.69–120.00) | 105.00 (63.83–123.94) | 0.640 |
| Day 4 | 95.00 (63.74–111.25) | 100.00 (65.36–113.06) | 0.403 |
| At the time of discharge | 90.52 (72.11–105.00) | 70.66 (58.85–104.02) | 0.806 |
| Duration of hospital stay, days | 4.00 (4.00–9.00) | 4.00 (4.00–8.00) | 0.820 |
| Duration of stay in intensive care unit, days | 3.00 (3.00–4.00) | 4.00 (3.00–4.00) | 0.156 |
| Number of pleural and pericardial punctures performed during hospitalization, n (%) | 2 (1.0%) | 0 (0%) | 0.999 |
| Assessment of clinical statement score, points | 4.00 (2.5–7.00) | 4.00 (2.00–7.00) | 0.632 |
| 6 min walk test at discharge, m | 309.50 (282.50–404.50) | 300.00 (214.50–347.00) | 0.250 |
| Death from any cause within 90 days, n (%) | 3 (1.4%) | 3 (1.4%) | 0.996 |
| Death from cardiovascular cause within 90 days | 1 (0.5%) | 1 (0.5%) | |
| Death from heart failure decompensation within 90 days | 0 (0%) | 2 (1%) | |
| Adverse Events | Standard Therapy Acetazolamide (−) n = 209 | Standard Therapy Acetazolamide (+) n = 207 | p |
|---|---|---|---|
| During treatment phase—n (%) | |||
| Doubling of serum creatinine level from baseline | 0 (0%) | 1 (0.5%) | 0.999 |
| Renal replacement therapy | 0 (0%) | 0 (0%) | - |
| Hypotension (SBP < 90 mm Hg) | 2 (1.0%) | 3 (1.4%) | 0.644 |
| Hypokalemia | 28 (13.4%) | 29 (14.0%) | 0.918 |
| Severe metabolic acidosis | 0 (0%) | 0 (0%) | - |
| Syncope | 0 (0%) | 0 (0%) | - |
| During hospitalization phase—n (%) | |||
| Doubling of serum creatinine level from baseline | 0 (0%) | 1 (0.5%) | 0.999 |
| Renal replacement therapy | 0 (0%) | 0 (0%) | - |
| Hypotension (SBP < 90 mm Hg) | 2 (1.0%) | 4 (1.9%) | 0.400 |
| Hypokalemia | 36 (17.2%) | 37 (17.9%) | 0.862 |
| Severe metabolic acidosis | 0 (0%) | 0 (0%) | - |
| Syncope | 0 (0%) | 0 (0%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabirov, I.; Rubanenko, O.; Villevalde, S.; Rubanenko, A.; Veselovskaya, N.; Ivanenko, V.; Kosheleva, N.; Menzorov, M.; Pochinka, I.; Protasov, K.; et al. Acetazolamide per os in Decompensated Chronic Heart Failure: Randomized Multicenter Trial ORION-A. J. Clin. Med. 2025, 14, 6517. https://doi.org/10.3390/jcm14186517
Sabirov I, Rubanenko O, Villevalde S, Rubanenko A, Veselovskaya N, Ivanenko V, Kosheleva N, Menzorov M, Pochinka I, Protasov K, et al. Acetazolamide per os in Decompensated Chronic Heart Failure: Randomized Multicenter Trial ORION-A. Journal of Clinical Medicine. 2025; 14(18):6517. https://doi.org/10.3390/jcm14186517
Chicago/Turabian StyleSabirov, Ibragim, Olesya Rubanenko, Svetlana Villevalde, Anatoly Rubanenko, Nadezhda Veselovskaya, Vitaly Ivanenko, Natalia Kosheleva, Maksim Menzorov, Ilya Pochinka, Konstantin Protasov, and et al. 2025. "Acetazolamide per os in Decompensated Chronic Heart Failure: Randomized Multicenter Trial ORION-A" Journal of Clinical Medicine 14, no. 18: 6517. https://doi.org/10.3390/jcm14186517
APA StyleSabirov, I., Rubanenko, O., Villevalde, S., Rubanenko, A., Veselovskaya, N., Ivanenko, V., Kosheleva, N., Menzorov, M., Pochinka, I., Protasov, K., Khasanov, N., Yakushin, S., Medvedeva, E., & Duplyakov, D., on behalf of ORION-A Investigators. (2025). Acetazolamide per os in Decompensated Chronic Heart Failure: Randomized Multicenter Trial ORION-A. Journal of Clinical Medicine, 14(18), 6517. https://doi.org/10.3390/jcm14186517








