A Machine Learning Approach to Modify the Neurocognitive Frailty Index for the Prediction of Cognitive Status in the Canadian Population
Abstract
1. Introduction
2. Methods
3. Results
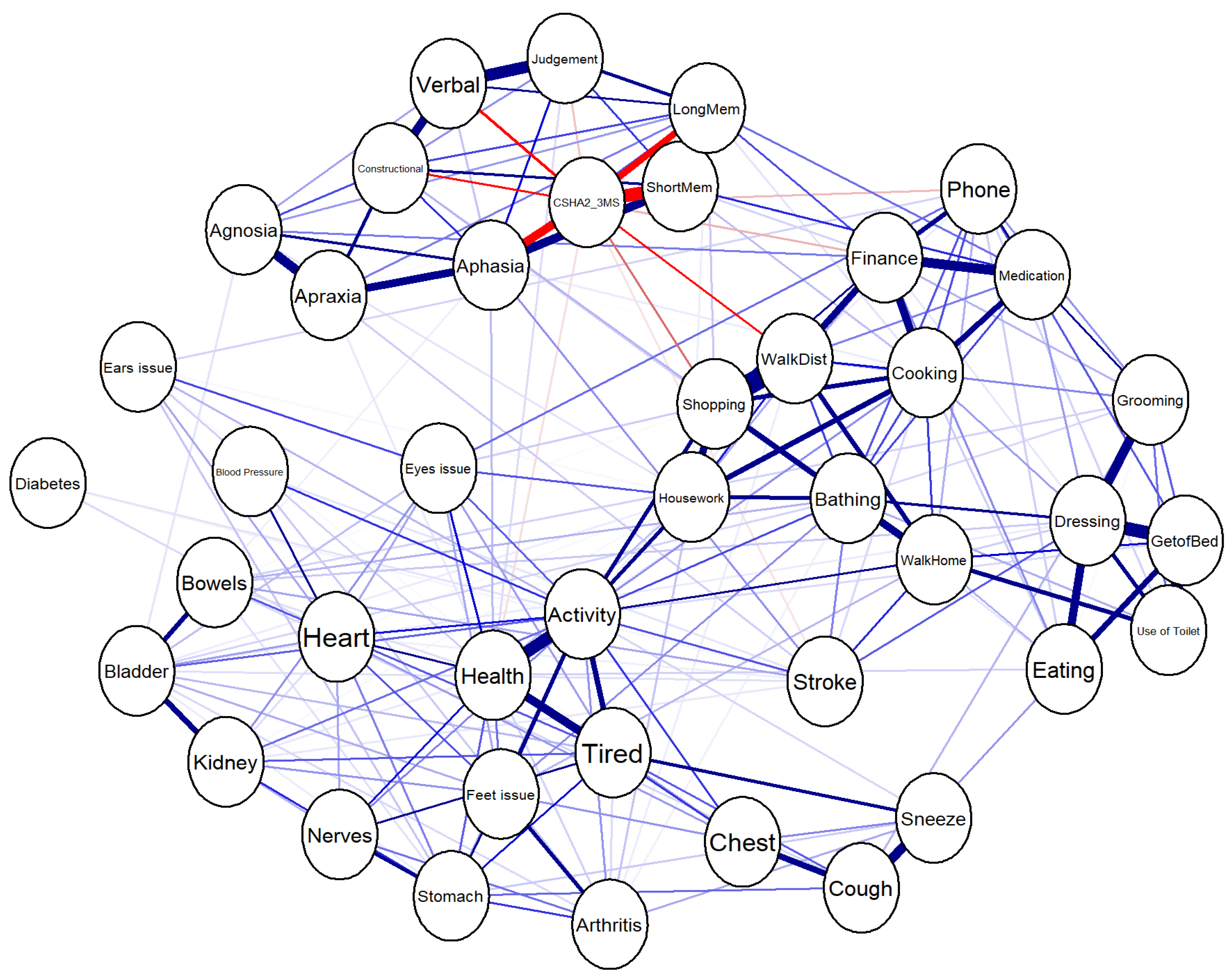
3.1. Network Analysis
- Strength: Sum of absolute edge weights connected to a node.
- Betweenness: Number of shortest paths that pass through a given node.
- Closeness: Inverse of the sum of shortest path distances from a node to all other nodes.
3.1.1. Visualization of the Network
3.1.2. Centrality Analysis
3.1.3. Identification of Less Impactful Variables
3.2. Neural Networks
3.2.1. Model Performance
3.2.2. Variable Importance
3.3. Least Absolute Shrinkage and Selection Operator (LASSO)
3.4. Random Forest
3.5. eXtreme Gradient Boosting (Xgboost)
3.6. Process of Final Variables Selection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz, A.M.; Perez, H.; Jantzi, M.; Liu, L.; Hirdes, J.P. Pan-Canadian estimates of the prevalence and risks associated with critical wandering among home care clients. Alzheimers Dement. 2024, 20, 7079–7089. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Kronmal, R.; Newman, A.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Boada, M.; Borson, S.; Chilukuri, M.; Doraiswamy, P.M.; Dubois, B.; Fillit, H.; Grossberg, G.T.; Hampel, H.; Iwata, A.; et al. Rationale for Early Diagnosis of Mild Cognitive Impairment (MCI) supported by Emerging Digital Technologies. J. Prev. Alzheimer’s Dis. 2020, 7, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Y.; Sun, Z.; Tan, C.C.; Tan, L.; Xu, W. Multi-Concept Frailty Predicts the Late-Life Occurrence of Cognitive Decline or Dementia: An Updated Systematic Review and Meta-Analysis of Longitudinal Studies. Front. Aging Neurosci. 2022, 14, 855553. [Google Scholar] [CrossRef]
- Borges, M.K.; Canevelli, M.; Cesari, M.; Aprahamian, I. Frailty as a Predictor of Cognitive Disorders: A Systematic Review and Meta-Analysis. Front. Med. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Doi, T.; Lee, S.; Suzuki, T. Cognitive Frailty and Incidence of Dementia in Older Persons. J. Prev. Alzheimer’s Dis. 2018, 5, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef]
- Pakzad, S.; Bourque, P.; Feldman, H.; Fallah, N. Toward developing a novel Neurocognitive Frailty Index in the elderly. J. Geriatr. Med. Gerontol. 2017, 3, 032. [Google Scholar] [CrossRef][Green Version]
- Pakzad, S.; Bourque, P.; Fallah, N. Prediction of Cognitive Status and 5-Year Survival Rate for Elderly with Cardiovascular Diseases: A Canadian Study of Health and Aging Secondary Data Analysis. J. Frailty Aging 2021, 10, 31–37. [Google Scholar] [CrossRef]
- Pakzad, S.; Bourque, P.; Fallah, N. The Predictive Status of the Neurocognitive Frailty Index in a Canadian Sample of Elderly with Hypertension. J. Geriatr. Med. Gerontol. 2020, 6, 087. [Google Scholar] [CrossRef]
- Kim, M.J.; Song, S.H.; Park, Y.J.; Lee, Y.H.; Kim, J.; Jeon, J.; Woo, K.; Kim, J.; Kim, J.Y.; Park, S.J.; et al. Comparison of Artificial Intelligence-Derived Heart Age with Chronological Age Using Normal Sinus Electrocardiograms in Patients with No Evidence of Cardiac Disease. J. Clin. Med. 2025, 14, 5548. [Google Scholar] [CrossRef]
- Ansari, A.; Ansari, N.; Khalid, U.; Markov, D.; Bechev, K.; Aleksiev, V.; Markov, G.; Poryazova, E. The Role of Artificial Intelligence in the Diagnosis and Management of Diabetic Retinopathy. J. Clin. Med. 2025, 14, 5150. [Google Scholar] [CrossRef]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.A. Ruptured Posterior Inferior Cerebellar Artery Aneurysms: Integrating Microsurgical Expertise, Endovascular Challenges, and AI-Driven Risk Assessment. J. Clin. Med. 2025, 14, 5374. [Google Scholar] [CrossRef] [PubMed]
- Pantilimonescu, T.F.; Damian, C.; Radu, V.D.; Hogea, M.; Costachescu, O.A.; Onofrei, P.; Toma, B.; Zelinschi, D.; Roca, I.C.; Ursu, R.G.; et al. Use of Artificial Intelligence Methods for Improved Diagnosis of Urinary Tract Infections and Urinary Stone Disease. J. Clin. Med. 2025, 14, 4942. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wu, Z.; Zhao, W.; Jia, L.; Li, S.; Wei, W.; Chen, X. The performance of artificial intelligence in image-based prediction of hematoma enlargement: A systematic review and meta-analysis. Ann. Med. 2025, 57, 2515473. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, A.A.; Aderinto, N.; Popoola, M.R.; Adeyanju, I.A.; Osonuga, A.; Olawade, D.B. Machine learning techniques for stroke prediction: A systematic review of algorithms, datasets, and regional gaps. Int. J. Med. Inform. 2025, 203, 106041. [Google Scholar] [CrossRef]
- Xie, Y.; Xiang, X.; Fan, M.; Li, H.; Du, L.; Gao, W.; Chen, T.; Shi, Z.; Yu, X.; Liu, F. Development of a machine learning-based prognostic model for survival prediction in patients with lung cancer brain metastases using multicenter clinical data. Int. J. Med. Inform. 2025, 203, 106025. [Google Scholar] [CrossRef]
- Al-Jabri, M.M.; Anshasi, H. Performance of machine and deep learning models for predicting delirium in adult ICU patients: A systematic review. Int. J. Med. Inform. 2025, 203, 106008. [Google Scholar] [CrossRef]
- Ghorbian, M.; Ghorbian, S. The potential of machine learning to personalized medicine in Neurogenetics: Current trends and future directions. Comput. Biol. Med. 2025, 196 Pt A, 110756. [Google Scholar] [CrossRef]
- Sánchez-Moreno, L.; Perez-Peña, A.; Duran-Lopez, L.; Dominguez-Morales, J.P. Ensemble-based Convolutional Neural Networks for brain tumor classification in MRI: Enhancing accuracy and interpretability using explainable AI. Comput. Biol. Med. 2025, 195, 110555. [Google Scholar] [CrossRef]
- Fallah, N.; Mitnitski, A.; Rockwood, K. Applying Neural Network Poisson Regression to Predict Cognitive Score Changes. J. Appl. Stat. 2011, 38, 2051–2062. [Google Scholar] [CrossRef]
- Kaur, H.; Malhi, A.K.; Pannu, H.S. Machine learning ensemble for neurological disorders. Neural Comput. Appl. 2020, 32, 5463–5476. [Google Scholar] [CrossRef]
- Latha, M.; Kavitha, G. Detection of schizophrenia in brain MR images based on segmented ventricle region and deep belief networks. Neural Comput. Appl. 2019, 31, 5195–5206. [Google Scholar] [CrossRef]
- Luo, S.; Li, X.; Li, J. Automatic Alzheimer’s Disease Recognition from MRI Data Using Deep Learning Method. J. Appl. Math. Phys.. 2017, 5, 1892–1898. [Google Scholar] [CrossRef]
- Fallah, N.; Noonan, V.K.; Waheed, Z.; Rivers, C.S.; Plashkes, T.; Bedi, M.; Etminan, M.; Thorogood, N.P.; Ailon, T.; Chan, E.; et al. Development of a machine learning algorithm for predicting in-hospital and 1-year mortality after traumatic spinal cord injury. Spine J. 2022, 22, 329–336. [Google Scholar] [CrossRef]
- Yousefi, M.; Akhbari, M.; Mohamadi, Z.; Karami, S.; Dasoomi, H.; Atabi, A.; Sarkeshikian, S.A.; Abdoullahi Dehaki, M.; Bayati, H.; Mashayekhi, N.; et al. Machine learning based algorithms for virtual early detection and screening of neurodegenerative and neurocognitive dis-orders: A systematic review. Front. Neurol. 2024, 15, 1413071. [Google Scholar] [CrossRef]
- McFall, G.P.; Bohn, L.; Gee, M.; Drouin, S.M.; Fah, H.; Han, W.; Li, L.; Camicioli, R.; Dixon, R.A. Identifying key multi-modal predictors of incipient dementia in Parkinson’s disease: A machine learning analysis and Tree SHAP interpretation. Front. Neurol. 2023, 15, 1124232. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.; Shi, L.; Han, C.; He, Q.; Che, Y.; Luo, L. A novel framework of MOPSO-GDM in recognition of Alzheimer’s EEG-based functional network. Front. Neurol. 2023, 15, 1160534. [Google Scholar] [CrossRef]
- Pergantis, P.; Bamicha, V.; Doulou, A.; Christou, A.I.; Bardis, N.; Skianis, C.; Drigas, A. Assistive and Emerging Technologies to Detect and Reduce Neurophysiological Stress and Anxiety in Children and Adolescents with Autism and Sensory Processing Disorders: A Systematic Review. Technologies 2025, 13, 144. [Google Scholar] [CrossRef]
- Xiao, X.; Yi, Y.; Soe, N.; Lat, P.; Lin, L.; Chen, X.; Song, H.; Sun, B.; Zhao, H.; Xu, X. A web-based tool for cancer risk prediction for middle-aged and elderly adults using machine learning algorithms and self-reported questions. Ann. Epidemiol. 2025, 101, 27–35. [Google Scholar] [CrossRef] [PubMed]

| Model I | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | ||
|---|---|---|---|---|---|---|
| Beta | Std. Error | Beta | ||||
| (Constant) | 48.174 | 8.745 | 5.509 | <0.001 | ||
| Age | −0.539 | 0.079 | −0.202 | −6.797 | <0.001 | |
| Gender | −1.595 | 1.078 | −0.043 | −1.480 | 0.139 | |
| Education | 2.387 | 1.156 | 0.066 | 2.066 | 0.039 | |
| 3MS at baseline | 0.902 | 0.061 | 0.515 | 14.676 | <0.001 | |
| Neurocognitive Frailty Index | −0.579 | 0.110 | −0.176 | −5.285 | <0.001 | |
| Model II | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | ||
| Beta | Std. Error | Beta | ||||
| (Constant) | 49.079 | 8.757 | 5.604 | <0.001 | ||
| Age | −0.541 | 0.079 | −0.203 | −6.829 | <0.001 | |
| Gender | −1.652 | 1.077 | −0.045 | −1.534 | 0.126 | |
| Education | 2.381 | 1.154 | 0.066 | 2.063 | 0.040 | |
| 3MS at baseline | 0.895 | 0.062 | 0.511 | 14.529 | <0.001 | |
| Modified Neurocognitive Frailty Index | −0.610 | 0.112 | −0.183 | −5.455 | <0.001 | |
| Model III | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | ||
| Beta | Std. Error | Beta | ||||
| (Constant) | 51.529 | 8.830 | 5.835 | <0.001 | ||
| Age | −0.556 | 0.079 | −0.209 | −7.001 | <0.001 | |
| Gender | −1.739 | 1.076 | −0.047 | −1.616 | 0.107 | |
| Education | 2.436 | 1.152 | 0.067 | 2.115 | 0.035 | |
| 3MS at baseline | 0.882 | 0.062 | 0.504 | 14.267 | <0.001 | |
| Neurocognitive Frailty Index | 2.752 | 1.431 | 0.838 | 1.923 | 0.055 | |
| Modified Neurocognitive Frailty Index | −3.412 | 1.462 | −1.021 | −2.334 | 0.020 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallah, N.; Pakzad, S.; Bourque, P.-É.; Goodarzynejad, H. A Machine Learning Approach to Modify the Neurocognitive Frailty Index for the Prediction of Cognitive Status in the Canadian Population. J. Clin. Med. 2025, 14, 6509. https://doi.org/10.3390/jcm14186509
Fallah N, Pakzad S, Bourque P-É, Goodarzynejad H. A Machine Learning Approach to Modify the Neurocognitive Frailty Index for the Prediction of Cognitive Status in the Canadian Population. Journal of Clinical Medicine. 2025; 14(18):6509. https://doi.org/10.3390/jcm14186509
Chicago/Turabian StyleFallah, Nader, Sarah Pakzad, Paul-Émile Bourque, and Hamidreza Goodarzynejad. 2025. "A Machine Learning Approach to Modify the Neurocognitive Frailty Index for the Prediction of Cognitive Status in the Canadian Population" Journal of Clinical Medicine 14, no. 18: 6509. https://doi.org/10.3390/jcm14186509
APA StyleFallah, N., Pakzad, S., Bourque, P.-É., & Goodarzynejad, H. (2025). A Machine Learning Approach to Modify the Neurocognitive Frailty Index for the Prediction of Cognitive Status in the Canadian Population. Journal of Clinical Medicine, 14(18), 6509. https://doi.org/10.3390/jcm14186509




