Predictive Value of [18F]FDG PET/CT for Neoadjuvant Chemoradiotherapy Response in Nasopharyngeal Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population and Inclusion Criteria
2.3. PET/CT Acquisition Protocol
2.4. Image Analysis and Quantitative PET Parameters
2.5. NAC Response Assessment
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Baseline PET/CT Parameters
3.3. NAC Response
3.4. Correlation Between PET Parameters and NAC Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AUC | Area Under the Curve |
| CCRT | Concurrent Chemoradiotherapy |
| CR | Complete Response |
| CT | Computed Tomography |
| EBER | Epstein–Barr Virus Early RNA |
| EBV | Epstein–Barr Virus |
| FDG | Fluorodeoxyglucose |
| IC | Induction Chemotherapy |
| MRI | Magnetic Resonance Imaging |
| MTV | Metabolic Tumor Volume |
| NAC | Neoadjuvant Chemotherapy |
| NPC | Nasopharyngeal Carcinoma |
| OSEM | Ordered Subset Expectation Maximization |
| PD | Progressive Disease |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| PR | Partial Response |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| ROC | Receiver Operating Characteristic |
| SD | Stable Disease |
| SUVmax | Maximum Standardized Uptake Value |
| SUVmean | Mean Standardized Uptake Value |
| TLG | Total Lesion Glycolysis |
References
- Quartuccio, N.; Ialuna, S.; Poma, S.; Lentini, V.L.; Pitruzzella, A.; Galfano, G.M.; Moreci, A.M.; Modica, D.M. An uncommon case of pediatric nasopharyngeal carcinoma with bone metastases and enchondromas evaluated using (18)f-fdg pet/ct. Mol. Imaging Radionucl. Ther. 2024, 33, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, N.; Pulizzi, S.; Modica, D.M.; Nicolosi, S.; D’Oppido, D.; Moreci, A.M.; Ialuna, S. Head-to-head comparison of [(18)f]fdg pet imaging and mri for the detection of recurrence or residual tumor in patients with nasopharyngeal carcinoma: A meta-analysis. Cancers 2024, 16, 3011. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Ribassin-Majed, L.; Marguet, S.; Lee, A.W.M.; Ng, W.T.; Ma, J.; Chan, A.T.C.; Huang, P.Y.; Zhu, G.; Chua, D.T.T.; Chen, Y.; et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J. Clin. Oncol. 2017, 35, 498–505. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Zhang, B.; Mo, Z.; Du, W.; Wang, Y.; Liu, L.; Wei, Y. Intensity-modulated radiation therapy versus 2d-rt or 3d-crt for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. Oral Oncol. 2015, 51, 1041–1046. [Google Scholar] [CrossRef]
- Sun, Y.; Li, W.F.; Chen, N.Y.; Zhang, N.; Hu, G.Q.; Xie, F.Y.; Sun, Y.; Chen, X.Z.; Li, J.G.; Zhu, X.D.; et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016, 17, 1509–1520. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Hu, G.Q.; Zhang, N.; Zhu, X.D.; Yang, K.Y.; Jin, F.; Shi, M.; Chen, Y.P.; Hu, W.H.; et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N. Engl. J. Med. 2019, 381, 1124–1135. [Google Scholar]
- Cao, S.M.; Yang, Q.; Guo, L.; Mai, H.Q.; Mo, H.Y.; Cao, K.J.; Qian, C.N.; Zhao, C.; Xiang, Y.Q.; Zhang, X.P.; et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase iii multicentre randomised controlled trial. Eur. J. Cancer 2017, 75, 14–23. [Google Scholar] [CrossRef]
- Hong, R.L.; Hsiao, C.F.; Ting, L.L.; Ko, J.Y.; Wang, C.W.; Chang, J.T.C.; Lou, P.J.; Wang, H.M.; Tsai, M.H.; Lai, S.C.; et al. Final results of a randomized phase iii trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage iva and ivb nasopharyngeal carcinoma-taiwan cooperative oncology group (tcog) 1303 study. Ann. Oncol. 2018, 29, 1972–1979. [Google Scholar] [CrossRef]
- Yang, Q.; Cao, S.M.; Guo, L.; Hua, Y.J.; Huang, P.Y.; Zhang, X.L.; Lin, M.; You, R.; Zou, X.; Liu, Y.P.; et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: Long-term results of a phase iii multicentre randomised controlled trial. Eur. J. Cancer 2019, 119, 87–96. [Google Scholar] [CrossRef]
- Hong, Y.J.; Kim, M.J.; Jeong, E.; Kim, J.E.; Hwang, J.; Lee, J.I.; Lee, J.H.; Na, D.L. Preoperative biomarkers in patients with idiopathic normal pressure hydrocephalus showing a favorable shunt surgery outcome. J. Neurol. Sci. 2018, 387, 21–26. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, Q.; Zhang, Y.; Pan, H.; Yao, Z.; Hu, S.; Shi, W.; Zhu, B.; Zhang, Y.; Hu, C. Pretreatment (18)f-fdg uptake heterogeneity can predict survival in patients with locally advanced nasopharyngeal carcinoma--a retrospective study. Radiat. Oncol. 2015, 10, 4. [Google Scholar] [CrossRef]
- Pak, K.; Cheon, G.J.; Nam, H.Y.; Kim, S.J.; Kang, K.W.; Chung, J.K.; Kim, E.E.; Lee, D.S. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: A systematic review and meta-analysis. J. Nucl. Med. 2014, 55, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, P.; Merlotti, A.; Olmetto, E.; Bianchi, A.; Desideri, I.; Bacigalupo, A.; Franco, P.; Franzese, C.; Orlandi, E.; Livi, L.; et al. What is the prognostic impact of fdg pet in locally advanced head and neck squamous cell carcinoma treated with concomitant chemo-radiotherapy? A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2122–2138. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, J.; Cheng, W.; Zhu, C.; Chen, L.; Xia, F.; Wang, M.; Yang, F.; Ma, X. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. Medicine 2017, 96, e8084. [Google Scholar] [CrossRef]
- Long, Z.C.; Ding, X.C.; Zhang, X.B.; Shui, Y.; Zheng, F.; Sun, P.P.; Hao, F.R.; Li, Z.R.; Hu, M. The efficacy of pretreatment (18)f-fdg pet-ct-based deep learning network structure to predict survival in nasopharyngeal carcinoma. Clin. Med. Insights Oncol. 2023, 17, 11795549231171793. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.M.; Welch, A.; McKiddie, F.; Nath, M. A systematic review and meta-analysis of predictive and prognostic models for outcome prediction using positron emission tomography radiomics in head and neck squamous cell carcinoma patients. Cancer Med. 2023, 12, 16181–16194. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition ajcc cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Niu, X.; Xue, F.; Ou, D.; Zheng, Y.; Hu, C.; Shen, C.; He, X. Pretreatment fdg pet in prognosis of locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Int. J. Med. Sci. 2025, 22, 933–939. [Google Scholar] [CrossRef]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- Quartuccio, N.; Caobelli, F.; Di Mauro, F.; Cammaroto, G.; Group, Y.A.W. Non-18f-fdg pet/ct in the management of patients affected by hnc: State-of-the-art. Nucl. Med. Commun. 2016, 37, 891–898. [Google Scholar] [CrossRef]
- Chan, S.C.; Chang, J.T.; Lin, C.Y.; Ng, S.H.; Wang, H.M.; Liao, C.T.; Chang, C.J.; Lin, S.Y.; Yen, T.C. Clinical utility of 18f-fdg pet parameters in patients with advanced nasopharyngeal carcinoma: Predictive role for different survival endpoints and impact on prognostic stratification. Nucl. Med. Commun. 2011, 32, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, L.; Zhang, J.; Li, W.F.; Mao, Y.P.; Zhang, Y.; Liu, L.Z.; Tian, L.; Lin, A.H.; Sun, Y.; et al. Induction chemotherapy improved long-term outcomes of patients with locoregionally advanced nasopharyngeal carcinoma: A propensity matched analysis of 5-year survival outcomes in the era of intensity-modulated radiotherapy. J. Cancer 2017, 8, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, Y.; Lin, Y.; Yang, Y.; Wan, J.; Gong, X.; Zhang, F.; Zhang, W.; Marks, T.; Wang, S.; et al. The role of pretreatment (18)f-fdg pet/ct for early prediction of neoadjuvant chemotherapy response in patients with locoregionally advanced nasopharyngeal carcinoma. Drug Des. Dev. Ther. 2021, 15, 4157–4166. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Murphy, J.D.; Khong, B.; La, T.H.; Kong, C.; Fischbein, N.J.; Colevas, A.D.; Iagaru, A.H.; Graves, E.E.; Loo, B.W., Jr.; et al. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1514–1520. [Google Scholar] [CrossRef]
- Alami, I.E.; Gihbid, A.; Charoute, H.; Khaali, W.; Brahim, S.M.; Tawfiq, N.; Cadi, R.; Belghmi, K.; El Mzibri, M.; Khyatti, M. Prognostic value of epstein-barr virus DNA load in nasopharyngeal carcinoma: A meta-analysis. Pan Afr. Med. J. 2022, 41, 6. [Google Scholar] [CrossRef]
- Cammaroto, G.; Quartuccio, N.; Sindoni, A.; Di Mauro, F.; Caobelli, F.; Young, A.W.G. The role of pet/ct in the management of patients affected by head and neck tumors: A review of the literature. Eur. Arch. Otorhinolaryngol. 2016, 273, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- Bogowicz, M.; Vuong, D.; Huellner, M.W.; Pavic, M.; Andratschke, N.; Gabrys, H.S.; Guckenberger, M.; Tanadini-Lang, S. Ct radiomics and pet radiomics: Ready for clinical implementation? Q. J. Nucl. Med. Mol. Imaging 2019, 63, 355–370. [Google Scholar] [CrossRef]
- Avanzo, M.; Wei, L.; Stancanello, J.; Vallières, M.; Rao, A.; Morin, O.; Mattonen, S.A.; El Naqa, I. Machine and deep learning methods for radiomics. Med. Phys. 2020, 47, e185–e202. [Google Scholar] [CrossRef] [PubMed]
- Cergan, R.; Dumitru, M.; Costache, A. Diagnostic and interventional imaging in various diseases. Medicina 2024, 60, 1810. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | N (%) or Mean ± SD |
|---|---|
| Age (years) | 60.8 ± 15.2 |
| Sex—Male | 20 (74.1%) |
| Sex—Female | 7 (25.9%) |
| Histotype—Undifferentiated non-keratinizing | 25 (92.6%) |
| Histotype—Squamous cell carcinoma | 2 (7.4%) |
| T Stage—T3 | 20 (74.1%) |
| T Stage—T4 | 7 (25.9%) |
| N Stage—N1 | 4 (14.8%) |
| N Stage—N2 | 21 (77.8%) |
| N Stage—N3 | 2 (7.4%) |
| AJCC Stage—III | 27 (100%) |
| EBER Positive | 7 (25.9%) |
| EBER Negative | 20 (74.1%) |
| Ki-67 Proliferation Index | Median: 50% (range 20–80%) |
| Parameter | Mean ± SD | Range |
|---|---|---|
| SUVmax | 12.4 ± 5.2 | 4.1–33.0 |
| SUVmean | 6.8 ± 2.8 | 3.6–12.9 |
| MTV (cm3) | 19.8 ± 16.2 | 5.7–63.2 |
| TLG (g/mL × cm3) | 156.4 ± 128.7 | 22.2–423.9 |
| Parameter | Responders (n = 19) | Non-Responders (n = 8) | p-Value |
|---|---|---|---|
| SUVmax | 10.9 ± 4.8 | 15.8 ± 4.1 | 0.021 |
| SUVmean | 6.1 ± 2.1 | 9.3 ± 2.8 | ns |
| MTV (cm3) | 16.2 ± 12.4 | 27.8 ± 19.5 | 0.045 |
| TLG (g/mL × cm3) | 128.6 ± 98.2 | 218.7 ± 152.4 | 0.038 |
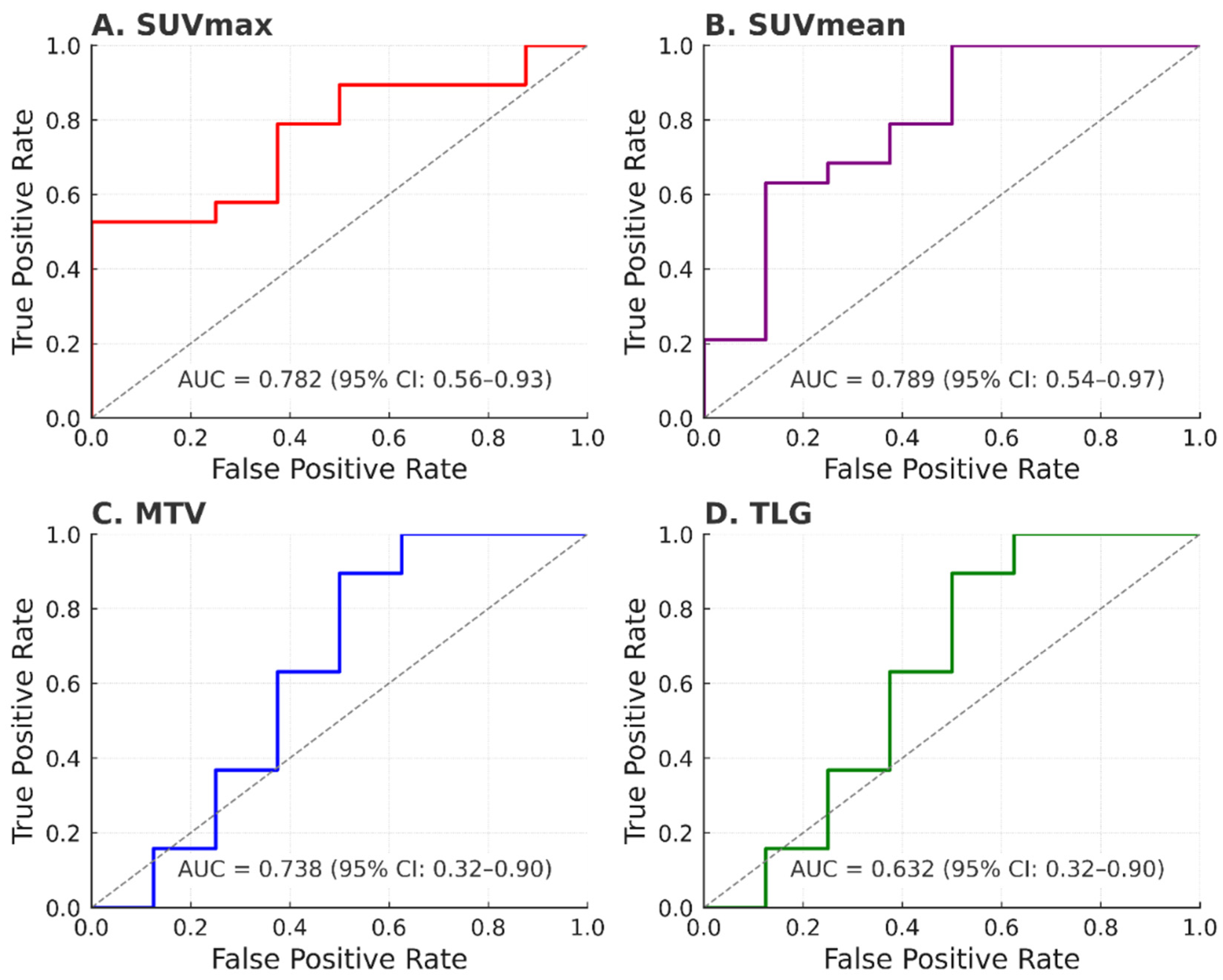
| Parameter | Cut-Off | Sensitivity (%) | Specificity (%) | AUC (95% CI) |
|---|---|---|---|---|
| SUVmax | 12.5 | 75.0 | 78.9 | 0.782 (0.598–0.966) |
| SUVmean | 7.8 | 62.5 | 78.9 | 0.724 (0.521–0.928) |
| MTV (cm3) | 20.0 | 62.5 | 84.2 | 0.738 (0.539–0.936) |
| TLG | 145.0 | 75.0 | 63.2 | 0.632 (0.405–0.860) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quartuccio, N.; Sireci, F.; Pulizzi, S.; Nicolosi, S.; D’Oppido, D.; Ialuna, S. Predictive Value of [18F]FDG PET/CT for Neoadjuvant Chemoradiotherapy Response in Nasopharyngeal Carcinoma. J. Clin. Med. 2025, 14, 6508. https://doi.org/10.3390/jcm14186508
Quartuccio N, Sireci F, Pulizzi S, Nicolosi S, D’Oppido D, Ialuna S. Predictive Value of [18F]FDG PET/CT for Neoadjuvant Chemoradiotherapy Response in Nasopharyngeal Carcinoma. Journal of Clinical Medicine. 2025; 14(18):6508. https://doi.org/10.3390/jcm14186508
Chicago/Turabian StyleQuartuccio, Natale, Federico Sireci, Sabina Pulizzi, Stefania Nicolosi, Dante D’Oppido, and Salvatore Ialuna. 2025. "Predictive Value of [18F]FDG PET/CT for Neoadjuvant Chemoradiotherapy Response in Nasopharyngeal Carcinoma" Journal of Clinical Medicine 14, no. 18: 6508. https://doi.org/10.3390/jcm14186508
APA StyleQuartuccio, N., Sireci, F., Pulizzi, S., Nicolosi, S., D’Oppido, D., & Ialuna, S. (2025). Predictive Value of [18F]FDG PET/CT for Neoadjuvant Chemoradiotherapy Response in Nasopharyngeal Carcinoma. Journal of Clinical Medicine, 14(18), 6508. https://doi.org/10.3390/jcm14186508







