Cholesterol Embolization Syndrome Presenting with Multifocal Cerebral Infarction After Thoracic Endovascular Aortic Repair: A Case Report
Abstract
1. Introduction
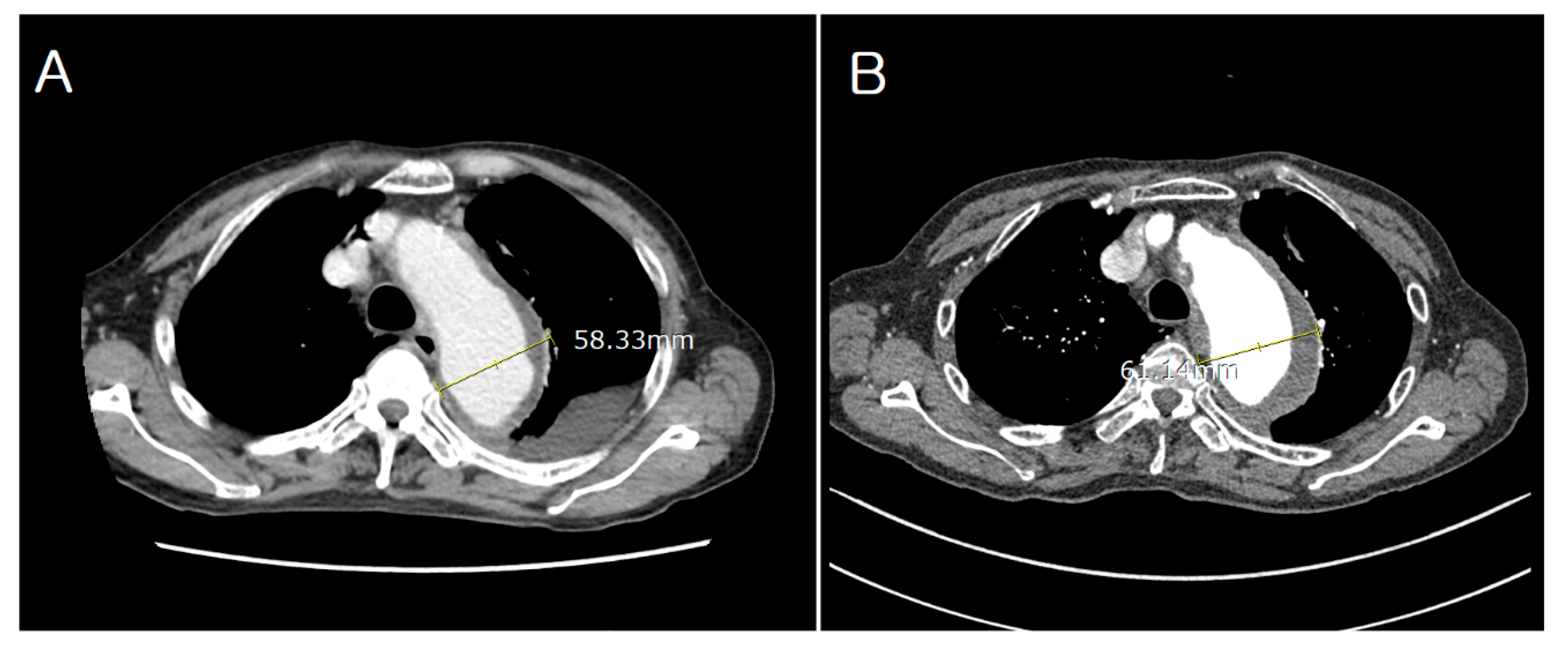
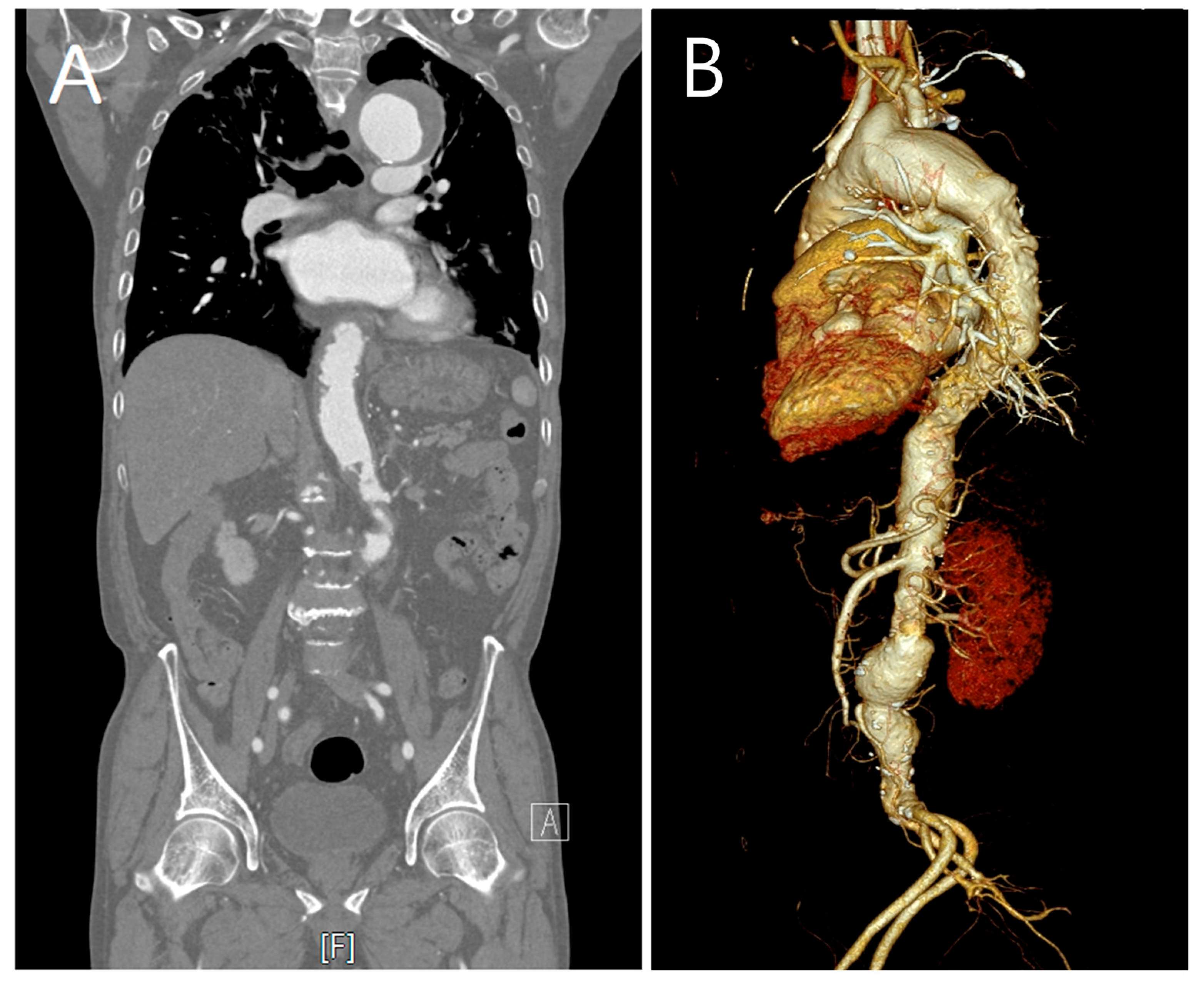
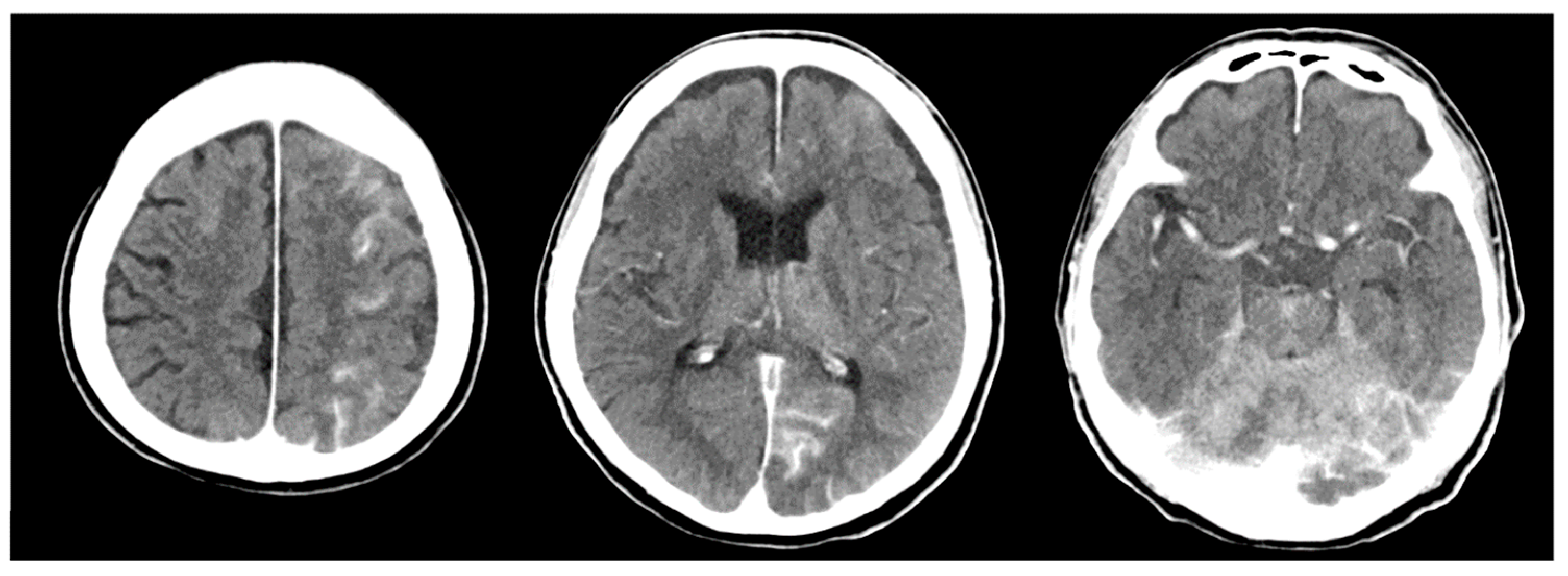
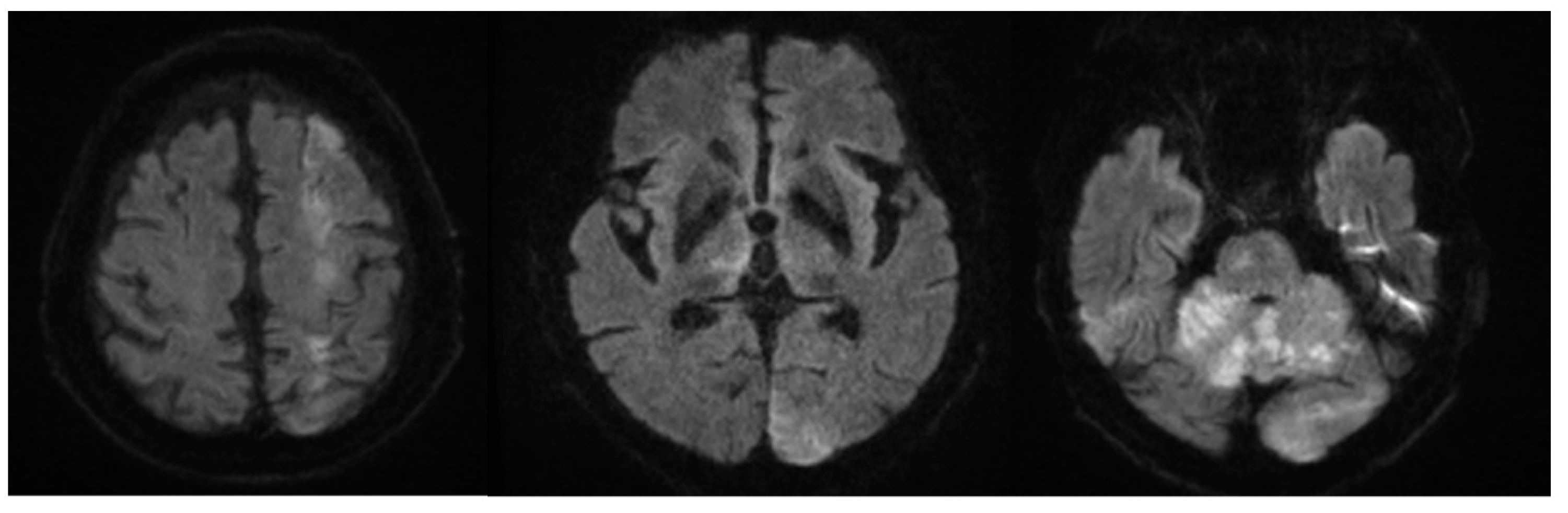
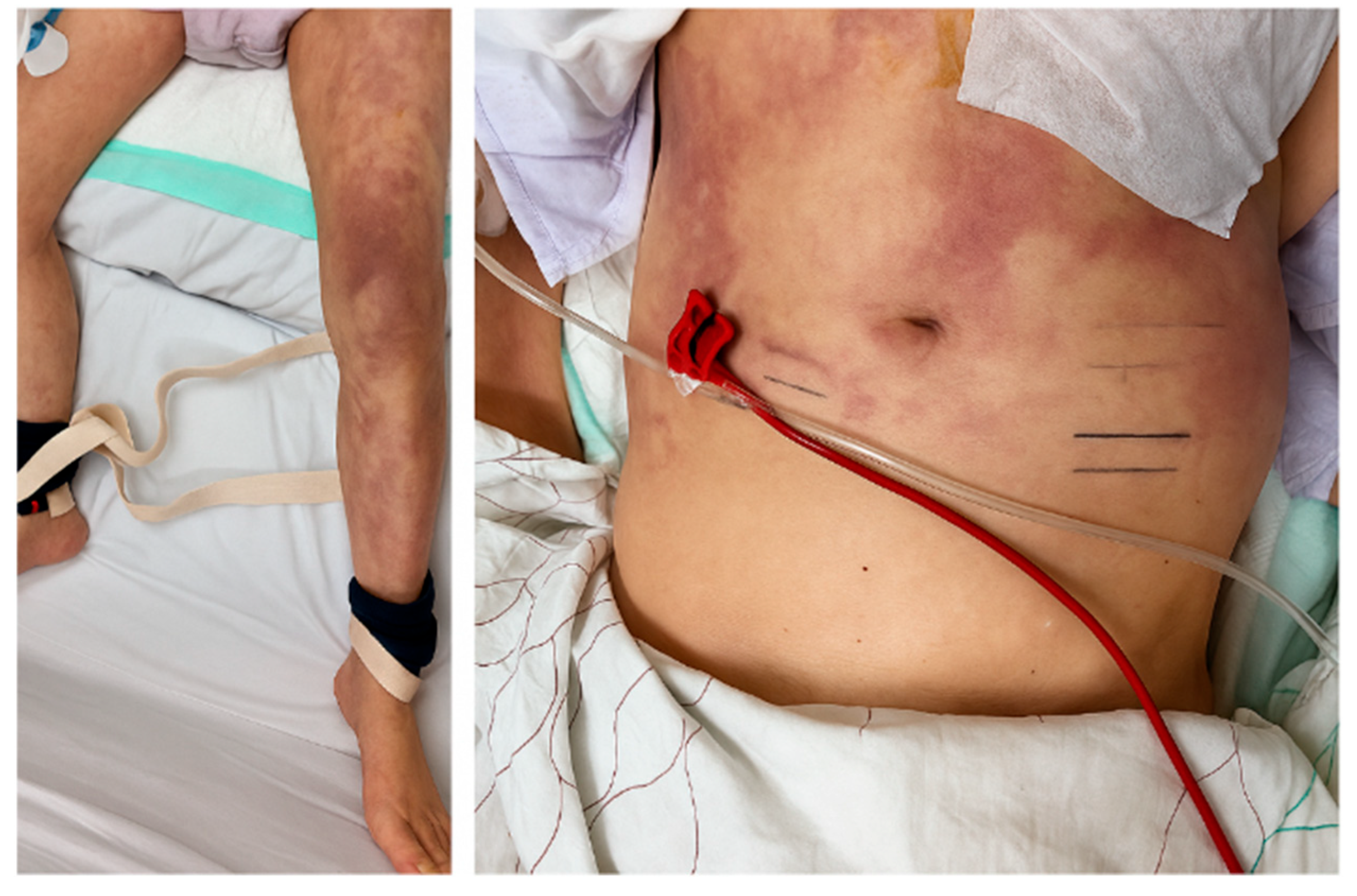
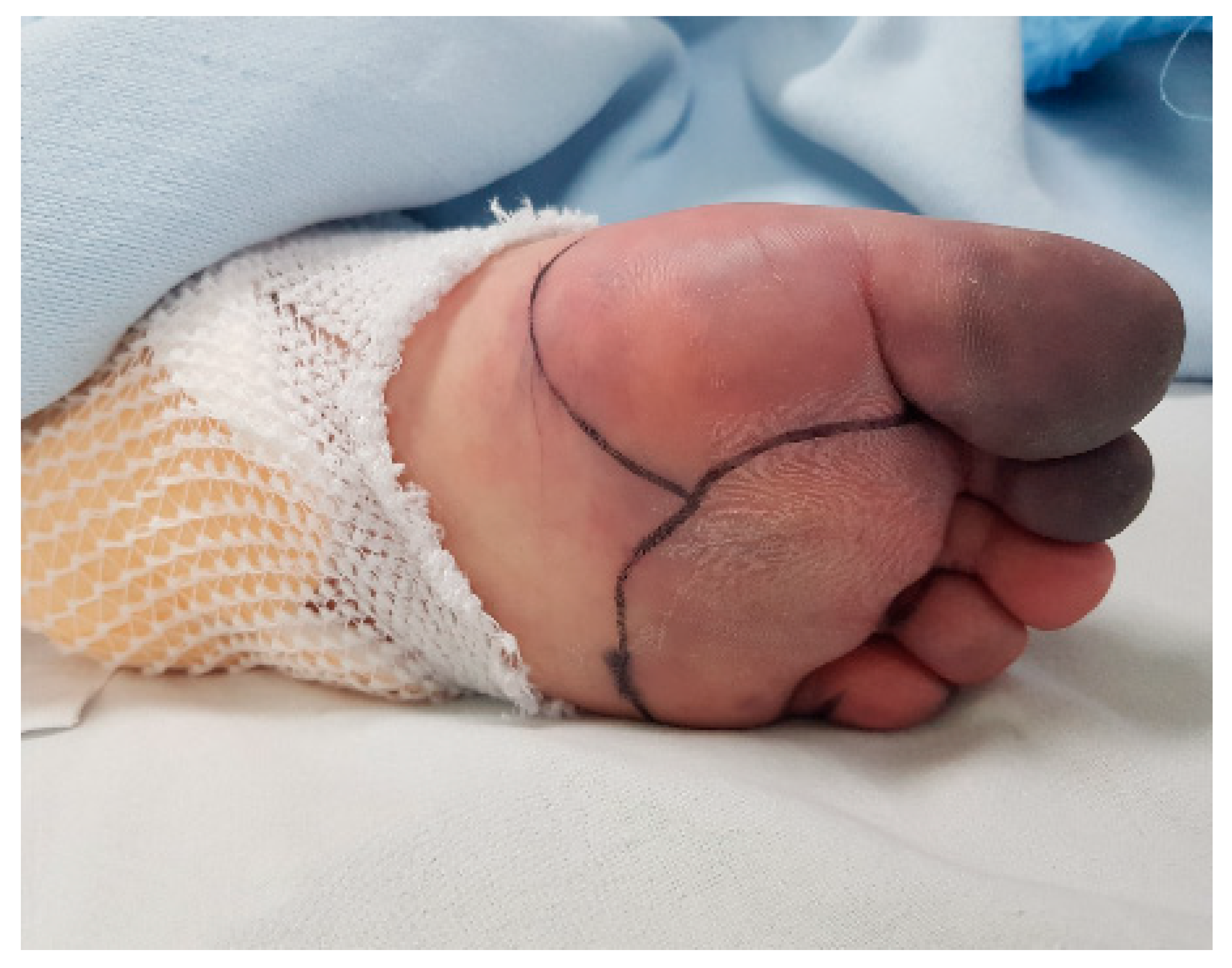
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CES | Cholesterol embolization syndrome |
| CI | Cerebral infarction |
| CRRT | Continuous renal replacement therapy |
| EVD | External ventricular drainage |
| ICU | Intensive care unit |
| LCCA | Left common carotid artery |
| LSCA | Left subclavian artery |
| ROSC | Return of spontaneous circulation |
| TEVAR | Thoracic endovascular aortic repair |
References
- Kronzon, I.; Saric, M. Cholesterol embolization syndrome. Circulation 2010, 122, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Tsutsui, H.; Tsuchihashi, M.; Masumoto, A.; Takeshita, A. The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: A prospective study. J. Am. Coll. Cardiol. 2003, 42, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeem, B.; Ayad, S.; Osman, A.-F.; Gjeka, R.; Kunadi, A. An Unusual Presentation of Cholesterol Embolization Syndrome. Cureus 2021, 13, e16993. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Kanzaki, M.; Ishima, D.; Usui, R.; Kimura, A.; Usui, K.; Amoh, Y.; Takeuchi, Y.; Kumabe, T.; Ako, J.; et al. Cholesterol crystal embolism-related cerebral infarction: Magnetic resonance imaging and clinical characteristics. eNeurologicalSci 2021, 25, 100388. [Google Scholar] [CrossRef]
- Bavaria, J.E.; Appoo, J.J.; Makaroun, M.S.; Verter, J.; Yu, Z.-F.; Mitchell, R.S. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: A multicenter comparative trial. J. Thorac. Cardiovasc. Surg. 2007, 133, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Martin, J.; Shennib, H.; Dunning, J.; Muneretto, C.; Schueler, S.; von Segesser, L.; Sergeant, P.; Turina, M. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. J. Am. Coll. Cardiol. 2010, 55, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.K.; Lu, Q.; Roselli, E.E.; Svensson, L.G.; Moon, M.C.; Hernandez, A.V.; Dowdall, J.; Cury, M.; Francis, C.; Pfaff, K.; et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: A comparison of endovascular and open techniques. Circulation 2008, 118, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Spanos, K.; Kölbel, T. Strategies for Stroke Prevention During TEVAR: From Embolic Protection to Device Preparation and Delivery. Endovasc. Today 2019, 18, 95–97. [Google Scholar]
- Herman, C.R.; Rosu, C.; Abraham, C.Z. Cerebral embolic protection during endovascular arch replacement. Ann. Cardiothorac. Surg. 2018, 7, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Ohki, T.; Kanaoka, Y.; Shukuzawa, K.; Baba, T.; Momose, M. A Novel Shaggy Aorta Scoring System to Predict Embolic Complications Following Thoracic Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, J.T.; Cheung, A.T.; McGarvey, M.L.; Moser, W.G.; Szeto, W.; Carpenter, J.P.; Fairman, R.M.; Pochettino, A.; Bavaria, J.E. Risk factors for perioperative stroke after thoracic endovascular aortic repair. Ann. Thorac. Surg. 2007, 84, 1195–1200; discussion 1200. [Google Scholar] [CrossRef] [PubMed]
- Ozkok, A. Cholesterol-embolization syndrome: Current perspectives. Vasc. Health Risk Manag. 2019, 15, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Mochida, Y.; Ohtake, T.; Ishioka, K.; Oka, M.; Maesato, K.; Moriya, H.; Hidaka, S.; Kobayashi, S. Association between eosinophilia and renal prognosis in patients with pathologically proven cholesterol crystal embolism. Clin. Exp. Nephrol. 2020, 24, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Akhmerov, A.; Gupta, N.; Chakravarty, T.; Makkar, R.R.; Azizzadeh, A. Use of a Dual-Filter Cerebral Embolic Protection Device in Thoracic Endovascular Aortic Repair. Ann. Vasc. Surg. 2020, 65, 54.e1–54.e4. [Google Scholar] [CrossRef]
- Martinez, G.J.; Celermajer, D.S.; Patel, S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis 2018, 269, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Verneuil, L.; Bekolo, R.Z.; Dompmartin, A.; Comoz, F.; Marcelli, C.; Leroy, D. Efficiency of colchicine and corticosteroids in a leg ulceration with cholesterol embolism in a woman with rheumatoid arthritis. Rheumatology 2003, 42, 1014–1016. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bayliss, G.; Zhuang, S. Cholesterol Crystal Embolism and Chronic Kidney Disease. Int. J. Mol. Sci. 2017, 18, 1120. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ziccardi, M.R.; Witzke, C.; Palacios, I.; Rangaswami, J. Cholesterol embolization syndrome: An under-recognized entity in cardiovascular interventions. J. Interv. Cardiol. 2018, 31, 407–415. [Google Scholar] [PubMed]
- Bainey, K.R.; Marquis-Gravel, G.; Belley-Côté, E.; Turgeon, R.D.; Ackman, M.L.; Babadagli, H.E.; Bewick, D.; Boivin-Proulx, L.-A.; Cantor, W.J.; Fremes, S.E.; et al. Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology 2023 Focused Update of the Guidelines for the Use of Antiplatelet Therapy. Can. J. Cardiol. 2024, 40, 160–181. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M. Treatment of Cholesterol Embolism with Corticosteroids and Alprostadil. Ann. Geriatr. Med. Res. 2019, 23, 31–34. [Google Scholar] [CrossRef] [PubMed]

| Lab | Hospital Day 1 | During Clinical Deterioration | Reference |
|---|---|---|---|
| WBC | 9.63 | 21.1 | 4.0~10.0 × 103/µL |
| Eosinophils | 4.2 | 0.2 | 0~5.8% |
| Hemoglobin | 13.1 | 8.5 | 14.0~16.5 g/dL |
| Platelets | 215 | 107 | 150~450 × 103/µL |
| BUN | 18 | 40 | 6~20 mg/dL |
| Creatinine | 1.07 | 3.81 | 0.84~1.23 mg/dL |
| Estimated GFR | 67 | 16 | ~≥90 mL/min/1.73 m2 |
| Troponin | 0.043 | 0.106 | ~0.014 ng/mL |
| CRP | 1.01 | 11.4 | ~0.5 mg/dL |
| ESR | Not performed | 23 | 0~20 mm/h |
| Serum cholesterol level | 151 | 83 | ~200 mg/dL |
| C4 Complement | Not performed | 26.8 | 10~40 mg/dL |
| C3 Complement | Not performed | 80.1 | 90~180 mg/dL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.W.; Kang, I.S. Cholesterol Embolization Syndrome Presenting with Multifocal Cerebral Infarction After Thoracic Endovascular Aortic Repair: A Case Report. J. Clin. Med. 2025, 14, 6507. https://doi.org/10.3390/jcm14186507
Lee SW, Kang IS. Cholesterol Embolization Syndrome Presenting with Multifocal Cerebral Infarction After Thoracic Endovascular Aortic Repair: A Case Report. Journal of Clinical Medicine. 2025; 14(18):6507. https://doi.org/10.3390/jcm14186507
Chicago/Turabian StyleLee, Seung Woo, and In Sook Kang. 2025. "Cholesterol Embolization Syndrome Presenting with Multifocal Cerebral Infarction After Thoracic Endovascular Aortic Repair: A Case Report" Journal of Clinical Medicine 14, no. 18: 6507. https://doi.org/10.3390/jcm14186507
APA StyleLee, S. W., & Kang, I. S. (2025). Cholesterol Embolization Syndrome Presenting with Multifocal Cerebral Infarction After Thoracic Endovascular Aortic Repair: A Case Report. Journal of Clinical Medicine, 14(18), 6507. https://doi.org/10.3390/jcm14186507






