Comparison of High-Charge Protocol vs. Dose Titration Protocol in Bilateral ECT: Evaluation of Antidepressant Effectiveness and EEG Parameters
Abstract
1. Introduction
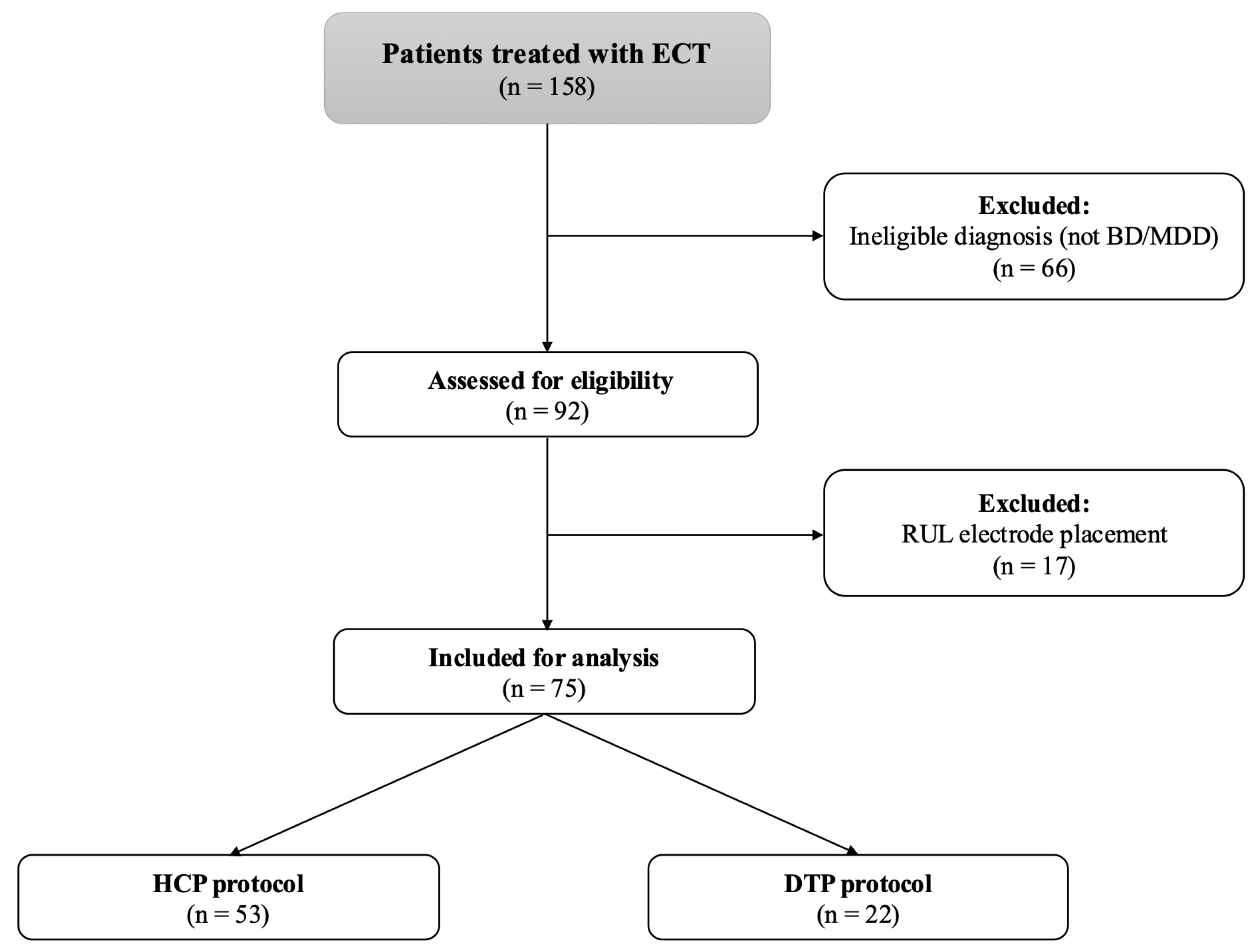
2. Materials and Methods
2.1. Study Design
2.2. High-Charge Protocol
2.3. Dose Titration Protocol
2.4. Clinical Outcome
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Effectiveness of ECT
3.3. Characteristics of Two Different Dosing Protocols
4. Discussion
4.1. Clinical Implications
4.2. Strengths and Limitations
4.3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Espinoza, R.T.; Kellner, C.H. Electroconvulsive Therapy. N. Engl. J. Med. 2022, 386, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Sackeim, H.A. Modern Electroconvulsive Therapy: Vastly Improved yet Greatly Underused. JAMA Psychiatry 2017, 74, 779–780. [Google Scholar] [CrossRef]
- Lemasson, M.; Rochette, L.; Galvão, F.; Poulet, E.; Lacroix, A.; Lecompte, M.; Auriacombe, M.; Patry, S.; Haesebaert, F. Pertinence of Titration and Age-Based Dosing Methods for Electroconvulsive Therapy: An International Retrospective Multicenter Study. J. ECT 2018, 34, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Bergsholm, P.; Bjølseth, T.M. Dosing methods in electroconvulsive therapy: Should the Scandinavian time-titration method be resumed? Nord. J. Psychiatry 2022, 76, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Brus, O.; Cao, Y.; Gustafsson, E.; Hultén, M.; Landen, M.; Lundberg, J.; Nordanskog, P.; Nordenskjöld, A. Self-assessed remission rates after electroconvulsive therapy of depressive disorders. Eur. Psychiatry 2017, 45, 154–160. [Google Scholar] [CrossRef]
- McCall, W.V.; Reboussin, D.M.; Weiner, R.D.; Sackeim, H.A. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: Acute antidepressant and cognitive effects. Arch. Gen. Psychiatry 2000, 57, 438–444. [Google Scholar] [CrossRef]
- Sackeim, H.A.; Prudic, J.; Devanand, D.P.; Nobler, M.S.; Lisanby, S.H.; Peyser, S.; Fitzsimons, L.; Moody, B.J.; Clark, J. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch. Gen. Psychiatry 2000, 57, 425–434. [Google Scholar] [CrossRef]
- Sackeim, H.A.; Prudic, J.; Devanand, D.P.; Nobler, M.S.; Haskett, R.F.; Mulsant, B.H.; Rosenquist, P.B.; McCall, W.V. The benefits and costs of changing treatment technique in electroconvulsive therapy due to insufficient improvement of a major depressive episode. Brain Stimul. 2020, 13, 1284–1295. [Google Scholar] [CrossRef]
- Sackeim, H.A.; Devanand, D.P.; Prudic, J. Stimulus intensity, seizure threshold, and seizure duration: Impact on the efficacy and safety of electroconvulsive therapy. Psychiatr. Clin. N. Am. 1991, 14, 803–843. [Google Scholar] [CrossRef]
- Sackeim, H.A.; Prudic, J.; Devanand, D.P.; Kiersky, J.E.; Fitzsimons, L.; Moody, B.J.; McElhiney, M.C.; Coleman, E.A.; Settembrino, J.M. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N. Engl. J. Med. 1993, 328, 839–846. [Google Scholar] [CrossRef]
- Chanpattana, W.; Chakrabhand, M.L.; Buppanharun, W.; Sackeim, H.A. Effects of stimulus intensity on the efficacy of bilateral ECT in schizophrenia: A preliminary study. Biol. Psychiatry 2000, 48, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, W.; Birkenhäger, T.K.; Boer, D. Guideline Electroconvulsive Therapy [Richtlijn Elektroconvulsie Therapie]; Dutch Association for Psychiatry: Utrecht, The Netherlands, 2010. [Google Scholar]
- Petrides, G.; Fink, M. The “half-age” stimulation strategy for ECT dosing. Convuls. Ther. 1996, 12, 138–146. [Google Scholar]
- Sackeim, H.A.; Decina, P.; Kanzler, M.; Kerr, B.; Malitz, S. Effects of electrode placement on the efficacy of titrated, low-dose ECT. Am. J. Psychiatry 1987, 144, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Plemper, J.; Sartorius, A.; Karl, S. Age-Dependent Dose Increase During an Acute Electroconvulsive Therapy Series. J. ECT 2023, 39, 193–196. [Google Scholar] [CrossRef]
- Østergaard, S.D.; Rothschild, A.J.; Flint, A.J.; Mulsant, B.H.; Whyte, E.M.; Vermeulen, T.; Bech, P.; Meyers, B.S. Establishing the cut-off score for remission and severity-ranges on the Psychotic Depression Assessment Scale (PDAS). J Affect. Disord. 2016, 190, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.S.; Flint, A.J.; Rothschild, A.J.; Mulsant, B.H.; Whyte, E.M.; Peasley-Miklus, C.; Papademetriou, E.; Leon, A.C.; Heo, M. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: The study of pharmacotherapy of psychotic depression (STOP-PD). Arch. Gen. Psychiatry 2009, 66, 838–847. [Google Scholar] [CrossRef]
- Leucht, S.; Fennema, H.; Engel, R.; Kaspers-Janssen, M.; Lepping, P.; Szegedi, A. What does the HAMD mean? J. Affect. Disord. 2013, 148, 243–248. [Google Scholar] [CrossRef]
- Ju, M.R.; Birkenhäger, T.K.; van den Broek, W.W. Does power matter with ECT? J. Affect. Disord. 2005, 89, 213–216. [Google Scholar] [CrossRef]
- Ottosson, J.O. Experimental studies of the mode of action of electroconvulsive therapy: Introduction. Acta. Psychiatr. Scand. Suppl. 1960, 35, 5–6. [Google Scholar]
- Quante, A.; Luborzewski, A.; Brakemeier, E.L.; Merkl, A.; Danker-Hopfe, H.; Bajbouj, M. Effects of 3 different stimulus intensities of ultrabrief stimuli in right unilateral electroconvulsive therapy in major depression: A randomized, double-blind pilot study. J. Psychiatr. Res. 2011, 45, 174–178. [Google Scholar] [CrossRef]
- Krystal, A.D.; Dean, M.D.; Weiner, R.D.; Tramontozzi, L.A., 3rd; Connor, K.M.; Lindahl, V.H.; Massie, R.W. ECT stimulus intensity: Are present ECT devices too limited? Am. J. Psychiatry 2000, 157, 963–967. [Google Scholar] [CrossRef]
- Peterchev, A.V.; Rosa, M.A.; Deng, Z.D.; Prudic, J.; Lisanby, S.H. Electroconvulsive therapy stimulus parameters: Rethinking dosage. J. ECT 2010, 26, 159–174. [Google Scholar] [CrossRef]
- Fridgeirsson, E.A.; Deng, Z.D.; Denys, D.; van Waarde, J.A.; van Wingen, G.A. Electric field strength induced by electroconvulsive therapy is associated with clinical outcome. Neuroimage Clin. 2021, 30, 102581. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Lee, E.; Cardoner, N.; Martínez-Zalacaín, I.; Pujol, J.; Makris, N.; Henry, M.; Via, E.; Hernández-Ribas, R.; Contreras-Rodríguez, O.; et al. Brain Volumetric Correlates of Right Unilateral Versus Bitemporal Electroconvulsive Therapy for Treatment-Resistant Depression. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Durand, D.M.; Jensen, A.; Bikson, M. Suppression of neural activity with high frequency stimulation. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), New York, NY, USA, 30 August–3 September 2006; pp. 1624–1625. [Google Scholar] [CrossRef]
- Kritzer, M.D.; Peterchev, A.V.; Camprodon, J.A. Electroconvulsive Therapy: Mechanisms of Action, Clinical Considerations, and Future Directions. Harv. Rev. Psychiatry 2023, 31, 101–113. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association; Task Force on Electroconvulsive Therapy. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging, 3rd ed.; American Psychiatric Association Publishing: Arlington, VA, USA, 2024. [Google Scholar]
- de Arriba-Arnau, A.; Soria, V.; Salvat-Pujol, N.; Menchón, J.M.; Urretavizcaya, M. Similar clinical improvement of depression using 0.5-ms and 1-ms pulse widths in bilateral electroconvulsive therapy. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 465–473. [Google Scholar] [CrossRef]
- Eser, D.; Nothdurfter, C.; Schüle, C.; Damm, J.; Steng, Y.; Möller, H.J.; Rupprecht, R.; Baghai, T. The influence of anaesthetic medication on safety, tolerability and clinical effectiveness of electroconvulsive therapy. World J. Biol. Psychiatry 2010, 11, 447–456. [Google Scholar] [CrossRef]
- Canbek, O.; Ipekcıoglu, D.; Menges, O.O.; Atagun, M.I.; Karamustafalıoglu, N.; Cetinkaya, O.Z.; Ilnem, M.C. Comparison of Propofol, Etomidate, and Thiopental in Anesthesia for Electroconvulsive Therapy: A Randomized, Double-blind Clinical Trial. J. ECT 2015, 31, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, C.; Kranaster, L.; Janke, C.; Sartorius, A. Impact of the anesthetic agents ketamine, etomidate, thiopental, and propofol on seizure parameters and seizure quality in electroconvulsive therapy: A retrospective study. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 255–261. [Google Scholar] [CrossRef]
- Singh, P.M.; Arora, S.; Borle, A.; Varma, P.; Trikha, A.; Goudra, B.G. Evaluation of Etomidate for Seizure Duration in Electroconvulsive Therapy: A Systematic Review and Meta-analysis. J. ECT 2015, 31, 213–225. [Google Scholar] [CrossRef]

| High-Charge Protocol [n = 53] | Dose Titration Protocol [n = 22] | p-Value | |
|---|---|---|---|
| Female [%; n] | 62.3% [n = 33] | 72.7% [n = 16] | p = 0.28 |
| Mean age in years, [mean ± SD] | 54 ± 15 | 41 ± 17 | p = 0.001 |
| Diagnosis of MDD * [%; n] | 50.9% [n = 27] | 50% [n = 11] | p = 0.57 |
| Index episode in weeks | 4.5 [2–11.8] | 8 [3–24] | p = 0.27 |
| Duration of disease in years [Median; IQR] | 15 [7–20] | 9 [3–18] | p = 0.04 |
| Psychotic features [%; n] | 26.4% [n = 14] | 45.5% [n = 10] | p = 0.09 |
| Comorbid substance use disorder [%; n] | 9.4% [n = 5] | 13.6% [n = 3] | p = 0.59 |
| Comorbid anxiety disorder [%; n] | 7.5% [n = 4] | 13.6% [n = 3] | p = 0.41 |
| Comorbid personality disorder [%; n] | 13.2% [n = 7] | 13.6% [n = 3] | p = 0.96 |
| History of previous ECT [%; n] | 15.1% [n = 8] | 13.6% [n = 3] | p = 0.87 |
| CGI-S—baseline [Median; IQR] | 5 [5–6] | 6 [5–6] | p = 0.003 |
| Groups | High-Charge Protocol (n = 53) | Dose Titration Protocol (n = 22) | p-Value | Adjusted p-Value |
|---|---|---|---|---|
| CGI-S—endpoint [Median; IQR] | 3 [2–3] | 2 [1.8–2.3] | p = 0.003 | p = 0.02 * |
| CGI-I [Median; IQR] | 2 [1–2] | 1 [1–2] | p = 0.007 | p = 0.004 * |
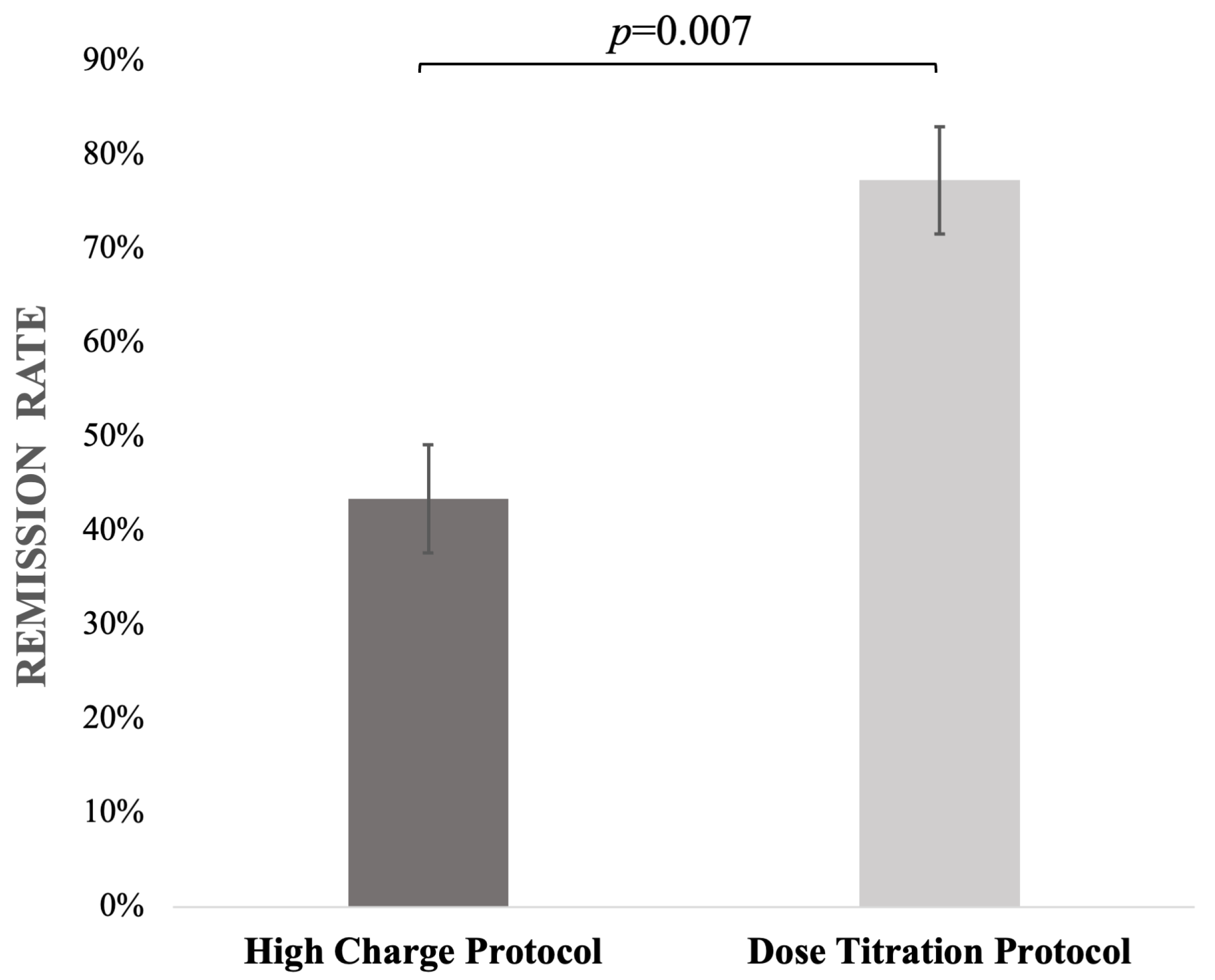
| Remission rate [%] | 43.4% (n = 23) | 77.3% (n = 17) | p = 0.007 | - |
| B | OR | 95% CI | p-Value | |
|---|---|---|---|---|
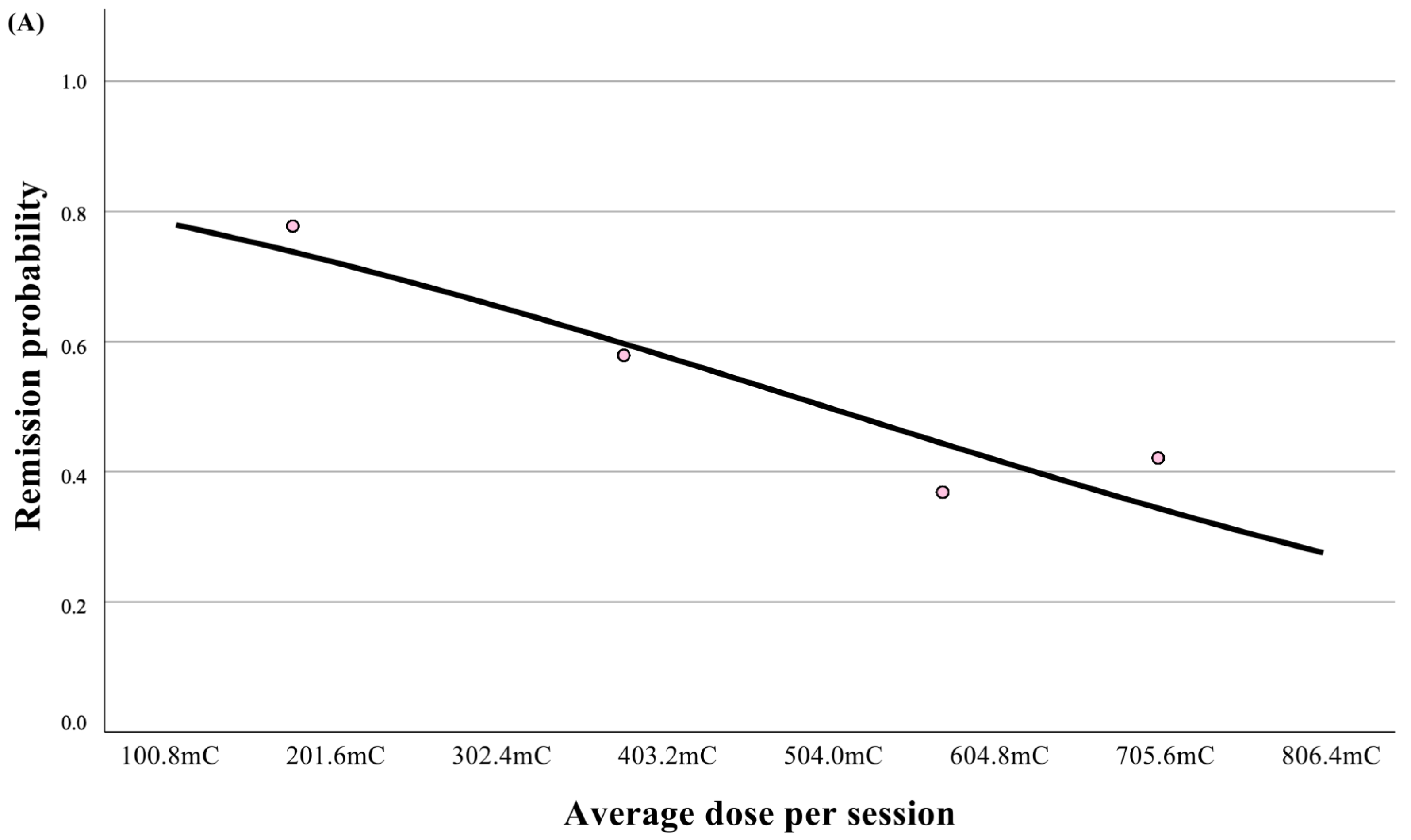
| Dose Titration Protocol | 1.76 | 5.81 | 1.48–22.86 | p = 0.01 |
| Comorbid Anxiety Disorder | −1.78 | 0.17 | 0.02–1.17 | p = 0.07 |
| High-Charge Protocol (n = 53) | Dose Titration Protocol (n = 22) | p-Value | Adjusted p-Value | |
|---|---|---|---|---|
| Total Charge over course (mC) [Median; IQR] | 5663.1 [3894.6–7492.7] | 2088.4 [1375.3–2914.7] | p < 0.001 | p = 0.005 * |
| Total number of sessions | 10.3 ± 2.3 | 11 ± 2.3 | p = 0.23 | p = 0.98 * |
| Average charge/session (mC) [mean ± SD] | 555.7 ± 147.1 | 208.5 ± 88.9 | p < 0.001 | p < 0.001 * |
| Total EEG seizure length (s) [Median; IQR] | 399 [300–480.5] | 675.5 [547.5–943.5] | p < 0.001 | p < 0.001 * |
| EEG seizure length/session (s) [Median; IQR] | 38.4 [33.8–47.1] | 68.6 [52.7–84.7] | p < 0.001 | p < 0.001 * |
| Total PSI (%) [Median; IQR] | 460.3 [255–523.1] | 457.6 [322.8–606.7] | p = 0.94 | p = 0.72 * |
| PSI/session (%) [Median; IQR] | 74.2 [65.8–82.0] | 63.6 [55.8–74.4] | p = 0.02 | p = 0.003 * |
| MIA (uV) [Median; IQR] | 1699.5 [1445.9–2200.5] | 2108.9 [1681.3–2495.5] | p = 0.02 | p = 0.98 * |
| MIA/session(uV) [Median; IQR] | 183 [158.1–216.4] | 208.8 [171.9–239.3] | p = 0.06 | p = 0.84 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jażdżyk, P.; Kuc, A.; Stachura, A.; Segiet-Święcicka, A.; Kosmalski, M.; Święcicki, Ł.; van Exel, E.; Pietras, T. Comparison of High-Charge Protocol vs. Dose Titration Protocol in Bilateral ECT: Evaluation of Antidepressant Effectiveness and EEG Parameters. J. Clin. Med. 2025, 14, 6490. https://doi.org/10.3390/jcm14186490
Jażdżyk P, Kuc A, Stachura A, Segiet-Święcicka A, Kosmalski M, Święcicki Ł, van Exel E, Pietras T. Comparison of High-Charge Protocol vs. Dose Titration Protocol in Bilateral ECT: Evaluation of Antidepressant Effectiveness and EEG Parameters. Journal of Clinical Medicine. 2025; 14(18):6490. https://doi.org/10.3390/jcm14186490
Chicago/Turabian StyleJażdżyk, Piotr, Agnieszka Kuc, Albert Stachura, Agnieszka Segiet-Święcicka, Marcin Kosmalski, Łukasz Święcicki, Eric van Exel, and Tadeusz Pietras. 2025. "Comparison of High-Charge Protocol vs. Dose Titration Protocol in Bilateral ECT: Evaluation of Antidepressant Effectiveness and EEG Parameters" Journal of Clinical Medicine 14, no. 18: 6490. https://doi.org/10.3390/jcm14186490
APA StyleJażdżyk, P., Kuc, A., Stachura, A., Segiet-Święcicka, A., Kosmalski, M., Święcicki, Ł., van Exel, E., & Pietras, T. (2025). Comparison of High-Charge Protocol vs. Dose Titration Protocol in Bilateral ECT: Evaluation of Antidepressant Effectiveness and EEG Parameters. Journal of Clinical Medicine, 14(18), 6490. https://doi.org/10.3390/jcm14186490







