Delay in Celiac Disease Diagnosis Among Patients with High-Risk Screening Conditions: Results from a United States Claims Database
Abstract
1. Introduction
2. Methods
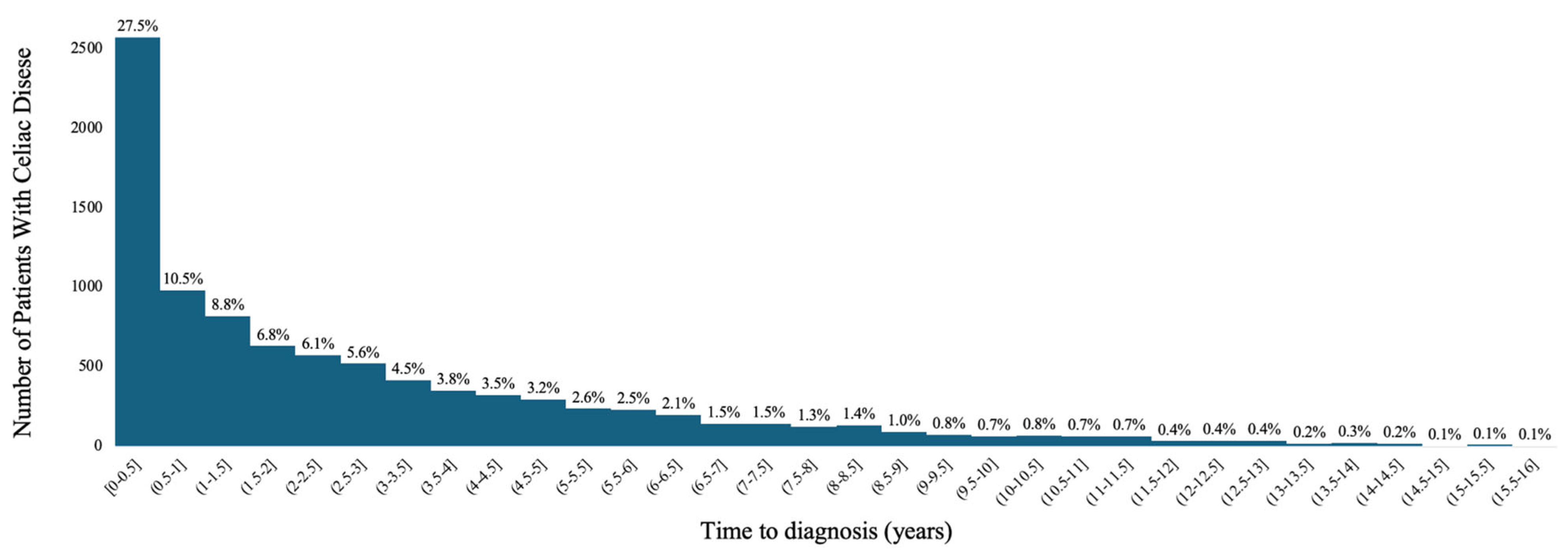
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CeD | Celiac Disease |
| IBS | Irritable Bowel Syndrome |
| IQR | Interquartile Range |
| TTG IgA | Tissue Transglutaminase Immunoglobulin A |
| SD | Standard Deviation |
| US | United States |
References
- Makharia, G.K.; Singh, P.; Catassi, C.; Sanders, D.S.; Leffler, D.; Ali, R.A.R.; Bai, J.C. The global burden of coeliac disease: Opportunities and challenges. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 313–327. [Google Scholar] [CrossRef]
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, Presentation, and Diagnosis of Celiac Disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; Debruyn, J.; Ronksley, P.E.; Shaheen, A.-A.; et al. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef]
- Cichewicz, A.B.; Mearns, E.S.; Taylor, A.; Boulanger, T.; Gerber, M.; Leffler, D.A.; Drahos, J.; Sanders, D.S.; Thomas Craig, K.J.; Lebwohl, B. Diagnosis and Treatment Patterns in Celiac Disease. Dig. Dis. Sci. 2019, 64, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Card, T.R.; Siffledeen, J.; West, J.; Fleming, K.M. An excess of prior irritable bowel syndrome diagnoses or treatments in Celiac disease: Evidence of diagnostic delay. Scand. J. Gastroenterol. 2013, 48, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, V.; Kurppa, K.; Huhtala, H.; Collin, P.; Maki, M.; Kaukinen, K. Factors associated with long diagnostic delay in celiac disease. Scand. J. Gastroenterol. 2014, 49, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.; Stavropoulos, S.N.; Panagi, S.G.; Goldstein, S.L.; McMahon, D.J.; Absan, H.; Neugut, A.I. Characteristics of Adult Celiac Disease in the USA: Results of a National Survey. Am. J. Gastroenterol. 2001, 96, 126. [Google Scholar] [CrossRef]
- Karhus, L.L.; Hansen, S.; Rumessen, J.J.; Linneberg, A. Diagnostic Delay in Coeliac Disease: A Survey among Danish Patients. Can. J. Gastroenterol. Hepatol. 2022, 2022, 5997624. [Google Scholar] [CrossRef]
- Lenti, M.V.; Aronico, N.; Bianchi, P.I.; D’AGate, C.C.; Neri, M.; Volta, U.; Mumolo, M.G.; Astegiano, M.; Calabrò, A.S.; Zingone, F.; et al. Diagnostic delay in adult coeliac disease: An Italian multicentre study. Dig. Liver Dis. 2023, 55, 743–750. [Google Scholar] [CrossRef]
- Mehta, S.; Agarwal, A.; Pachisia, A.V.; Singh, A.; Dang, S.; Vignesh, D.; Ahmed, A.; Chaudhari, B.R.; Prasad, S.; Goyal, R.M.; et al. Impact of delay in the diagnosis on the severity of celiac disease. J. Gastroenterol. Hepatol. 2024, 39, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Norstrom, F.; Lindholm, L.; Sandstrom, O.; Nordyke, K.; Ivarsson, A. Delay to celiac disease diagnosis and its implications for health-related quality of life. BMC Gastroenterol. 2011, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Paez, M.A.; Gramelspacher, A.M.; Sinacore, J.; Winterfield, L.; Venu, M. Delay in Diagnosis of Celiac Disease in Patients Without Gastrointestinal Complaints. Am. J. Med. 2017, 130, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Pulido, O.; Zarkadas, M.; Dubois, S.; MacIsaac, K.; Cantin, I.; La Vieille, S.; Godefroy, S.; Rashid, M. Clinical features and symptom recovery on a gluten-free diet in Canadian adults with celiac disease. Can. J. Gastroenterol. 2013, 27, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.S.; Hurlstone, D.P.; Stokes, R.O.; Rashid, F.; Milford-Ward, A.; Hadjivassiliou, M.; Lobo, A.J. Changing face of adult coeliac disease: Experience of a single university hospital in South Yorkshire. Postgrad. Med. J. 2002, 78, 31–33. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Vadasz, N.; Stotz, M.; Lehmann, R.; Studerus, D.; Greuter, T.; Frei, P.; Zeitz, J.; Scharl, M.; Misselwitz, B.; et al. Celiac disease diagnosis still significantly delayed–Doctor’s but not patients’ delay responsive for the increased total delay in women. Dig. Liver Dis. 2016, 48, 1148–1154. [Google Scholar] [CrossRef]
- Zipser, R.D.; Patel, S.; Yahya, K.Z.; Baisch, D.W.; Monarch, E. Presentations of adult celiac disease in a nationwide patient support group. Dig. Dis. Sci. 2003, 48, 761–764. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Greer, K.B.; Limketkai, B.N.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 59–76. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Hill, I.D.; Fasano, A.; Guandalini, S.; Hoffenberg, E.; Levy, J.; Reilly, N.; Verma, R. NASPGHAN Clinical Report on the Diagnosis and Treatment of Gluten-related Disorders. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 156–165. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W.; Herzstein, J.; Kemper, A.R.; et al. Screening for Celiac Disease: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 317, 1252–1257. [Google Scholar] [PubMed]
- National Institute for Health and Care Excellence. Coeliac Disease: Recognition, Assessment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2015. [Google Scholar]
- Hill, I.D.; Dirks, M.H.; Liptak, G.S.; Colletti, R.B.; Fasano, A.; Guandalini, S.; Hoffenberg, E.J.; Horvath, K.; Murray, J.A.; Pivor, M.; et al. Guideline for the Diagnosis and Treatment of Celiac Disease in Children: Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 1–19. [Google Scholar] [CrossRef]
- Pelkowski, T.D.; Viera, A.J. Celiac disease: Diagnosis and management. Am. Fam. Physician 2014, 89, 99–105. [Google Scholar]
- Zingone, F.; Bai, J.C.; Cellier, C.; Ludvigsson, J.F. Celiac Disease-Related Conditions: Who to Test? Gastroenterology 2024, 167, 64–78. [Google Scholar] [CrossRef]
- Adams, D.W.; Moleski, S.; Jossen, J.; Tye-Din, J.A. Clinical Presentation and Spectrum of Gluten Symptomatology in Celiac Disease. Gastroenterology 2024, 167, 51–63. [Google Scholar] [CrossRef]
- Jericho, H.; Sansotta, N.; Guandalini, S. Extraintestinal Manifestations of Celiac Disease: Effectiveness of the Gluten-Free Diet. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 75–79. [Google Scholar] [CrossRef]
- Dhar, J.; Samanta, J.; Sharma, M.; Kumar, S.; Sinha, S.K.; Kochhar, R. Impact of delay in diagnosis in patients with celiac disease: A study of 570 patients at a tertiary care center. Indian. J. Gastroenterol. 2022, 41, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Long, K.H.; Rubio-Tapia, A.; Wagie, A.E.; Iii, L.J.M.; Lahr, B.D.; Van Dyke, C.T.; Murray, J.A. The economics of coeliac disease: A population-based study. Aliment. Pharmacol. Ther. 2010, 32, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Optum. Clinformatics Data Mart, Version 8.1; OptumInsight: Eden Prairie, MN, USA, 2020. [Google Scholar]
- Bianchi, P.I.; Lenti, M.V.; Petrucci, C.; Gambini, G.; Aronico, N.; Varallo, M.; Rossi, C.M.; Pozzi, E.; Groppali, E.; Siccardo, F.; et al. Diagnostic Delay of Celiac Disease in Childhood. JAMA Netw. Open 2024, 7, e245671. [Google Scholar] [CrossRef]
- Ching, C.K.; Lebwohl, B. Celiac Disease in the Elderly. Curr. Treat. Options Gastroenterol. 2022, 20, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Rashtak, S.; Murray, J.A. Celiac disease in the elderly. Gastroenterol. Clin. N. Am. 2009, 38, 433–446. [Google Scholar] [CrossRef]
- Lebwohl, B.; Tennyson, C.A.; Holub, J.L.; Lieberman, D.A.; Neugut, A.I.; Green, P.H. Sex and racial disparities in duodenal biopsy to evaluate for celiac disease. Gastrointest. Endosc. 2012, 76, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Rampertab, S.D.; Pooran, N.; Brar, P.; Singh, P.; Green, P.H. Trends in the presentation of celiac disease. Am. J. Med. 2006, 119, 355.e9–355.e14. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.; Bernabeu, P.; Cameo, J.; Gutiérrez, A.; García, M.G.; Aguas, M.; Belén, O.; Zapater, P.; Jover, R.; Hofstadt, C.V.-D.; et al. Gender Biases and Diagnostic Delay in Inflammatory Bowel Disease: Multicenter Observational Study. Inflamm. Bowel Dis. 2023, 29, 1886–1894. [Google Scholar] [CrossRef]
- eClinicalMedicine. Gendered pain: A call for recognition and health equity. EClinicalMedicine 2024, 69, 102558. [Google Scholar] [CrossRef]
- Zylberberg, H.M.; Miller, E.B.; Ratner, A.; Hammill, B.G.; Mehta, P.; Alesci, S.; Lebwohl, B. Correlations Between Relative Prevalence of Celiac Disease and Sociodemographic Variables in the United States. Am. J. Gastroenterol. 2023, 119, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.; Lopez, M.H. Hispanic Americans’ Trust In and Engagement With Science. Pew Research Center. 14 June 2022. Available online: https://www.pewresearch.org/science/2022/06/14/hispanic-americans-experiences-with-health-care/ (accessed on 16 July 2025).
- The Celiac Society Celiac Disease Unit Recognition Program (CDURP). The Celiac Society. Available online: https://theceliacsociety.org/membership/celiac-disease-unit-recognition-program-cdrup/ (accessed on 3 July 2025).
| Variable | N (%) | Mean Time in Months (Standard Deviation) | p Value |
|---|---|---|---|
| Gender: | <0.0001 | ||
| Female | 6771 (72.46) | 34.74 (37.08) | |
| Male | 2571 (27.51) | 31.08 (36.08) | |
| Age: | <0.0001 | ||
| 1–20 years | 1488 (15.92) | 24.11 (31.39) | |
| 21–60 years | 5225 (55.92) | 32.49 (36.70) | |
| 61–100 years | 2630 (28.15) | 41.65 (38.35) | |
| Region: | <0.0001 | ||
| Midwest | 2138 (22.88) | 31.65 (36.10) | |
| Northeast | 1832 (19.61) | 30.59 (33.21) | |
| South | 3337 (35.71) | 36.35 (38.81) | |
| West | 2013 (21.54) | 34.44 (36.94) | |
| Unknown | 24 (0.26) | 37.32 (47.75) | |
| Race: | <0.0001 | ||
| Asian, non-Hispanic | 185 (1.98) | 32.41 (35.48) | |
| Black, non-Hispanic | 430 (4.60) | 36.30 (38.22) | |
| Hispanic | 685 (7.33) | 38.45 (37.79) | |
| White, non-Hispanic | 7447 (79.70) | 34.41 (37.13) | |
| Unknown | 597 (6.39) | 18.52 (26.56) |
| High Risk Screening Condition | Presence | Absence | p Value | ||
|---|---|---|---|---|---|
| N (%) | Mean (SD) | N (%) | Mean (SD) | ||
| Abdominal pain (recurrent) | 7018 (75.11) | 36.20 (37.89) | 2326 (24.89) | 26.30 (32.36) | <0.0001 |
| Ataxia | 1007 (10.78) | 49.13 (40.58) | 8337 (89.22) | 31.88 (35.92) | <0.0001 |
| Autoimmune thyroid disease | 868 (9.29) | 44.43 (39.43) | 8476 (90.71) | 32.65 (36.39) | <0.0001 |
| Constipation (chronic, unexplained) | 376 (4.02) | 42.09 (39.11) | 8968 (95.98) | 33.39 (36.70) | <0.0001 |
| Dental enamel defects | 0 (0.00) | - | 9344 (100.00) | - | - |
| Dermatitis herpetiformis | 238 (2.55) | 30.78 (33.17) | 9106 (97.45) | 33.82 (36.93) | 0.2089 |
| Diarrhea (chronic) | 5833 (62.43) | 35.72 (37.32) | 3511 (37.57) | 30.46 (35.80) | <0.0001 |
| Down syndrome | 46 (0.49) | 38.55 (41.03) | 9298 (99.51) | 33.72 (36.82) | 0.3751 |
| Failure to thrive | 233 (2.49) | 20.69 (28.68) | 9111 (97.51) | 34.07 (36.97) | <0.0001 |
| Family history | 471 (5.04) | 30.96 (38.14) | 8873 (94.96) | 33.89 (36.77) | 0.0926 |
| Hypertransaminasemia (cryptogenic) | 183 (1.96) | 36.67 (39.59) | 9161 (98.04) | 33.68 (36.78) | 0.2770 |
| Intestinal malabsorption (unspecified) | 859 (9.19) | 35.59 (37.47) | 8485 (90.81) | 33.55 (36.77) | 0.1220 |
| Iron deficiency anemia | 2568 (27.48) | 39.75 (38.23) | 6776 (72.52) | 31.46 (36.04) | <0.0001 |
| Irritable bowel syndrome | 2662 (28.49) | 40.42 (38.03) | 6682 (71.51) | 31.08 (36.02) | <0.0001 |
| Oral aphthous ulcers (severe or persistent) | 249 (3.84) | 48.79 (41.90) | 9095 (97.34) | 33.33 (36.61) | <0.0001 |
| Osteomalacia or premature osteoporosis | 359 (1.56) | 42.46 (37.38) | 8985 (96.16) | 33.39 (36.78) | <0.0001 |
| Peripheral neuropathy | 146 (2.75) | 46.57 (41.18) | 9198 (98.44) | 33.54 (36.73) | <0.0001 |
| Short stature | 257 (0.21) | 30.06 (37.86) | 9087 (97.25) | 33.84 (36.81) | 0.1048 |
| Turner syndrome | 20 (6.88) | 37.34 (33.02) | 9324 (99.79) | 33.73 (36.85) | 0.6616 |
| Type 1 diabetes | 643 (6.88) | 43.25 (37.22) | 8701 (93.12) | 33.04 (36.72) | <0.0001 |
| Weight loss (unexplained) | 2357 (25.22) | 37.58 (38.32) | 6987 (74.78) | 32.45 (36.24) | <0.0001 |
| Williams syndrome | 0 (0.0) | - | 9344 (100.00) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zylberberg, H.M.; Miller, E.B.P.; Reidy, D.; Avery, K.; Newberry, C.; Ratner, A.; Silberg, D.G. Delay in Celiac Disease Diagnosis Among Patients with High-Risk Screening Conditions: Results from a United States Claims Database. J. Clin. Med. 2025, 14, 6471. https://doi.org/10.3390/jcm14186471
Zylberberg HM, Miller EBP, Reidy D, Avery K, Newberry C, Ratner A, Silberg DG. Delay in Celiac Disease Diagnosis Among Patients with High-Risk Screening Conditions: Results from a United States Claims Database. Journal of Clinical Medicine. 2025; 14(18):6471. https://doi.org/10.3390/jcm14186471
Chicago/Turabian StyleZylberberg, Haley M., Erin B. P. Miller, Deirdre Reidy, Kate Avery, Carolyn Newberry, Amy Ratner, and Debra G. Silberg. 2025. "Delay in Celiac Disease Diagnosis Among Patients with High-Risk Screening Conditions: Results from a United States Claims Database" Journal of Clinical Medicine 14, no. 18: 6471. https://doi.org/10.3390/jcm14186471
APA StyleZylberberg, H. M., Miller, E. B. P., Reidy, D., Avery, K., Newberry, C., Ratner, A., & Silberg, D. G. (2025). Delay in Celiac Disease Diagnosis Among Patients with High-Risk Screening Conditions: Results from a United States Claims Database. Journal of Clinical Medicine, 14(18), 6471. https://doi.org/10.3390/jcm14186471







