The Impact of Cerebral Small Vessel Disease on Functional Recovery After Intracerebral Hemorrhage: Stratified Analysis by Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Neuroimaging Classification of ICH and cSVD
2.2. Functional Evaluation
2.3. Statistical Analysis
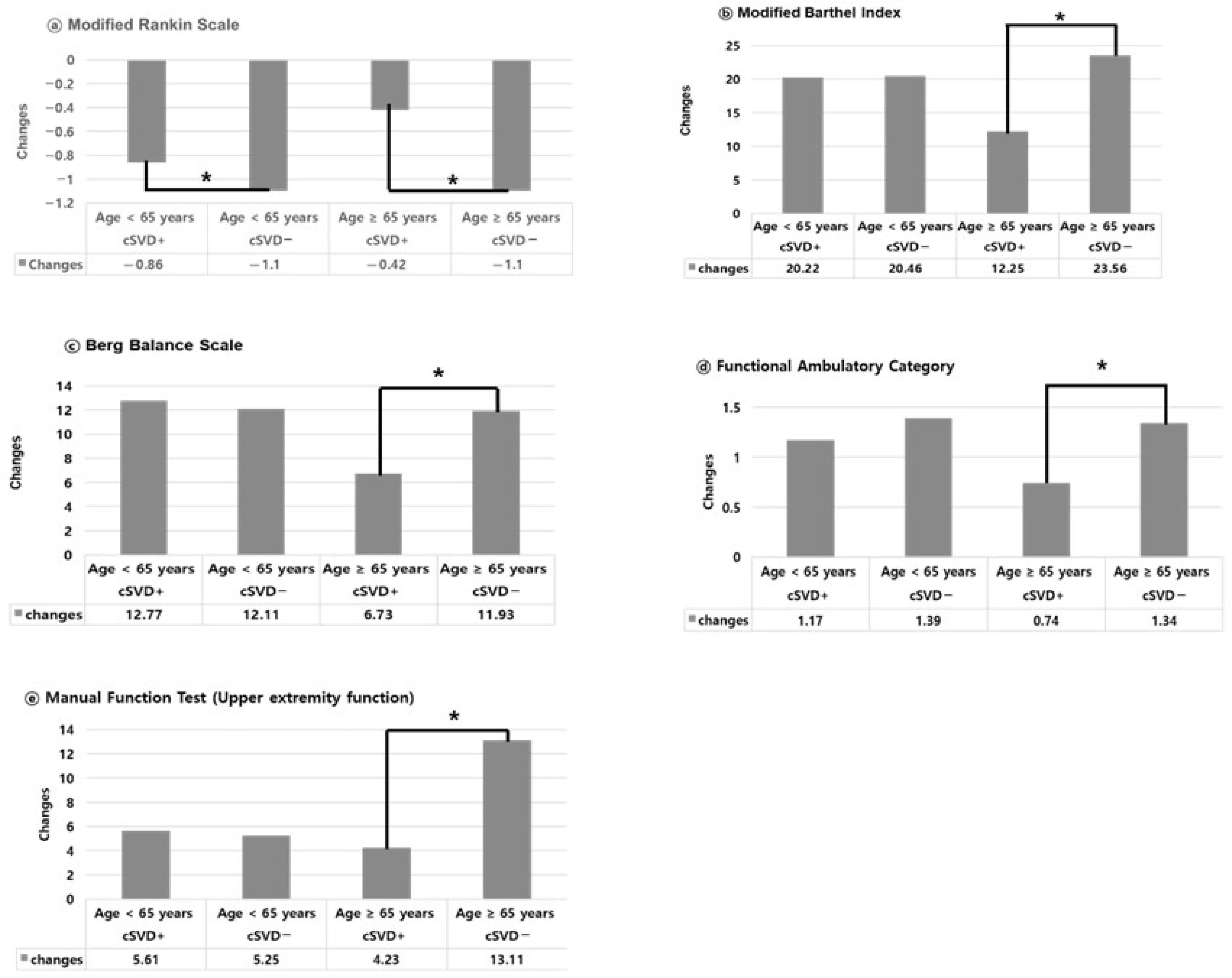
3. Results
4. Discussion
4.1. Distinct Contributions Compared to Previous Studies
4.2. Age-Dependent Impact of cSVD on Functional Recovery After ICH
4.3. Considerations for the Elderly Without cSVD Subgroup
4.4. Functional Impact of cSVD in Younger Adults
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADL | activities of daily living |
| BBS | Berg balance scale |
| cSVD | cerebral small vessel disease |
| FAC | functional ambulatory category |
| MBI | modified Barthel index |
| MFT | manual function test |
| mRS | modified Rankin scale |
| ICH | intracerebral hemorrhage |
| WMHs | white matter hyperintensities |
References
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C., 3rd; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: A Guideline from the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef]
- Duering, M.; Biessels, G.J.; Brodtmann, A.; Chen, C.; Cordonnier, C.; de Leeuw, F.E.; Debette, S.; Frayne, R.; Jouvent, E.; Rost, N.S.; et al. Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol. 2023, 22, 602–618. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Shan, Y.; Cai, W.; Liu, S.; Hu, M.; Liao, S.; Huang, X.; Zhang, B.; Wang, Y.; et al. Cerebral small vessel disease: Neuroimaging markers and clinical implication. J. Neurol. 2019, 266, 2347–2362. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Frosch, M.P.; Baron, J.C.; Pasi, M.; Albucher, J.F.; Banerjee, G.; Barbato, C.; Bonneville, F.; Brandner, S.; et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022, 21, 714–725. [Google Scholar] [CrossRef]
- Gao, Y.; Zong, C.; Liu, H.; Zhang, K.; Yang, H.; Wang, Y.; Li, Y.; Song, B.; Xu, Y. Clinical features and associated factors of coexisting intracerebral hemorrhage in patients with cerebral small vessel disease: A cross-sectional study. Sci. Rep. 2024, 14, 5596. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef]
- Gurol, M.E.; Biessels, G.J.; Polimeni, J.R. Advanced Neuroimaging to Unravel Mechanisms of Cerebral Small Vessel Diseases. Stroke 2020, 51, 29–37. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef]
- Hakim, A.M. Small Vessel Disease. Front. Neurol. 2019, 10, 1020. [Google Scholar] [CrossRef]
- Uniken Venema, S.M.; Marini, S.; Lena, U.K.; Morotti, A.; Jessel, M.; Moomaw, C.J.; Kourkoulis, C.; Testai, F.D.; Kittner, S.J.; Brouwers, H.B.; et al. Impact of Cerebral Small Vessel Disease on Functional Recovery After Intracerebral Hemorrhage. Stroke 2019, 50, 2722–2728. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, W.; Zhan, Z.; Xia, L.; Han, Z. Cerebral small vessel disease and prognosis in intracerebral haemorrhage: A systematic review and meta-analysis of cohort studies. Eur. J. Neurol. 2022, 29, 2511–2525. [Google Scholar] [CrossRef]
- Best, J.G.; Jesuthasan, A.; Werring, D.J. Cerebral small vessel disease and intracranial bleeding risk: Prognostic and practical significance. Int. J. Stroke 2023, 18, 44–52. [Google Scholar] [CrossRef]
- Markus, H.S.; de Leeuw, F.E. Cerebral small vessel disease: Recent advances and future directions. Int. J. Stroke 2023, 18, 4–14. [Google Scholar] [CrossRef]
- Cliteur, M.P.; Sondag, L.; Wolsink, A.; Rasing, I.; Meijer, F.J.A.; Jolink, W.M.T.; Wermer, M.J.H.; Klijn, C.J.M.; Schreuder, F. Cerebral small vessel disease and perihematomal edema formation in spontaneous intracerebral hemorrhage. Front. Neurol. 2022, 13, 949133. [Google Scholar] [CrossRef]
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Tuhrim, S.; Broderick, J.P.; Batjer, H.H.; Hondo, H.; Hanley, D.F. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001, 344, 1450–1460. [Google Scholar] [CrossRef]
- Hemphill, J.C., 3rd; Bonovich, D.C.; Besmertis, L.; Manley, G.T.; Johnston, S.C. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001, 32, 891–897. [Google Scholar] [CrossRef]
- Staals, J.; Makin, S.D.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef]
- Newcommon, N.J.; Green, T.L.; Haley, E.; Cooke, T.; Hill, M.D. Improving the assessment of outcomes in stroke: Use of a structured interview to assign grades on the modified Rankin Scale. Stroke 2003, 34, 377–378. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83 (Suppl. S2), S7–S11. [Google Scholar]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kondo, T.; Suzukamo, Y.; Michimata, A.; Izumi, S. Reliability and validity of the Manual Function Test in patients with stroke. Am. J. Phys. Med. Rehabil. 2009, 88, 247–255. [Google Scholar] [CrossRef]
- Vikner, T.; Karalija, N.; Eklund, A.; Malm, J.; Lundquist, A.; Gallewicz, N.; Dahlin, M.; Lindenberger, U.; Riklund, K.; Bäckman, L.; et al. 5-Year Associations Among Cerebral Arterial Pulsatility, Perivascular Space Dilation, and White Matter Lesions. Ann. Neurol. 2022, 92, 871–881. [Google Scholar] [CrossRef]
- Schulz, M.; Malherbe, C.; Cheng, B.; Thomalla, G.; Schlemm, E. Functional connectivity changes in cerebral small vessel disease—A systematic review of the resting-state MRI literature. BMC Med. 2021, 19, 103. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Sato, T.; Nito, C.; Nishiyama, Y.; Suda, S.; Matsumoto, N.; Aoki, J.; Saito, T.; Suzuki, K.; Katano, T.; et al. The Effect of Aging and Small-Vessel Disease Burden on Hematoma Location in Patients with Acute Intracerebral Hemorrhage. Cerebrovasc. Dis. 2021, 50, 526–534. [Google Scholar] [CrossRef]
- Uniken Venema, S.M.; Marini, S.; Brouwers, H.B.; Morotti, A.; Woo, D.; Anderson, C.D.; Rosand, J. Associations of Radiographic Cerebral Small Vessel Disease with Acute Intracerebral Hemorrhage Volume, Hematoma Expansion, and Intraventricular Hemorrhage. Neurocrit. Care 2020, 32, 383–391. [Google Scholar] [CrossRef]
- Cannistraro, R.J.; Badi, M.; Eidelman, B.H.; Dickson, D.W.; Middlebrooks, E.H.; Meschia, J.F. CNS small vessel disease: A clinical review. Neurology 2019, 92, 1146–1156. [Google Scholar] [CrossRef]
- Dupré, N.; Drieu, A.; Joutel, A. Pathophysiology of cerebral small vessel disease: A journey through recent discoveries. J. Clin. Investig. 2024, 134, e172841. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D.; Lin, J.; Thomas, A.M.; Miao, J.; Chen, D.; Li, S.; Chu, C. Cerebral small vessel disease: Pathological mechanisms and potential therapeutic targets. Front. Aging Neurosci. 2022, 14, 961661. [Google Scholar] [CrossRef]
- Das, A.S.; Regenhardt, R.W.; Vernooij, M.W.; Blacker, D.; Charidimou, A.; Viswanathan, A. Asymptomatic Cerebral Small Vessel Disease: Insights from Population-Based Studies. J. Stroke 2019, 21, 121–138. [Google Scholar] [CrossRef]
- Rodriguez, L.; Araujo, A.T.; Vera, D.D.; Rodríguez Gelvez, A.; Camacho, P.A.; Mantilla, D.E.; Mantilla, J.C. Prevalence and imaging characteristics of cerebral small vessel disease in a Colombian population aged 40 years and older. Brain Commun. 2024, 6, fcae057. [Google Scholar] [CrossRef]
- Watson, N.; Bonsack, F.; Sukumari-Ramesh, S. Intracerebral Hemorrhage: The Effects of Aging on Brain Injury. Front. Aging Neurosci. 2022, 14, 859067. [Google Scholar] [CrossRef]
- Jang, J.S.; Park, Y.S. Contributing factors of spontaneous intracerebral hemorrhage development in young adults. J. Cerebrovasc. Endovasc. Neurosurg. 2024, 26, 274–283. [Google Scholar] [CrossRef]
- Periole, C.; Blanc, C.; Calvière, L.; Fontaine, L.; Viguier, A.; Albucher, J.F.; Chollet, F.; Bonneville, F.; Olivot, J.M.; Raposo, N. Prevalence and characterization of cerebral small vessel disease in young adults with intracerebral hemorrhage. Int. J. Stroke 2023, 18, 102–108. [Google Scholar] [CrossRef]


| Age < 65 Years (n = 161) | Age ≥ 65 Years (n = 195) | |||||
|---|---|---|---|---|---|---|
| cSVD+ (n = 64) | cSVD− (n = 97) | p-Value | cSVD+ (n = 179) | cSVD− (n = 16) | p-Value | |
| Age (year) | 54.67 ± 8.44 | 48.68 ± 10.82 | <0.001 | 78.90 ± 7.59 | 67.1 ± 3.9 | <0.001 |
| Male, n (%) | 45 (70.31) | 56 (57.73) | 0.03 | 104 (58.10) | 7 (43.75) | 0.051 |
| Hypertension, n (%) | 57 (89.06) | 61 (62.89) | 0.022 | 173 (96.65) | 8 (50.0) | <0.001 |
| Diabetes, n (%) | 31 (48.44) | 16 (16.49) | <0.001 | 105 (58.66) | 4 (25.0) | 0.342 |
| Hyperlipidemia, n (%) | 32 (50.0) | 27 (27.84) | 0.077 | 114 (63.69) | 5 (31.25) | 0.353 |
| Atrial fibrillation, n (%) | 3 (4.69) | 2 (2.06) | 0.744 | 35 (19.55) | 1 (6.25) | 0.564 |
| Heart failure, n (%) | 6 (9.38) | 4 (4.12) | 0.541 | 43 (24.02) | 0 (0.0) | 0.569 |
| Chronic kidney disease, n (%) | 4 (6.25) | 0 (0.0) | 0.020 | 21 (11.73) | 2 (12.5) | <0.001 |
| Antiplatelet use, n (%) | 11 (17.19) | 7 (7.22) | 0.807 | 89 (49.72) | 4 (25.0) | 0.049 |
| Anticoagulant use, n (%) | 7 (10.94) | 4 (4.12) | 0.122 | 27 (15.08) | 2 (12.5) | 0.553 |
| Alcohol use, n (%) | 51 (79.69) | 59 (60.82) | 0.067 | 61 (34.08) | 5 (31.25) | 0.014 |
| Smoker, n (%) | 35 (54.69) | 43 (44.33) | 0.037 | 41 (22.91) | 4 (25.0) | 0.006 |
| History of cognitive impairment, n (%) | 4 (6.25) | 0 (0.0) | <0.001 | 62 (34.64) | 2 (12.5) | <0.001 |
| ICH score (0–4) | 1.84 ± 1.50 | 1.54 ± 1.38 | 0.041 | 2.20 ± 1.31 | 1.5 ± 1.35 | 0.007 |
| SVD score (0–6) | 1.73 ± 0.67 | NA | NA | 2.18 ± 0.90 | NA | NA |
| Good prognosis (mRS 0–2) 3 month after ICH, n (%) | 33 (51.56) | 69 (71.13) | 0.017 | 56 (31.28) | 9 (56.25) | 0.712 |
| Poor prognosis (mRS 3–5) 3 month after ICH, n (%) | 31 (48.44) | 28 (28.87) | 0.035 | 123 (68.72) | 7 (43.75) | 0.042 |
| Mortality (mRS 6) within 3 months after ICH, n (%) | 6 (9.38) | 0 | NA | 35 | 0 | NA |
| Brain MRI performed, n (%) | 64 (100) | 70 (72.16) | NA | 109 (60.89) | 9 (56.25) | NA |
| Age < 65 Years (n = 161) | Age ≥ 65 Years (n = 195) | |||||
|---|---|---|---|---|---|---|
| cSVD+ (n = 64) | cSVD− (n = 97) | p-Value | cSVD+ (n = 179) | cSVD− (n = 16) | p-Value | |
| Age (year) | 54.67 ± 8.44 | 48.68 ± 10.82 | <0.001 | 78.90 ± 7.59 | 67.1 ± 3.9 | <0.001 |
| Male, n (%) | 45 (70.31) | 56 (57.73) | 0.03 | 104 (58.10) | 7 (43.75) | 0.051 |
| ICH score (0–4) | 1.84 ± 1.50 | 1.54 ± 1.38 | 0.041 | 2.20 ± 1.31 | 1.5 ± 1.35 | 0.007 |
| ICH etiology | ||||||
| Hypertension, n (%) | 56 (87.5) | 72 (74.23) | 0.02 | 116 (64.8) | 9 (56.25) | 0.078 |
| Cerebral amyloid angiopathy, n (%) | 1 (1.56) | 1 (1.03) | 0.872 | 49 (27.37) | 4 (25.0) | <0.001 |
| Arteriovenous malformation, n (%) | 0 (0.0) | 11 (11.34) | 0.516 | 0 (0.0) | 0 (0.0) | 0.947 |
| Moyamoya disease, n (%) | 6 (9.36) | 4 (4.12) | 0.045 | 3 (1.68) | 0 (0.0) | 0.01 |
| Tumor-related hemorrhage, n (%) | 1 (1.56) | 4 (4.12) | 0.217 | 6 (3.35) | 1 (6.25) | 0.263 |
| Unknown cause, n (%) | 0 (0.0%) | 5 (5.15) | 0.889 | 5 (2.79) | 2 (12.5) | 0.747 |
| ICH location | ||||||
| Basal ganglia, n (%) | 26 (46.63) | 46 (47.42) | 0.641 | 49 9 (27.37) | 4 (25.0) | 0.562 |
| Thalamus, n (%) | 14 (21.88) | 10 (10.31) | 0.032 | 38 (21.23) | 2 (12.5) | 0.153 |
| Cerebral lobe, n (%) | 11 (17.19) | 23 (23.71) | 0.525 | 64 (35.75) | 5 (31.25) | 0.082 |
| Pons and brainstem, n (%) | 9 (14.06) | 8 (8.25) | 0.094 | 12 (6.70) | 0 (0.0) | <0.001 |
| Cerebellum, n (%) | 4 (6.25) | 10 | <0.001 | 16 (8.94) | 5 (31.25) | 0.074 |
| cSVD classification | ||||||
| Presence of white matter hyperintensity, n (%) | 49 (76.56) | NA | 152 (84.92) | NA | ||
| Presence of lacunes, n (%) | 23 (35.94) | 91 (50.84) | ||||
| Presence of microbleeds, n (%) | 33 (51.56) | 76 (42.46) | ||||
| Presence of enlarged perivascular spaces, n (%) | 1 (1.56) | 19 (10.61) | ||||
| SVD score (0–6) | 1.73 ± 0.67 | 2.18 ± 0.90 | ||||
| Age < 65 Years (n = 161) | Age ≥ 65 Years (n = 195) | |||||
|---|---|---|---|---|---|---|
| Function | cSVD+ (n = 64) | cSVD− (n = 97) | p-Value | cSVD+ (n = 179) | cSVD− (n = 16) | p-Value |
| Initial Evaluation | ||||||
| mRS | 3.78 ± 1.20 | 3.41 ± 0.92 | 0.018 | 4.13 ± 0.92 | 3.8 ± 0.92 | <0.001 |
| MBI (daily activity) | 26.98 ± 27.33 | 20.5 ± 24.27 | 0.006 | 16.25 ± 20.60 | 20.5 ± 24.27 | <0.001 |
| BBS (balance and gait) | 14.17 ± 18.65 | 10.7 ± 15.37 | 0.032 | 7.07 ± 12.84 | 10.7 ± 15.37 | <0.001 |
| FAC (gait) | 1.20 ± 1.62 | 0.6 ± 0.97 | 0.187 | 0.56 ± 1.01 | 0.6 ± 0.97 | 0.754 |
| MFT (hand function) | 13.25 ± 10.92 | 9.4 ± 9.71 | 0.019 | 10.41 ± 9.40 | 9.4 ± 9.71 | 0.912 |
| Swallowing function (non-oral diet/limited diet/normal diet) | 16/13/35 (25.0/20.31/ 54.69%) | 2/35/60 (2.06/36.08/ 61.86%) | 0.415 | 117/43/19 (65.36/24.02/10.61%) | 2/9/5 (12.5/56.25/ 31.25%) | 0.076 |
| Follow-Up Evaluation at three months after ICH | ||||||
| mRS | 2.92 ± 1.75 | 2.13 ± 1.60 | 0.013 | 3.71 ± 1.62 | 2.8 ± 0.82 | <0.001 |
| MBI (daily activity) | 47.20 ± 34.98 | 60.62 ± 33.29 | 0.019 | 28.5 ± 28.67 | 44.21 ± 29.42 | <0.001 |
| BBS (balance and gait) | 26.94 ± 20.95 | 33.33 ± 21.53 | 0.072 | 13.80 ± 17.53 | 22.62 ± 20.19 | <0.001 |
| FAC (gait) | 2.38 ± 1.84 | 2.95 ± 1.70 | 0.05 | 1.30 ± 1.43 | 1.92 ± 1.20 | <0.001 |
| MFT (hand function) | 18.86 ± 12.51 | 22.82 ± 11.07 | 0.043 | 14.64 ± 11.34 | 22.51 ± 10.22 | <0.001 |
| Swallowing function (non-oral diet/limited diet/normal diet) | 8/11/45 (12.5/17.19/ 70.31%) | 0/8/89 (0.0/8.25/91.75%) | 0.002 | 98/50/31 (54.75/27.93/17.32%) | 0/5/11 (0.0/31.25/68.75%) | 0.021 |
| Hospital admission status at 3 months after ICH, n (%) | 12 (18.75%) | 3 (3.09%) | 0.179 | 138 (77.19%) | 8 (50.0%) | 0.523 |
| Age < 65 Years (n = 161) | Age ≥ 65 Years (n = 195) | |||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio | 95% CI | p-Value | OR | 95% CI | p-Value |
| cSVD present | 3.82 | 1.23–8.76 | 0.004 | 7.44 | 2.40–15.35 | <0.001 |
| ICH score | 3.95 | 2.51–6.21 | <0.001 | 3.29 | 2.36–4.89 | <0.001 |
| Age (per year) | 1.04 | 1.01–1.09 | 0.02 | 0.96 | 0.91–1.01 | 0.117 |
| Chronic kidney disease | 4.21 | 1.35–7.61 | 0.02 | 5.48 | 1.43–13.72 | 0.03 |
| Hypertension | 2.95 | 1.05–4.26 | 0.03 | 2.17 | 1.74–3.49 | <0.001 |
| Diabetes | 3.16 | 1.12–6.94 | 0.02 | 1.96 | 0.97–10.91 | 0.152 |
| Heart failure | 0.74 | 0.61–4.96 | 0.527 | 1.35 | 0.82–5.34 | 0.415 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Kim, H.; Lee, S.J. The Impact of Cerebral Small Vessel Disease on Functional Recovery After Intracerebral Hemorrhage: Stratified Analysis by Age. J. Clin. Med. 2025, 14, 6450. https://doi.org/10.3390/jcm14186450
Lee H-J, Kim H, Lee SJ. The Impact of Cerebral Small Vessel Disease on Functional Recovery After Intracerebral Hemorrhage: Stratified Analysis by Age. Journal of Clinical Medicine. 2025; 14(18):6450. https://doi.org/10.3390/jcm14186450
Chicago/Turabian StyleLee, Hong-Jae, Haney Kim, and Sook Joung Lee. 2025. "The Impact of Cerebral Small Vessel Disease on Functional Recovery After Intracerebral Hemorrhage: Stratified Analysis by Age" Journal of Clinical Medicine 14, no. 18: 6450. https://doi.org/10.3390/jcm14186450
APA StyleLee, H.-J., Kim, H., & Lee, S. J. (2025). The Impact of Cerebral Small Vessel Disease on Functional Recovery After Intracerebral Hemorrhage: Stratified Analysis by Age. Journal of Clinical Medicine, 14(18), 6450. https://doi.org/10.3390/jcm14186450






