Evaluation of the Percentage of Monocyte Subpopulations with TLR2 and TLR4 Expression About Selected Skin Functional Parameters in Patients with Acne Vulgaris—Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Criteria for Inclusion and Exclusion from the Study Group
2.2. Assessment of Skin Function Parameters
2.3. Isolation of Peripheral Blood Mononuclear Cells (PBMC)
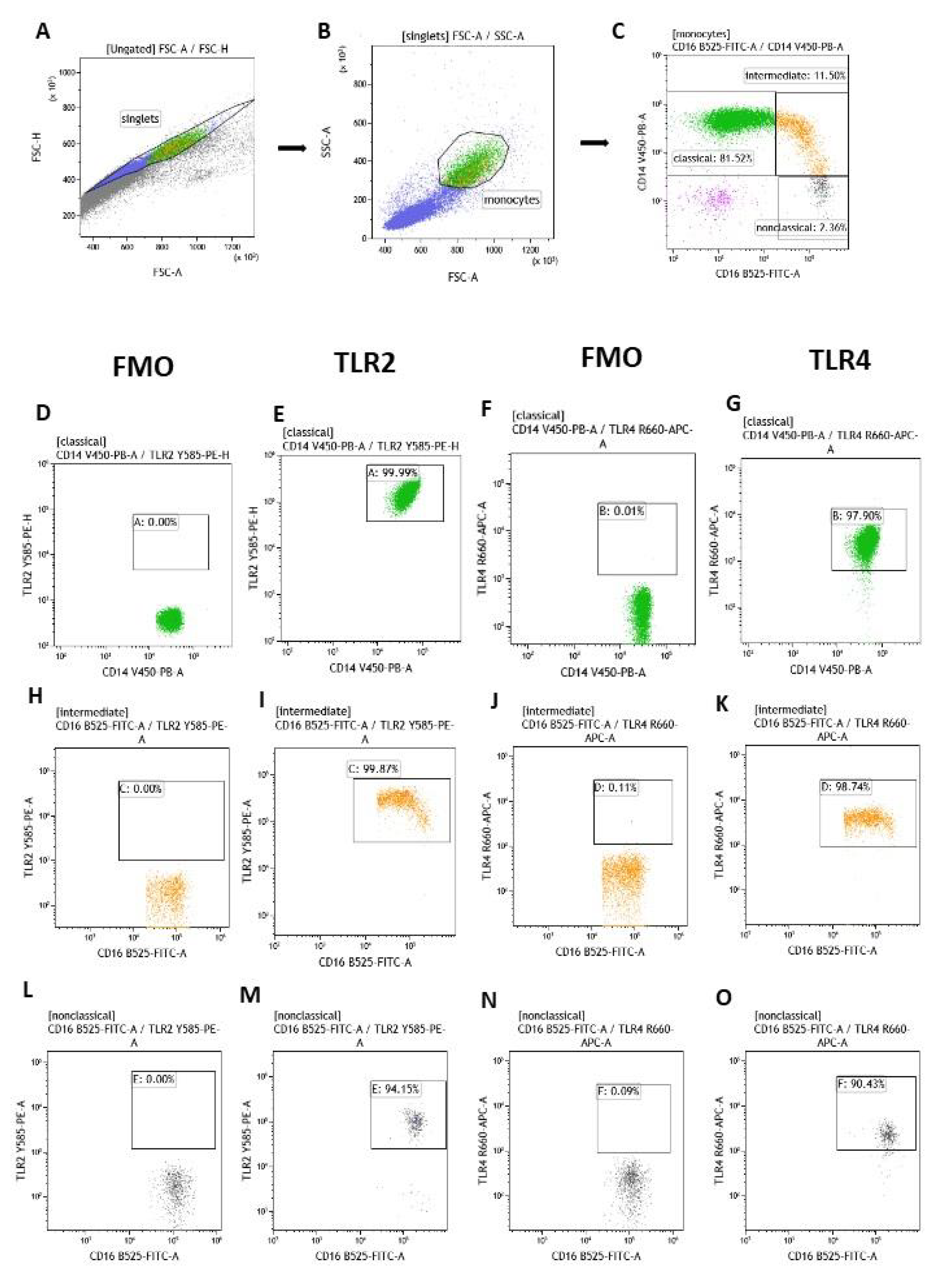
2.4. Flow Cytometry of Classical, Intermediate and Non-Classical Monocytes with Expression of TLR2 and TLR4
2.5. Statistical Methods
3. Results
3.1. Characteristics of the Study Group
3.2. Comparison of Skin Functional Parameters and Percentages of Classical, Intermediate and Non-Classical Monocytes with TLR2 and TLR4 Expression
3.3. Correlations Between the Percentage of Individual Monocyte Subpopulations with TLR2 and TLR4 Expression and Functional Skin Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burma, N.E.; Woo, T.E.; Parsons, L. Topical Clascoterone for Acne Vulgaris. Skin Ther. Lett. 2022, 27, 1–3. [Google Scholar]
- Rao, A.; Douglas, S.C.; Hall, J.M. Endocrine Disrupting Chemicals, Hormone Receptors, and Acne Vulgaris: A Connecting Hypothesis. Cells 2021, 10, 1439. [Google Scholar] [CrossRef]
- Chilicka, K.; Rogowska, A.M.; Szyguła, R.; Dzieńdziora-Urbińska, I.; Taradaj, J. A comparison of the effectiveness of azelaic and pyruvic acid peels in the treatment of female adult acne: A randomized controlled trial. Sci. Rep. 2020, 10, 12612. [Google Scholar] [CrossRef]
- Tanghetti, E.A. The role of inflammation in the pathology of acne. J. Clin. Aesthetic Dermatol. 2013, 6, 27–35. [Google Scholar]
- Noszczyk, M. Kosmetologia Pielęgnacyjna i Lekarska, 1st ed.; PZWL Medical Publishing House: Warsaw, Poland, 2018; pp. 50–53. [Google Scholar]
- Biegaj, M. Trądzik pospolity i jego leczenie. Kosmetologia Estetyczna 2017, 2, 155–157. [Google Scholar]
- Dréno, B.; Nguyen, J.M.; Hainaut, E.; Machet, L.; Leccia, M.T.; Beneton, N.; Claudel, J.P.; Célérier, P.; Moigne, M.; Naour, S.; et al. Efficacy of Spironolactone Compared with Doxycycline in Moderate Acne in Adult Females: Results of the Multicentre, Controlled, Randomized, Double-blind Prospective and Parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) Study. Acta Derm.-Venereol. 2024, 104, adv26002. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Choi, J.W.; Park, K.C.; Youn, S.W. Facial sebum affects the development of acne, especially the distribution of inflammatory acne. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 301–306. [Google Scholar]
- Santer, M.; Burden-The, E.; Ravenscroft, J. Managing acne vulgaris: An update. Drug Ther. Bull. 2023, 62, 6–10. [Google Scholar] [CrossRef]
- Hammoda, T.M.; Ahmed, N.A.; Hamdino, M. Fractional CO2 laser versus 1064-nm long-pulsed Nd: YAG laser for inflammatory acne vulgaris treatment: A randomized clinical trial. Lasers Med. Sci. 2023, 38, 187. [Google Scholar] [CrossRef]
- Liyanage, A.; Prathapan, S.; Jayarathne, C.; Ranaweera, L.S.; Perera, J. Validation and Cultural Adaptation of the Sinhala Translation of the Cardiff Acne Disability Index (CADI). Patient Relat. Outcome Meas. 2024, 15, 131–141. [Google Scholar] [CrossRef]
- Baroud, S.; Wu, J.; Zouboulis, C.C. Acne Syndromes and Mosaicism. Biomedicines 2021, 9, 1735. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.H.; Yang, S.H.; Lee, J.; Park, B.C.H.; Park, K.Y.; Jung, J.Y.; Bae, Y.; Park, G.H. Combination Treatment with Human Adipose Tissue Stem Cell-derived Exosomes and Fractional CO2 Laser for Acne Scars: A 12-week Prospective, Double-blind, Randomized, Split-face Study. Acta Derm.-Venereol. 2020, 100, adv00310. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, K.; Layton, A.M.; Ogawa, R. Updated Treatment for Acne: Targeted Therapy Based on Pathogenesis. Dermatol. Ther. 2021, 11, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Kulthanan, K.; Trakanwittayarak, S.; Tuchindaet, P.; Chularojanamontri, L.; Limphoka, P.; Varothai, S. A Double-Blinded, Randomized, Vehicle-Controlled Study of the Efficacy of Moisturizer Containing Licochalcone A, Decanediol, L-Carnitine, and Salicylic Acid for Prevention of Acne Relapse in Asian Population. Biomed Res. Int. 2020, 2020, 2857812. [Google Scholar] [CrossRef]
- Szepietowski, J.; Kapińska-Mrowiecka, M.; Kaszuba, A.; Langner, A.; Placek, W.; Wolska, H.; Matusiak, Ł. Trądzik zwyczajny: Patogeneza i leczenie. Konsensus Polskiego Towarzystwa Dermatologicznego. Prz. Dermatol. 2012, 99, 649–673. [Google Scholar]
- Bergler-Czop, B.; Bilewicz-Stebel, M.; Stańkowska, A. Trądzik pospolity—Najczęstsza dermatoza osób młodych. Wiad. Dermatol. 2019, 2, 6–24. [Google Scholar]
- Zouboulis, C.C. Endocrinology and immunology of acne: Two sides of the same coin. Exp. Dermatol. 2020, 29, 840–859. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.M.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Van Steensel, M.A.; Goh, B.C. Cutibacterium acnes: Much ado about maybe nothing much. Exp. Dermatol. 2021, 30, 1471–1476. [Google Scholar] [CrossRef]
- Huang, L.; Yang, S.; Yu, X.; Fang, F.; Zhu, L.; Wang, L.; Zhang, X.; Yang, C.; Qian, Q.; Zhu, T. Association of different cell types and inflammation in early acne vulgaris. Front. Immunol. 2024, 15, 1275269. [Google Scholar] [CrossRef]
- Eliasse, Y.; Leveque, E.; Garidou, L.; Battut, L.; McKenzie, B.; Nocera, T.; Redoules, D.; Espinosa, E. IL-17+ Mast Cell/T Helper Cell Axis in the Early Stages of Acne. Front. Immunol. 2021, 12, 740540. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.G.A.; Maraee, A.H.; Al-Sharaky, D.R.; Elshaib, M.E.; Kohla, M.S.M.; Shehata, W.A. Tissue expression of IL-17A and FOXP3 in acne vulgaris patients. J. Cosmet. Dermatol. 2021, 20, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Méhul, B.; Carlavan, I.; Genette, A.; Seraidaris, A.; Bertino, B.; Ménigot, C.; Bourdès, V.; Dréno, B.; Voegel, J.J.; Blanchet-Réthoré, S. Proteomic Analysis Reveals an Increase of Neutrophils and TH17-related Proteins Expression in Severe Nodular Acne Lesions of the Back. J. Skin Stem Cell 2020, 6, e101449. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Zhan, Y.; Yi, Y.; Jiang, Q.; Zhang, Q.; Wu, Y.; Wu, M. Neutrophil extracellular trap-related mechanisms in acne vulgaris inspire a novel treatment strategy with adipose-derived stem cells. Sci. Rep. 2024, 14, 1521. [Google Scholar] [CrossRef]
- Feng, Y.; Li, J.; Mo, X.; Ju, Q. Macrophages in acne vulgaris: Mediating phagocytosis, inflammation, scar formation, and therapeutic implications. Front. Immunol. 2024, 15, 1355455. [Google Scholar] [CrossRef]
- Nakai, K. Multiple roles of macrophage in skin. J. Dermatol. Sci. 2021, 104, 2–10. [Google Scholar] [CrossRef]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Zhang, B.; Choi, Y.M.; Lee, J.; An, I.S.; Li, L.; He, C.; Dong, Y.; Bae, S.; Meng, H. Toll-like receptor 2 plays a critical role in pathogenesis of acne vulgaris. Biomed. Dermatol. 2019, 3, 4. [Google Scholar] [CrossRef]
- Wunsch, E.; Koziarska, D.; Milkiewicz, M.; Naprawa, G.; Nowacki, P.; Hartleb, M.; Milkiewicz, P. In patients with liver cirrhosis, proinflammatory interleukins correlate with health-related quality of life irrespective of minimal hepatic encephalopathy. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1402–1407. [Google Scholar] [CrossRef]
- Branger, J.; Knapp, S.; Weijer, S.; Leemans, J.C.; Pater, J.M.; Speelman, P.; Florquin, S.; Poll, T. Role of Toll-Like Receptor 4 in Gram-Positive and Gram-Negative Pneumonia inMice. Infect. Immun. 2004, 72, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.H.; Shin, S.H.; Roh, Y.J.; Moon, N.J.; Seo, S.J.; Park, K.Y. Particulate matter increases Cutibacterium acnes-induced inflammation in human epidermal keratinocytes via the TLR4/NF-κB pathway. PLoS ONE 2022, 17, e0268595. [Google Scholar] [CrossRef] [PubMed]
- D’hautcourt, J.L.; Isaac, J. Mean Fluorescence Intensity of Dual Stained Cells. Cytometry 1999, 38, 44–46. [Google Scholar] [CrossRef]
- Tang, T.; Xu, Y.; Wang, L.; Zhang, P. In vitro acne disease model from inertial focusing effect for studying the interactions between sebocyte glands and macrophages. Biotechnol. J. 2023, 18, e2300108. [Google Scholar] [CrossRef]
- Sun, L.; Liu, W.; Zhang, L.J. The Role of Toll-like Receptors in Skin Host Defense, Psoriasis, and Atopic Dermatitis. J. Immunol. Res. 2019, 2019, 1824624. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, H.; Zhao, M.; Lu, Q. TLR2 and TLR4 in autoimmune diseases: A comprehensive review. Clin. Rev. Allergy Immunol. 2014, 47, 136–147. [Google Scholar] [CrossRef]
- Cejkova, P.; Nemeckova, I.; Broz, J.; Cerna, M. TLR2 and TLR4 expression on CD14++ and CD14+ monocyte subtypes in adult-onset autoimmune diabetes. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2016, 160, 76–83. [Google Scholar] [CrossRef]
- Bharti, S.; Vadlamudi, H.C. A strategic review on the involvement of receptors, transcription factors and hormones in acne pathogenesis. J. Recept. Signal Transduct. 2021, 41, 105–116. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, H.L.; Bu, X.L.; Zhang, J.B.; Lu, Y.G. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann. Transl. Med. 2022, 10, 645. [Google Scholar] [CrossRef]
- Ozlu, E.; Karadag, A.S.; Ozkanli, S.; Oguztuzun, S.; Kilic, M.; Zemheri, E.; Akbulak, O.; Akdeniz, N. Comparison of TLR-2, TLR-4, and antimicrobial peptide levels in different lesions of acne vulgaris. Cutan. Ocul. Toxicol. 2016, 35, 300–309. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, D.; Lee, M.; Lee, J.; Cho, S.; Kim, T.J.; Kim, H.S. Micro-Current Stimulation Suppresses Inflammatory Responses in Peptidoglycan-Treated Raw 264.7 Macrophages and Propionibacterium acnes-Induced Skin Inflammation via TLR2/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 2508. [Google Scholar] [CrossRef]
- Dispenza, M.C.; Wolpert, E.B.; Gilliland, K.L.; Dai, J.P.; Cong, Z.; Nelson, A.M.; Thiboutot, D.M. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J. Investig. Dermatol. 2012, 132, 2198–2205. [Google Scholar] [CrossRef]
| Skin Parameter | Location | Range | n | % |
|---|---|---|---|---|
| Sebumeter— Secretion of sebum | forehead | <100—dry skin, less sebum | 17 | 44.7% |
| 100–220—normal | 20 | 52.6% | ||
| >220—oily skin | 1 | 2.6% | ||
| Total | 38 | 100.0% | ||
| cheek | <70—dry skin, less sebum | 14 | 36.8% | |
| 70–180—normal | 23 | 60.5% | ||
| >180—oily skin | 1 | 2.6% | ||
| Total | 38 | 100.0% | ||
| Corneometer— Hydration | forehead | <30—very dry skin | 1 | 2.6% |
| 30–45—dry skin | 7 | 18.4% | ||
| >45—skin sufficiently moisturized | 30 | 78.9% | ||
| Total | 38 | 100.0% | ||
| cheek | <30—very dry skin | 9 | 23.7% | |
| 30–45—dry skin | 16 | 42.1% | ||
| >45—skin sufficiently moisturized | 13 | 34.2% | ||
| Total | 38 | 100.0% | ||
| Mexameter—phototype | forehead | 0–100—Photo type I (Nordic/Celtic skin) | 10 | 26.3% |
| 100–150—Photo type II (Light Caucasians) | 24 | 63.2% | ||
| 150–250—Photo type III (European mixed type/very fair Asian skin) | 4 | 10.5% | ||
| 250–350 Photo type IV (Mediterranean/fari Asian skin) | 0 | 0.0% | ||
| 350–450 Photo type V (Dark skin) | 0 | 0.0% | ||
| >450 Photo type VI (Black skin) | 0 | 0.0% | ||
| Total | 38 | 100.0% | ||
| Mexameter—phototype | cheek | 0–100—Photo type I (Nordic/Celtic skin) | 15 | 39.5% |
| 100–150—Photo type II (Light Caucasians) | 20 | 52.6% | ||
| 150–250—Photo type III (European mixed type/very fair Asian skin) | 3 | 7.9% | ||
| 250–350 Photo type IV (Mediterranean/fari Asian skin) | 0 | 0.0% | ||
| 350–450 Photo type V (Dark skin) | 0 | 0.0% | ||
| >450 Photo type VI (Black skin) | 0 | 0.0% | ||
| Total | 38 | 100.0% | ||
| Mexameter—erythema | forehead | 0–170—no erythema | 3 | 7.9% |
| 170–330—minimal erythema | 22 | 57.9% | ||
| 330–450—diffuse erythema | 12 | 31.6% | ||
| 450–570—high erythema | 1 | 2.6% | ||
| >570—extreme erythema | 0 | 0.0% | ||
| Total | 38 | 100.0% | ||
| Mexameter—erythema | cheek | 0–170—no erythema | 1 | 2.6% |
| 170–330—minimal erythema | 25 | 65.8% | ||
| 330–450—diffuse erythema | 8 | 21.1% | ||
| 450–570—high erythema | 4 | 10.5% | ||
| >570—extreme erythema | 0 | 0.0% | ||
| Total | 38 | 100.0% | ||
| Skin-pH-Meter—pH | forehead | + acidic range- | 2 | 5.3% |
| normal | 0 | 0.0% | ||
| − high skin pH value+ | 36 | 94.7% | ||
| Total | 38 | 100.0% | ||
| Skin-pH-Meter—pH | cheek | + acidic range − | 0 | 0.0% |
| normal | 0 | 0.0% | ||
| − high skin pH value + | 38 | 100.0% | ||
| Total | 38 | 100.0% |
| Occurrence of Post-Acne Changes (Erythema, Discoloration, Scars) | Z | p | ||||||
|---|---|---|---|---|---|---|---|---|
| Yes (n = 11) | No (n = 27) | |||||||
| M | Me | SD | M | Me | SD | |||
| Sebumeter—forehead | 129.64 | 143.00 | 54.75 | 106.00 | 95.00 | 49.39 | −1.22 | 0.22 |
| Sebumeter—cheek | 102.82 | 93.00 | 42.31 | 82.22 | 72.00 | 41.78 | −1.37 | 0.17 |
| Corneometer—forehead | 53.94 | 53.67 | 9.53 | 51.39 | 52.30 | 11.47 | −0.82 | 0.41 |
| Corneometer—cheek | 41.65 | 39.23 | 11.67 | 37.57 | 35.77 | 13.29 | −0.76 | 0.45 |
| Mexameter- phototype—forehead | 90.64 | 85.33 | 25.62 | 126.84 | 124.00 | 29.82 | −3.25 | 0.00 |
| Mexameter- phototype—cheek | 85.17 | 79.00 | 23.32 | 108.86 | 106.33 | 31.16 | −2.40 | 0.02 |
| Mexameter- erythema—forehead | 234.59 | 221.67 | 56.16 | 312.67 | 319.00 | 78.65 | −2.86 | 0.00 |
| Mexameter- erythema—cheek | 259.26 | 226.23 | 68.31 | 327.77 | 316.33 | 93.76 | −2.17 | 0.03 |
| pH—forehead | 6.96 | 6.56 | 2.57 | 5.85 | 5.87 | 0.97 | −1.38 | 0.17 |
| pH—cheek | 6.05 | 5.20 | 1.41 | 6.11 | 5.98 | 1.11 | −0.50 | 0.62 |
| Classical—CD14++CD16- | 86.05 | 86.39 | 6.14 | 86.53 | 86.80 | 5.50 | −0.18 | 0.86 |
| Classical with TLR2 (%) | 96.86 | 97.62 | 3.58 | 97.35 | 98.93 | 4.47 | −0.69 | 0.49 |
| Classical TLR2 (MFI) | 176,639.02 | 159,580.48 | 58728.15 | 189,566.68 | 194,171.20 | 64,745.11 | −0.47 | 0.64 |
| Classical with TLR4 (%) | 97.24 | 98.31 | 3.01 | 94.59 | 96.73 | 7.06 | −1.01 | 0.31 |
| Classical TLR4 (MFI) | 8545.39 | 6217.96 | 8979.67 | 45,942.43 | 3747.89 | 215,344.42 | −2.72 | 0.01 |
| Intermediate—CD14++CD16+ | 6.72 | 6.44 | 2.54 | 6.22 | 5.86 | 2.76 | −0.69 | 0.49 |
| Intermediate with TLR2 (%) | 96.07 | 97.24 | 3.61 | 96.24 | 98.89 | 10.93 | −1.32 | 0.19 |
| Intermediate TLR2 (MFI) | 407,542.37 | 258,617.46 | 371,861.53 | 574,693.01 | 265,604.26 | 1,121,976.14 | −0.34 | 0.74 |
| Intermediate with TLR4 (%) | 91.69 | 93.00 | 2.96 | 86.70 | 88.02 | 11.69 | −0.85 | 0.39 |
| Intermediate TLR4 (MFI) | 7758.55 | 7987.94 | 2174.22 | 6138.33 | 5177.02 | 2753.74 | −2.43 | 0.02 |
| Non-classical—CD14+/lowCD16++ | 2.64 | 2.55 | 1.02 | 2.51 | 2.02 | 1.25 | −0.95 | 0.34 |
| Non-classical with TLR2 (%) | 89.56 | 91.42 | 8.96 | 92.18 | 93.12 | 5.81 | −0.69 | 0.49 |
| Non-classical TLR2 (MFI) | 204,353.42 | 160,257.39 | 134,941.57 | 721,003.20 | 171,728.03 | 2,646,051.93 | −0.34 | 0.74 |
| Non-classical with TLR4 (%) | 94.51 | 94.33 | 3.20 | 87.16 | 89.77 | 9.92 | −2.62 | 0.01 |
| Non-classical TLR4 (MFI) | 10,675.59 | 5606.41 | 15,948.57 | 6652.76 | 4784.17 | 5087.21 | −1.69 | 0.09 |
| The Occurrence of Papulopustular Acne, with the Presence of Inflammatory Eruptions | Z | p | ||||||
|---|---|---|---|---|---|---|---|---|
| Yes (n = 29) | No (n = 9) | |||||||
| M | Me | SD | M | Me | SD | |||
| Sebumeter—forehead | 114.76 | 117.00 | 50.38 | 106.67 | 79.00 | 57.28 | −0.70 | 0.48 |
| Sebumeter—cheek | 91.60 | 88.00 | 43.66 | 77.15 | 66.33 | 38.43 | −0.76 | 0.45 |
| Corneometer—forehead | 52.94 | 53.47 | 11.52 | 49.51 | 50.90 | 8.53 | −1.18 | 0.24 |
| Corneometer—cheek | 38.84 | 39.23 | 14.33 | 38.49 | 37.27 | 6.48 | −0.02 | 0.99 |
| Mexameter- phototype—forehead | 114.01 | 118.67 | 30.35 | 123.93 | 113.67 | 41.01 | −0.05 | 0.96 |
| Mexameter- phototype—cheek | 104.92 | 105.23 | 26.88 | 92.59 | 85.00 | 41.54 | −0.74 | 0.46 |
| Mexameter- erythema—forehead | 298.97 | 279.33 | 72.51 | 261.37 | 247.67 | 101.91 | −1.25 | 0.21 |
| Mexameter- erythema—cheek | 308.51 | 297.50 | 86.25 | 306.07 | 263.00 | 113.79 | −0.22 | 0.82 |
| pH—forehead | 6.27 | 5.99 | 1.83 | 5.84 | 5.61 | 0.82 | −0.60 | 0.55 |
| pH—cheek | 6.08 | 5.98 | 1.27 | 6.11 | 5.69 | 0.91 | −0.22 | 0.82 |
| Classical-CD14++CD16- | 86.20 | 86.60 | 5.92 | 87.00 | 88.38 | 4.72 | −0.09 | 0.93 |
| Classical with TLR2 (%) | 96.66 | 97.90 | 4.65 | 98.98 | 99.42 | 1.03 | −1.60 | 0.11 |
| Classical TLR2 (MFI) | 188,518.70 | 191,080.59 | 67,725.44 | 177,143.02 | 194,171.20 | 43,972.91 | −0.26 | 0.80 |
| Classical with TLR4 (%) | 94.93 | 97.40 | 6.95 | 96.74 | 98.04 | 2.81 | −0.15 | 0.88 |
| Classical TLR4 (MFI) | 44,798.76 | 4469.56 | 207,532.37 | 3920.10 | 3683.52 | 1220.89 | −1.60 | 0.11 |
| Intermediate—CD14++CD16+ | 6.47 | 6.03 | 2.74 | 6.02 | 5.86 | 2.59 | −0.39 | 0.69 |
| Intermediate with TLR2 (%) | 95.49 | 98.57 | 10.62 | 98.46 | 98.89 | 1.55 | −0.62 | 0.54 |
| Intermediate TLR2 (MFI) | 600,954.50 | 265,604.26 | 109,4922.39 | 285,777.42 | 253,970.75 | 100,338.78 | −0.98 | 0.33 |
| Intermediate with TLR4 (%) | 87.03 | 89.71 | 11.04 | 91.75 | 93.51 | 5.82 | −1.12 | 0.26 |
| Intermediate TLR4 (MFI) | 6834.91 | 5830.45 | 2756.43 | 5874.07 | 5177.02 | 2394.47 | −1.08 | 0.28 |
| Non-classical—CD14+/lowCD16++ | 2.50 | 2.02 | 1.27 | 2.71 | 2.66 | 0.83 | −0.77 | 0.44 |
| Non-classical with TLR2 (%) | 90.17 | 91.42 | 7.12 | 95.44 | 95.29 | 3.98 | −2.35 | 0.02 |
| Non-classical TLR2 (MFI) | 696,514.14 | 162,954.85 | 255,2202.16 | 168,451.55 | 162,931.91 | 35,061.29 | −0.26 | 0.80 |
| Non-classical with TLR4 (%) | 89.04 | 92.48 | 10.11 | 90.09 | 90.95 | 5.15 | −0.46 | 0.64 |
| Non-classical TLR4 (MFI) | 8433.55 | 5240.34 | 10,660.78 | 5831.45 | 4431.87 | 3777.49 | −1.29 | 0.20 |
| Classical—CD14++CD16- | Classical with TLR2 (%) | Classical TLR2 (MFI) | Classical with TLR4 (%) | Classical TLR4 (MFI) | ||
|---|---|---|---|---|---|---|
| Sebumeter—forehead | rho | 0.14 | −0.02 | −0.07 | 0.22 | 0.10 |
| p | 0.41 | 0.90 | 0.66 | 0.18 | 0.57 | |
| Sebumeter—forehead | rho | 0.15 | −0.08 | −0.08 | −0.14 | −0.19 |
| p | 0.36 | 0.62 | 0.63 | 0.40 | 0.26 | |
| Corneometer—forehead | rho | 0.04 | 0.06 | −0.35 | −0.13 | −0.03 |
| p | 0.80 | 0.72 | 0.03 | 0.43 | 0.86 | |
| Corneometer—cheek | rho | −0.05 | 0.38 | −0.34 | −0.41 | 0.02 |
| p | 0.76 | 0.02 | 0.04 | 0.01 | 0.91 | |
| Mexameter- phototype—forehead | rho | 0.00 | 0.15 | 0.24 | −0.26 | −0.27 |
| p | 1.00 | 0.37 | 0.15 | 0.12 | 0.11 | |
| Mexameter- phototype—cheek | rho | −0.14 | 0.18 | 0.09 | −0.37 | −0.31 |
| p | 0.41 | 0.29 | 0.58 | 0.02 | 0.06 | |
| Mexameter- erythema—forehead | rho | 0.10 | −0.09 | 0.30 | −0.17 | −0.27 |
| p | 0.55 | 0.58 | 0.07 | 0.30 | 0.10 | |
| Mexameter- erythema—cheek | rho | 0.29 | −0.07 | 0.00 | 0.14 | −0.13 |
| p | 0.08 | 0.68 | 0.98 | 0.40 | 0.44 | |
| pH—forehead | rho | −0.04 | 0.20 | −0.20 | −0.26 | 0.25 |
| p | 0.82 | 0.22 | 0.22 | 0.12 | 0.13 | |
| pH—cheek | rho | −0.03 | 0.25 | 0.11 | −0.21 | 0.10 |
| p | 0.84 | 0.13 | 0.51 | 0.21 | 0.54 |
| Intermediate CD14++CD16+ | Intermediate z TLR2 (%) | Intermediate TLR2 (MFI) | Intermediate with TLR4 (%) | Intermediate TLR4 (MFI) | ||
|---|---|---|---|---|---|---|
| Sebumeter—forehead | rho | 0.05 | −0.08 | −0.07 | 0.06 | 0.12 |
| p | 0.76 | 0.64 | 0.66 | 0.71 | 0.47 | |
| Sebumeter—forehead | rho | −0.10 | 0.01 | −0.04 | −0.07 | −0.12 |
| p | 0.54 | 0.97 | 0.79 | 0.69 | 0.47 | |
| Corneometer—forehead | rho | 0.25 | 0.01 | −0.33 | −0.26 | −0.07 |
| p | 0.12 | 0.94 | 0.04 | 0.11 | 0.68 | |
| Corneometer—cheek | rho | 0.20 | 0.15 | −0.21 | −0.24 | −0.16 |
| p | 0.22 | 0.38 | 0.20 | 0.14 | 0.35 | |
| Mexameter- phototype—forehead | rho | 0.04 | 0.20 | 0.14 | −0.29 | −0.23 |
| p | 0.80 | 0.23 | 0.41 | 0.08 | 0.17 | |
| Mexameter- phototype—cheek | rho | 0.06 | 0.20 | 0.07 | −0.39 | −0.18 |
| p | 0.72 | 0.23 | 0.67 | 0.01 | 0.29 | |
| Mexameter- erythema—forehead | rho | −0.15 | 0.06 | 0.21 | −0.38 | −0.24 |
| p | 0.38 | 0.73 | 0.21 | 0.02 | 0.15 | |
| Mexameter- erythema—cheek | rho | −0.22 | 0.01 | −0.02 | −0.01 | −0.17 |
| p | 0.19 | 0.98 | 0.92 | 0.98 | 0.30 | |
| pH—forehead | rho | 0.16 | 0.09 | −0.14 | 0.20 | 0.06 |
| p | 0.33 | 0.60 | 0.41 | 0.22 | 0.71 | |
| pH—cheek | rho | 0.13 | 0.16 | 0.04 | 0.16 | 0.13 |
| p | 0.43 | 0.34 | 0.83 | 0.34 | 0.44 |
| Non-Classical CD14+/lowCD16++ | Non-Classical with TLR2 (%) | Non-Classical TLR2 (MFI) | Non-Classical with TLR4 (%) | Non-Classical TLR4 (MFI) | ||
|---|---|---|---|---|---|---|
| Sebumeter—forehead | rho | −0.05 | 0.08 | −0.18 | 0.09 | −0.10 |
| p | 0.77 | 0.62 | 0.29 | 0.59 | 0.54 | |
| Sebumeter—forehead | rho | 0.01 | 0.00 | −0.05 | −0.20 | −0.25 |
| p | 0.94 | 1.00 | 0.78 | 0.23 | 0.13 | |
| Corneometer—forehead | rho | 0.00 | 0.03 | −0.45 | −0.03 | −0.20 |
| p | 0.98 | 0.88 | 0.01 | 0.85 | 0.24 | |
| Corneometer—cheek | rho | −0.20 | −0.06 | 0.06 | −0.13 | 0.01 |
| p | 0.22 | 0.71 | 0.71 | 0.43 | 0.97 | |
| Mexameter- phototype—forehead | rho | −0.20 | −0.23 | 0.20 | −0.37 | −0.07 |
| p | 0.22 | 0.17 | 0.23 | 0.02 | 0.67 | |
| Mexameter- phototype—cheek | rho | −0.13 | −0.15 | 0.15 | −0.35 | −0.11 |
| p | 0.45 | 0.38 | 0.38 | 0.03 | 0.51 | |
| Mexameter- erythema—forehead | rho | −0.27 | −0.22 | 0.05 | −0.41 | −0.13 |
| p | 0.10 | 0.20 | 0.75 | 0.01 | 0.44 | |
| Mexameter- erythema—cheek | rho | −0.19 | −0.08 | −0.14 | −0.20 | −0.18 |
| p | 0.26 | 0.62 | 0.41 | 0.24 | 0.28 | |
| pH—forehead | rho | 0.26 | 0.10 | 0.10 | 0.32 | 0.14 |
| p | 0.12 | 0.57 | 0.53 | 0.05 | 0.40 | |
| pH—cheek | rho | −0.02 | −0.05 | 0.13 | 0.20 | 0.18 |
| p | 0.89 | 0.77 | 0.43 | 0.23 | 0.27 |
| Severity of Acne | Z | p | ||||||
|---|---|---|---|---|---|---|---|---|
| Mild (n = 13) | Medium Severity/Moderate or Severe (n = 25) | |||||||
| M | Me | SD | M | Me | SD | |||
| Sebumeter—forehead | 103.08 | 79.00 | 45.61 | 117.92 | 117.00 | 54.36 | −0.95 | 0.34 |
| Sebumeter—cheek | 70.72 | 59.00 | 39.06 | 97.26 | 93.00 | 41.97 | −1.89 | 0.06 |
| Corneometer—forehead | 50.14 | 53.63 | 14.21 | 53.16 | 52.30 | 8.85 | −0.11 | 0.91 |
| Corneometer—cheek | 36.76 | 35.63 | 14.33 | 39.79 | 39.23 | 12.15 | −0.54 | 0.59 |
| Mexameter- phototype—forehead | 122.95 | 120.33 | 22.72 | 112.93 | 112.00 | 37.01 | −0.97 | 0.33 |
| Mexameter- phototype—cheek | 96.74 | 104.67 | 31.20 | 104.73 | 105.23 | 30.85 | −0.40 | 0.69 |
| Mexameter- erythema—forehead | 308.90 | 321.67 | 94.55 | 280.27 | 271.00 | 72.37 | −1.12 | 0.26 |
| Mexameter- erythema—cheek | 324.56 | 282.67 | 101.93 | 299.29 | 297.50 | 87.03 | −0.75 | 0.45 |
| pH—forehead | 5.90 | 5.88 | 0.72 | 6.31 | 5.99 | 1.96 | −0.40 | 0.69 |
| pH—cheek | 5.85 | 5.69 | 0.76 | 6.22 | 6.04 | 1.35 | −0.89 | 0.37 |
| Classical—CD14++CD16- | 86.78 | 87.92 | 5.75 | 86.19 | 86.60 | 5.65 | −0.14 | 0.89 |
| Classical with TLR2 (%) | 97.89 | 98.13 | 2.42 | 96.85 | 98.56 | 4.88 | −0.08 | 0.94 |
| Classical TLR2 (MFI) | 193,675.25 | 198,579.74 | 82,354.79 | 181,742.05 | 185,268.68 | 50,900.51 | −0.23 | 0.82 |
| Classical with TLR4 (%) | 95.89 | 96.73 | 3.89 | 95.08 | 97.82 | 7.23 | −0.08 | 0.94 |
| Classical TLR4 (MFI) | 5333.95 | 4234.98 | 3473.94 | 50,604.14 | 4469.56 | 223,585.20 | −0.42 | 0.68 |
| Intermediate—CD14++CD16+ | 5.87 | 5.86 | 2.71 | 6.62 | 6.03 | 2.68 | −0.85 | 0.40 |
| Intermediate with TLR2 (%) | 94.45 | 99.08 | 15.66 | 97.09 | 98.45 | 3.13 | −0.91 | 0.36 |
| Intermediate TLR2 (MFI) | 758,851.56 | 265,604.26 | 1,589,525.36 | 405,384.28 | 261,178.94 | 350,185.74 | −0.42 | 0.68 |
| Intermediate with TLR4 (%) | 89.12 | 93.51 | 9.93 | 87.64 | 90.02 | 10.48 | −0.75 | 0.45 |
| Intermediate TLR4 (MFI) | 5942.71 | 5083.32 | 2372.85 | 6952.95 | 5910.13 | 2803.69 | −1.62 | 0.11 |
| Non-classical— CD14+/lowCD16++ | 2.34 | 2.45 | 0.74 | 2.66 | 2.05 | 1.35 | −0.68 | 0.50 |
| Non-classical with TLR2 (%) | 92.59 | 93.43 | 6.42 | 90.81 | 92.24 | 7.11 | −0.91 | 0.36 |
| Non-classical TLR2 (MFI) | 256,187.73 | 154,627.26 | 364,869.10 | 735,381.34 | 171,728.03 | 2,744,298.89 | −0.63 | 0.53 |
| Non-classical with TLR4 (%) | 89.83 | 90.95 | 5.95 | 89.01 | 91.38 | 10.51 | −0.48 | 0.63 |
| Non-classical TLR4 (MFI) | 5913.00 | 4784.17 | 3259.41 | 8807.48 | 5240.34 | 11,445.13 | −0.88 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firlej, E.; Grzegorzewska, W.; Jastrzębska-Pawłowska, K.; Janiszewska, M.; Gąbka-Flis, I.; Makarska-Białokoz, M.; Roliński, J.; Bartosińska, J. Evaluation of the Percentage of Monocyte Subpopulations with TLR2 and TLR4 Expression About Selected Skin Functional Parameters in Patients with Acne Vulgaris—Cross-Sectional Study. J. Clin. Med. 2025, 14, 6449. https://doi.org/10.3390/jcm14186449
Firlej E, Grzegorzewska W, Jastrzębska-Pawłowska K, Janiszewska M, Gąbka-Flis I, Makarska-Białokoz M, Roliński J, Bartosińska J. Evaluation of the Percentage of Monocyte Subpopulations with TLR2 and TLR4 Expression About Selected Skin Functional Parameters in Patients with Acne Vulgaris—Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(18):6449. https://doi.org/10.3390/jcm14186449
Chicago/Turabian StyleFirlej, Ewelina, Wioleta Grzegorzewska, Katarzyna Jastrzębska-Pawłowska, Mariola Janiszewska, Ilona Gąbka-Flis, Magdalena Makarska-Białokoz, Jacek Roliński, and Joanna Bartosińska. 2025. "Evaluation of the Percentage of Monocyte Subpopulations with TLR2 and TLR4 Expression About Selected Skin Functional Parameters in Patients with Acne Vulgaris—Cross-Sectional Study" Journal of Clinical Medicine 14, no. 18: 6449. https://doi.org/10.3390/jcm14186449
APA StyleFirlej, E., Grzegorzewska, W., Jastrzębska-Pawłowska, K., Janiszewska, M., Gąbka-Flis, I., Makarska-Białokoz, M., Roliński, J., & Bartosińska, J. (2025). Evaluation of the Percentage of Monocyte Subpopulations with TLR2 and TLR4 Expression About Selected Skin Functional Parameters in Patients with Acne Vulgaris—Cross-Sectional Study. Journal of Clinical Medicine, 14(18), 6449. https://doi.org/10.3390/jcm14186449







