Non-Ossifying Fibromas: A 2025 Review
Abstract
1. Introduction
2. Etiology
3. Epidemiology
4. Clinical Presentation
4.1. Asymptomatic Patient
4.2. Symptomatic Patient
4.3. Pathologic Fracture
- Cortical thickness < 2 mm on CT imaging
- Lesion involving > 50% of the bone diameter on orthogonal radiographs
- Size > 4 cm in weight-bearing bones
- Presence of “Pac-Man Sign” or “syndesmosis sign” in the distal tibia
- Ritschl Stage B lesions with persistent symptoms
- Pain with weight-bearing activities
5. Radiographic and Advanced Imaging
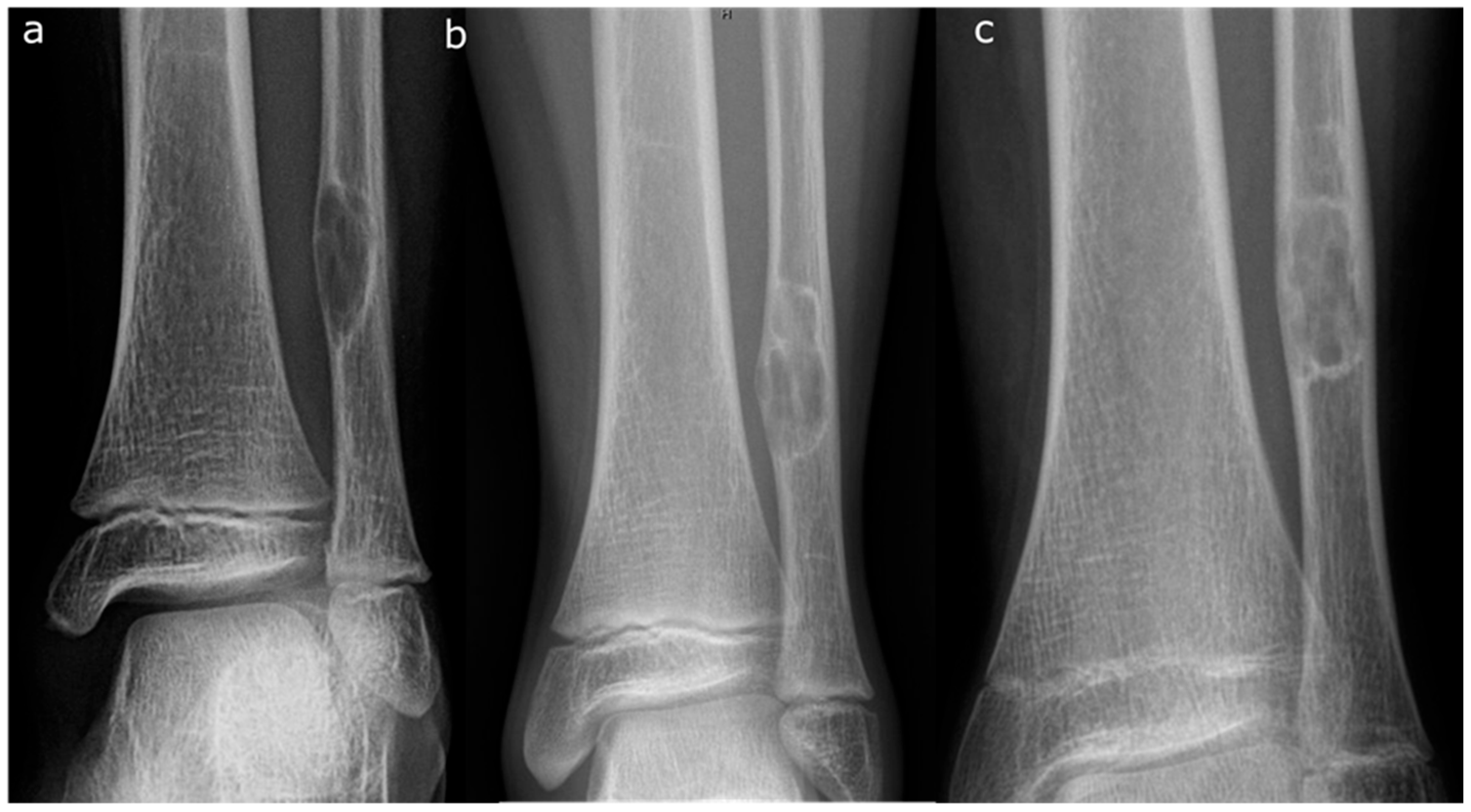
5.1. Radiographic Features
5.2. Staging
- Stage A: Small, eccentric, cortically based lesions near the epiphysis with smooth, round borders.
- Stage B: Lesions become polycystic with clear but thin sclerotic borders, increasing in size and exhibiting variable distances from the epiphysis. The most significant growth typically occurs during this stage, transitioning from Stage A or within Stage B.
- Stage C: Lesions demonstrate increased sclerosis and reduced growth potential. The radiographic features in Stage C can be quite variable and can be a source of confusion [19].
- Stage D: Complete and homogeneous sclerosis is observed, with no further growth.
5.3. Advanced Imaging
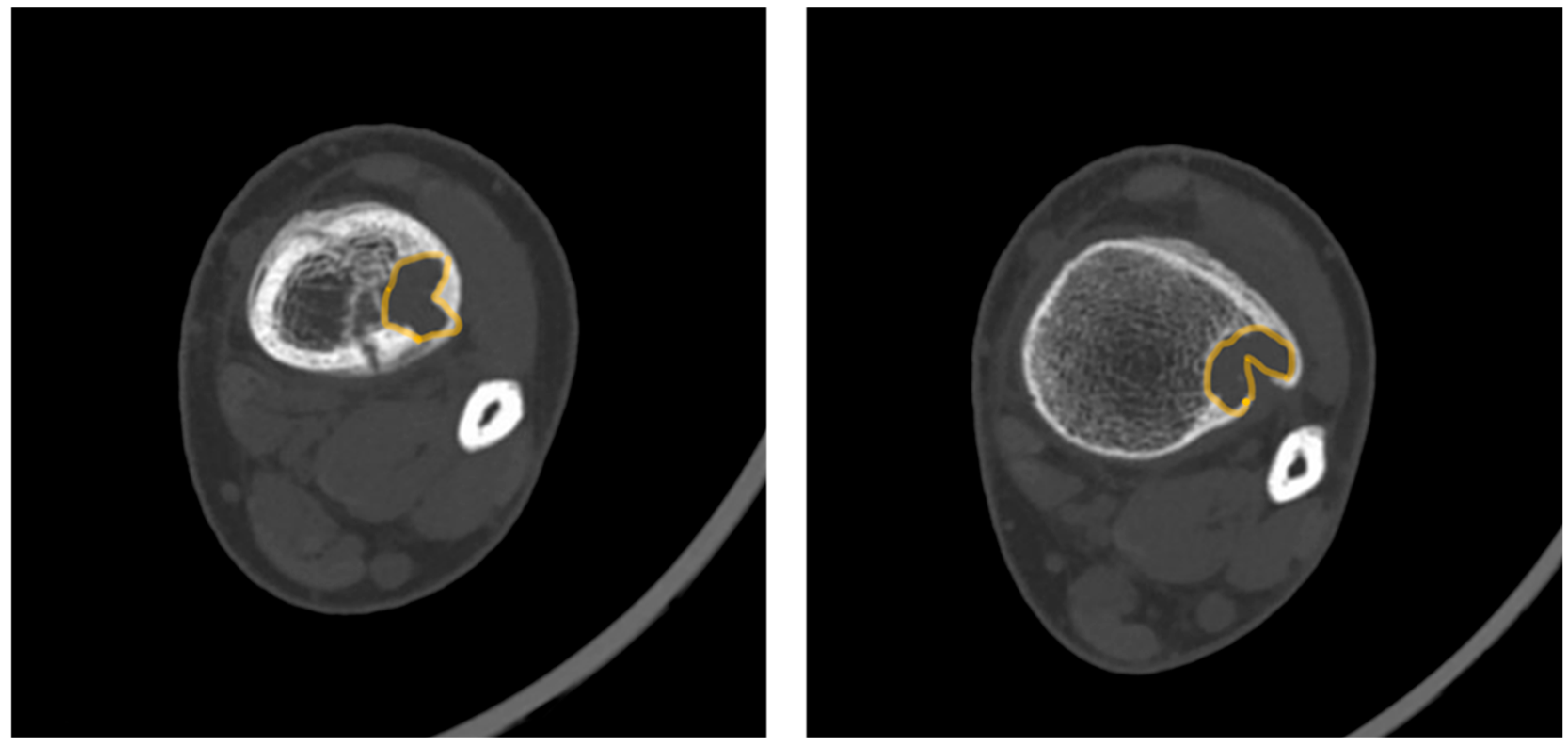
- Magnetic Resonance Imaging (MRI): MRI can help differentiate NOFs from other lesions such as fibrous dysplasia or malignancies [20]. On MRI, NOFs are typically T1-hypointense and T2-hyperintense. A peripheral, low-signal rim on all sequences corresponds to the sclerotic border seen on radiographs. Post-contrast sequences usually show minimal, peripheral enhancement, whereas more aggressive lesions often demonstrate avid, diffuse enhancement [21,22]. In 2021, Baghdadi et al. identified advanced imaging features, such as the “Pac-Man sign” and the “syndesmosis sign”, that may indicate increased fracture risk in distal tibia NOFs [23].
- Pac-Man Sign—Proliferation of bone anterior and posterior to the syndesmosis results in a shape that resembles the video game character “Pac-Man”. This sign was found to be highly specific (95%) but not very sensitive (47%) for predicting pathologic fracture (Figure 3).
- Syndesmosis Sign—Advanced imaging shows the syndesmosis inserting into the distal tibia lesion. This sign was found to be highly sensitive (94%) but less specific (48%) for predicting fracture risk.
- Computed Tomography (CT): CT can be useful for evaluating the degree of cortical thinning, which is important when assessing fracture risk [24]. CT is superior for delineating the precise cortical integrity. It allows for quantitative measurement of the cross-sectional area occupied by the lesion, which is a key factor in biomechanical models predicting fracture risk. The signs described above can also be visualized on a CT scan. Figure 3 demonstrates a “Pac-Man Sign” on CT of a 13-year-old boy who had a fracture through an NOF.
- Bone Scintigraphy: Demonstrates mild uptake, reflecting the lesion’s low metabolic activity [25].
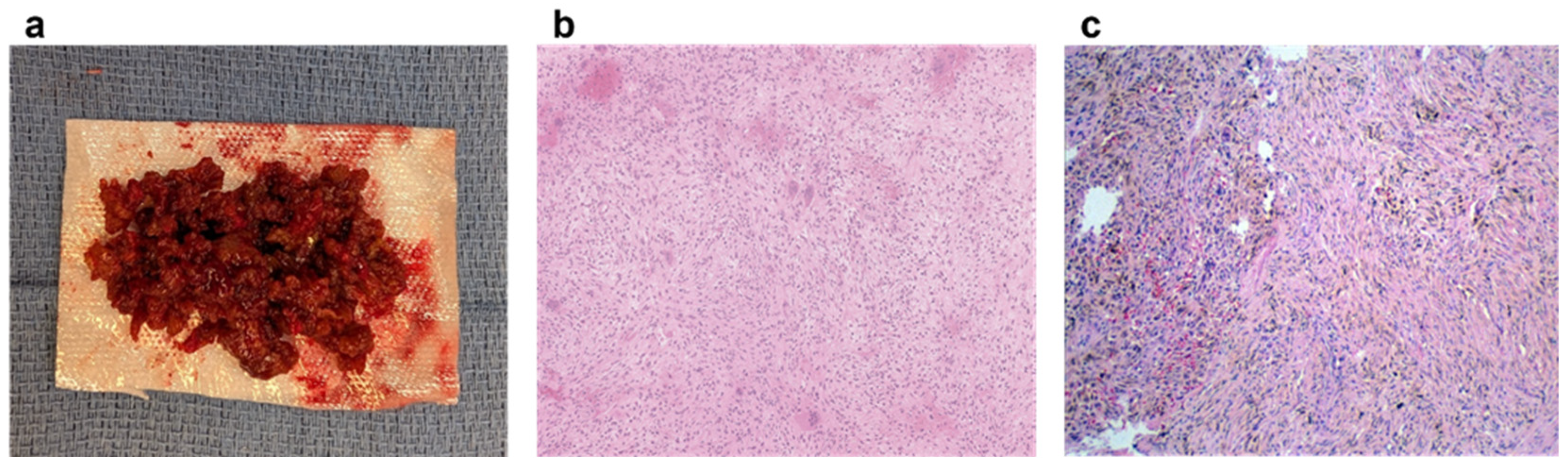
6. Pathology
7. Non-Operative Management
7.1. Observation and Monitoring
7.2. Activity Modification
7.3. Closed Reduction and Casting of Pathologic Fractures
8. Operative Intervention
8.1. Indications
8.2. Techniques
- Curettage and Bone Grafting: This is by far the most performed procedure. After curettage, the cavity is filled with autograft, allograft, or synthetic bone substitute [32]. While autograft is theoretically biologically superior, the donor site morbidity makes allograft the most used graft choice. In addition, the relatively high success rate and low risk of recurrence make the risks of an additional incision with autograft prohibitive. Synthetic substitutes such as calcium sulfate and calcium phosphate can provide structural support to allow earlier weight bearing. Again, the risks of cementation, in the setting of a benign disease with good operative results, make this a less common choice. Adjuvants are typically not used in the curettage stage for NOFs, as they are in giant cell tumors of bone or aneurysmal bone cysts. NOFs are not locally aggressive, and the use of phenol or argon beam coagulation is not typically indicated to reduce recurrence. Phenol and argon have a risk of local tissue damage, making them uncommonly used in the setting of NOFs. Figure 5 demonstrates pre-, intra-, and post-operative radiographic images of a 17-year-old boy with a large symptomatic distal tibia NOF who underwent a curettage and bone grafting procedure.
- Internal Fixation: For structural support in large lesions or fractures, internal fixation with plates or intramedullary nails may be necessary.
8.3. Outcomes
9. Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NOFs | Non-ossifying fibromas |
| CT | Computed tomography |
| MRI | Magnetic Resonance Imaging |
References
- Sontag, L.; Pyle, S. The Appearance and Nature of Cyst-like Areas in the Distal Femoral Metaphyses of Children. Am. J. Roentgenol. 1941, 46, 185–188. [Google Scholar]
- Dumitriu, D.; Menten, R.; Clapuyt, P. Pitfalls in the Diagnosis of Common Benign Bone Tumours in Children. Insights Imaging 2014, 5, 645–655. [Google Scholar] [CrossRef]
- Phemister, D.B. Chronic Fibrous Osteomyelitis. Ann. Surg. 1929, 90, 756–764. [Google Scholar]
- Merrow, C.; Hariharan, S. Fibroxanthoma. In Imaging in Pediatrics; Elsevier BV: Amsterdam, The Netherlands, 2018; p. 275. ISBN 9780323477789. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. In Soft Tissue and Bone Tumours, 5th ed.; IARC: Lyon, France, 2020; ISBN 9789283245025. [Google Scholar]
- Jaffe, H.L.; Lichtenstein, L. Non-Osteogenic Fibroma of Bone. Am. J. Pathol. 1942, 18, 205–221. [Google Scholar]
- Collier, C.D.; Nelson, G.B.; Conry, K.T.; Kosmas, C.; Getty, P.J.; Liu, R.W. The Natural History of Benign Bone Tumors of the Extremities in Asymptomatic Children: A Longitudinal Radiographic Study. J. Bone Jt. Surg. 2021, 103, 575. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Muzykewicz, D.A.; Dwek, J.; Mubarak, S.J. The Aetiology of the Non-Ossifying Fibroma of the Distal Femur and Its Relationship to the Surrounding Soft Tissues. J. Child. Orthop. 2017, 11, 373–379. [Google Scholar] [CrossRef]
- Baumhoer, D.; Kovac, M.; Sperveslage, J.; Ameline, B.; Strobl, A.-C.; Krause, A.; Trautmann, M.; Wardelmann, E.; Nathrath, M.; Höller, S.; et al. Activating Mutations in the MAP-Kinase Pathway Define Non-Ossifying Fibroma of Bone. J. Pathol. 2019, 248, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Bovée, J.V.; Hogendoorn, P.C. Non-ossifying Fibroma: A RAS-MAPK Driven Benign Bone Neoplasm. J. Pathol. 2019, 248, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Schindeler, A.; Little, D.G. Ras-MAPK Signaling in Osteogenic Differentiation: Friend or Foe? J. Bone Miner. Res. 2006, 21, 1331–1338. [Google Scholar] [CrossRef]
- Jamshidi, K.; Motaghi, P.; Bagherifard, A.; Eigi, M.; Al-Baseesee, H.H.; Mirzaei, A. Comparison of characteristic features and local recurrence in syndromic versus non-syndromic multifocal non-ossifying fibroma. J. Orthop. Sci. 2021, 26, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Ritschl, P.; Karnel, F.; Hajek, P. Fibrous Metaphyseal Defects—Determination of Their Origin and Natural History Using a Radiomorphological Study. Skelet. Radiol. 1988, 17, 8–15. [Google Scholar] [CrossRef]
- Emori, M.; Tsuchie, H.; Teramoto, A.; Shimizu, J.; Mizushima, E.; Murahashi, Y.; Nagasawa, H.; Miyakoshi, N.; Yamashita, T. Non-ossifying fibromas and fibrous cortical defects around the knee—An epidemiologic survey in a Japanese pediatric population. BMC Musculoskelet. Disord. 2022, 23, 378. [Google Scholar] [CrossRef]
- Moretti, V.M.; Slotcavage, R.L.; Crawford, E.A.; Lackman, R.D.; Ogilvie, C.M. Curettage and graft alleviates athletic-limiting pain in benign lytic bone lesions. Clin. Orthop. Relat. Res. 2011, 469, 283–288. [Google Scholar] [CrossRef]
- Shimal, A.; Davies, A.M.; James, S.L.; Grimer, R.J. Fatigue-type stress fractures of the lower limb associated with fibrous cortical defects/non-ossifying fibromas in the skeletally immature. Clin. Radiol. 2010, 65, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Tanaka, K.; Yoshida, T.; Iwamoto, Y. Nonossifying fibroma accompanied by patho-logical fracture in a 12-year-old runner. J. Orthop. Sports Phys. Ther. 2008, 38, 434–438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, C.; Garner, H.; Ahlawat, S. Society of Skeletal Radiology-White Paper. Guidelines for the Diagnostic Management of Incidental Solitary Bone Lesions on CT and MRI in Adults: Bone Reporting and Data System (Bone-RADS). Skeletal. Radiol. 2022, 51, 1743–1764. [Google Scholar] [CrossRef]
- Błaż, M.; Palczewski, P.; Swiątkowski, J.; Gołębiowski, M. Cortical Fibrous Defects and Non-Ossifying Fibromas in Children and Young Adults: The Analysis of Radiological Features in 28 Cases and a Review of Literature. Pol. J. Radiol. 2011, 76, 32–39. [Google Scholar]
- Rammanohar, J.; Zhang, C.; Thahir, A.; Krkovic, M. Imaging of Non-Ossifying Fibromas: A Case Series. Cureus 2021, 13, e14102. [Google Scholar] [CrossRef] [PubMed]
- Jee, W.; Choe, B.; Kang, H. Nonossifying Fibroma: Characteristics at MR Imaging with Pathologic Correlation. Radiology 1998, 209, 197–202. [Google Scholar] [CrossRef]
- Stacy, G.; Dixon, L. Pitfalls in MR Image Interpretation Prompting Referrals to an Orthopedic Oncology Clinic. Radiographics 2007, 27, 805–826. [Google Scholar] [CrossRef]
- Baghdadi, S.; Nguyen, J.C.; Arkader, A. Nonossifying Fibroma of the Distal Tibia: Predictors of Fracture and Management Algorithm. J. Pediatr. Orthop. 2021, 41, e671. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.N.; Muzykewicz, D.A.; Mubarak, S.J. Nonossifying Fibromas: A Computed Tomography–Based Criteria to Predict Fracture Risk. J. Pediatr. Orthop. 2020, 40, e149–e154. [Google Scholar] [CrossRef] [PubMed]
- Hod, N.; Levi, Y.; Fire, G. Scintigraphic Characteristics of Non-Ossifying Fibroma in Military Recruits Undergoing Bone Scintigraphy for Suspected Stress Fractures and Lower Limb Pains. Nucl. Med. Commun. 2007, 28, 25–33. [Google Scholar] [CrossRef]
- Choi, J.; Ro, J. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Adv. Anat. Pathol. 2021, 28, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.D.; Haley, J.C.; Ruiz, R.; Essary, L.; Moran, C.A.; Fletcher, C.D. “Juvenile” xanthogranuloma: An immunophenotypic study with a reappraisal of histogenesis. Am. J. Dermatopathol. 2001, 23, 104–111. [Google Scholar] [CrossRef]
- Yamamoto, H.; Iwasaki, T.; Yamada, Y.; Matsumoto, Y.; Otsuka, H.; Yoshimoto, M.; Kohashi, K.; Taguchi, K.; Yokoyama, R.; Nakashima, Y.; et al. Diagnostic utility of histone H3.3 G34W, G34R, and G34V mutant-specific antibodies for giant cell tumors of bone. Hum. Pathol. 2018, 73, 41–50. [Google Scholar] [CrossRef]
- Patel, R.R.; Damron, T.A. The Role of Surveillance in Predicting Fracture in Pediatric Patients with Incidentally Discovered Nonossifying Fibromas and Fibrous Cortical Defects: Is It Worth It? J. Pediatr. Orthop. 2024, 44, 395. [Google Scholar] [CrossRef]
- Herget, G.W.; Mauer, D.; Krauß, T.; El Tayeh, A.; Uhl, M.; Südkamp, N.P.; Hauschild, O. Non-Ossifying Fibroma: Natural History with an Emphasis on a Stage-Related Growth, Fracture Risk and the Need for Follow-Up. BMC Musculoskelet. Disord. 2016, 17, 147. [Google Scholar] [CrossRef]
- Easley, M.E.; Kneisl, J.S. Pathologic fractures through nonossifying fibromas: Is prophylactic treatment warranted? J. Pediatr. Orthop. 1997, 17, 808–813. [Google Scholar] [CrossRef]
- Arata, M.A.; Peterson, H.A.; Dahlin, D.C. Pathological Fractures through Non-Ossifying Fibromas. Review of the Mayo Clinic Experience. J. Bone Jt. Surg. 1981, 63, 980. [Google Scholar] [CrossRef]
- Andreacchio, A.; Alberghina, F.; Testa, G.; Canavese, F. Surgical Treatment for Symptomatic Non-Ossifying Fibromas of the Lower Extremity with Calcium Sulfate Grafts in Skeletally Immature Patients. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, A.; Foca, F.; Oboldi, D.; Diano, D.; Bazzocchi, A.; Fabbri, L.; Mercatali, L.; Vanni, S.; Maltoni, M.; Bianchini, D.; et al. 3-T magnetic resonance-guided high-intensity focused ultrasound (3 T-MR-HIFU) for the treatment of pain from bone metastases of solid tumors. Support Care Cancer 2022, 30, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.A.; Shah, S.U.; Yasin, N.F.; Abdullah, B.J.J. Magnetic resonance guided focused ultrasound for treatment of bone tumors. J. Orthop. Surg. 2017, 25, 2309499017716256. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Stauffer, P.R.; Vrba, D.; Hurwitz, M.D. Focused ultrasound for treatment of bone tumours. Int. J. Hyperth. 2015, 31, 260–271. [Google Scholar] [CrossRef] [PubMed]


| Lesion | Typical Age | Location | Radiographic Features | Histology Key Feature |
|---|---|---|---|---|
| Non-ossifying Fibroma | 5–20 years | Metaphysis (eccentric) | Lytic, scalloped, sclerotic rim, bubbly appearance | Storiform spindle cells, hemosiderin |
| Fibrous Dysplasia | <30 years | Metaphysis/Diaphysis (central) | Ground-glass, “Rind” sign, Shepherd’s Crook deformity | “Chinese character” woven bone |
| Aneurysmal Bone Cyst | <20 years | Metaphysis (central) | Expansile, lytic, fluid-fluid levels on MRI | Blood-filled spaces, giant cells |
| Simple Bone Cyst (UBC) | <20 years | Metaphysis (central) | Lytic, well-defined, “fallen leaf” sign after fracture | Thin fibrous lining, clear fluid |
| Giant Cell Tumor | 20–40 years | Epiphysis (abuts joint) | Lytic, expansile, non-sclerotic margin | Numerous osteoclast-like giant cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, K.; Smith, J.B.; Todi, N.; Brown, D.; Randall, R.L. Non-Ossifying Fibromas: A 2025 Review. J. Clin. Med. 2025, 14, 6428. https://doi.org/10.3390/jcm14186428
Walker K, Smith JB, Todi N, Brown D, Randall RL. Non-Ossifying Fibromas: A 2025 Review. Journal of Clinical Medicine. 2025; 14(18):6428. https://doi.org/10.3390/jcm14186428
Chicago/Turabian StyleWalker, Kyle, Jimmy B. Smith, Niket Todi, Danielle Brown, and Robert L. Randall. 2025. "Non-Ossifying Fibromas: A 2025 Review" Journal of Clinical Medicine 14, no. 18: 6428. https://doi.org/10.3390/jcm14186428
APA StyleWalker, K., Smith, J. B., Todi, N., Brown, D., & Randall, R. L. (2025). Non-Ossifying Fibromas: A 2025 Review. Journal of Clinical Medicine, 14(18), 6428. https://doi.org/10.3390/jcm14186428







