Dual Impacts of Lung Transplantation on the Recovery and Comorbidity of Interstitial Lung Diseases: A Longitudinal Assessment of the Benefits and Burden
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Endpoints
2.2. Statistical Analysis
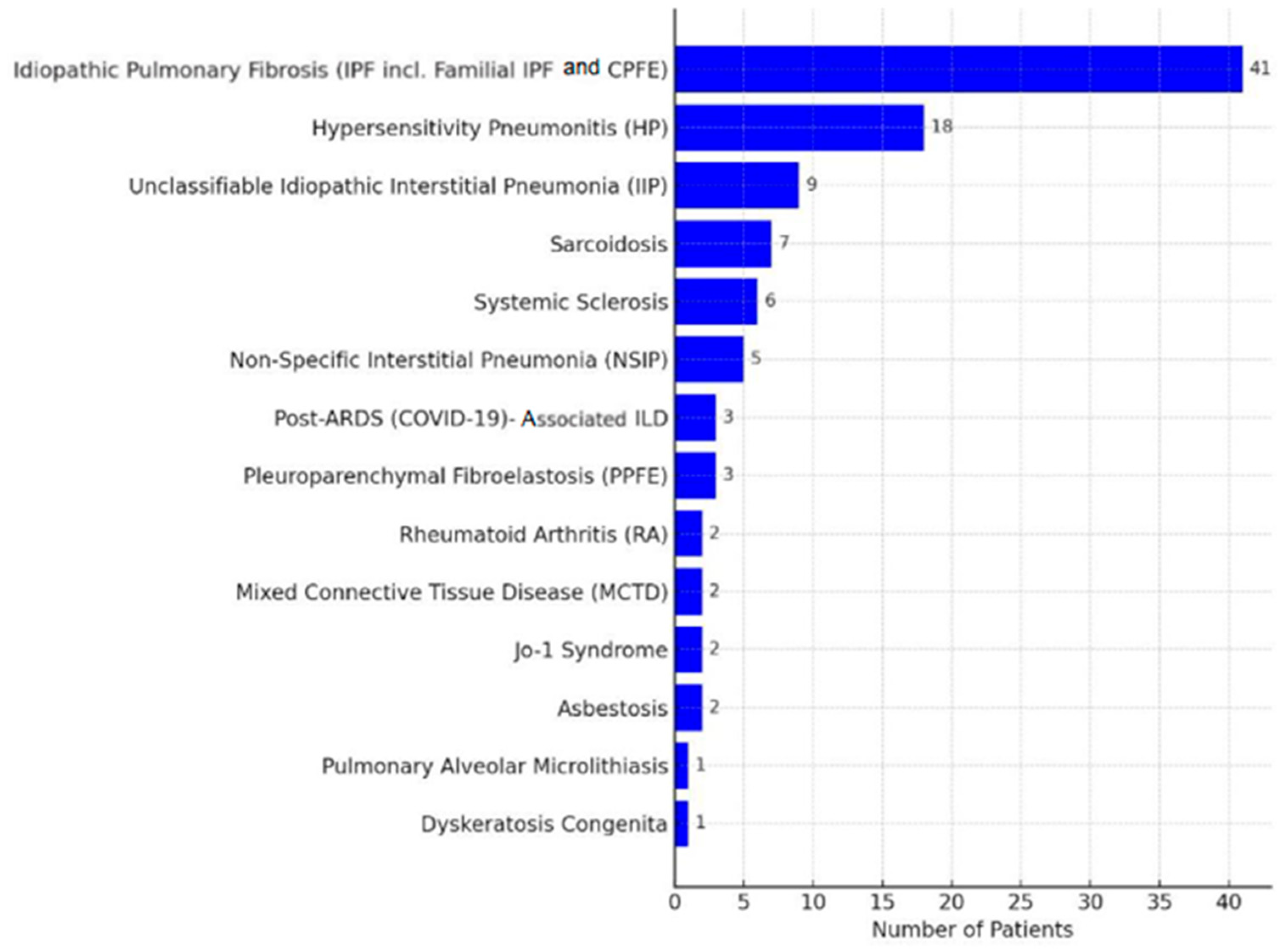
3. Results
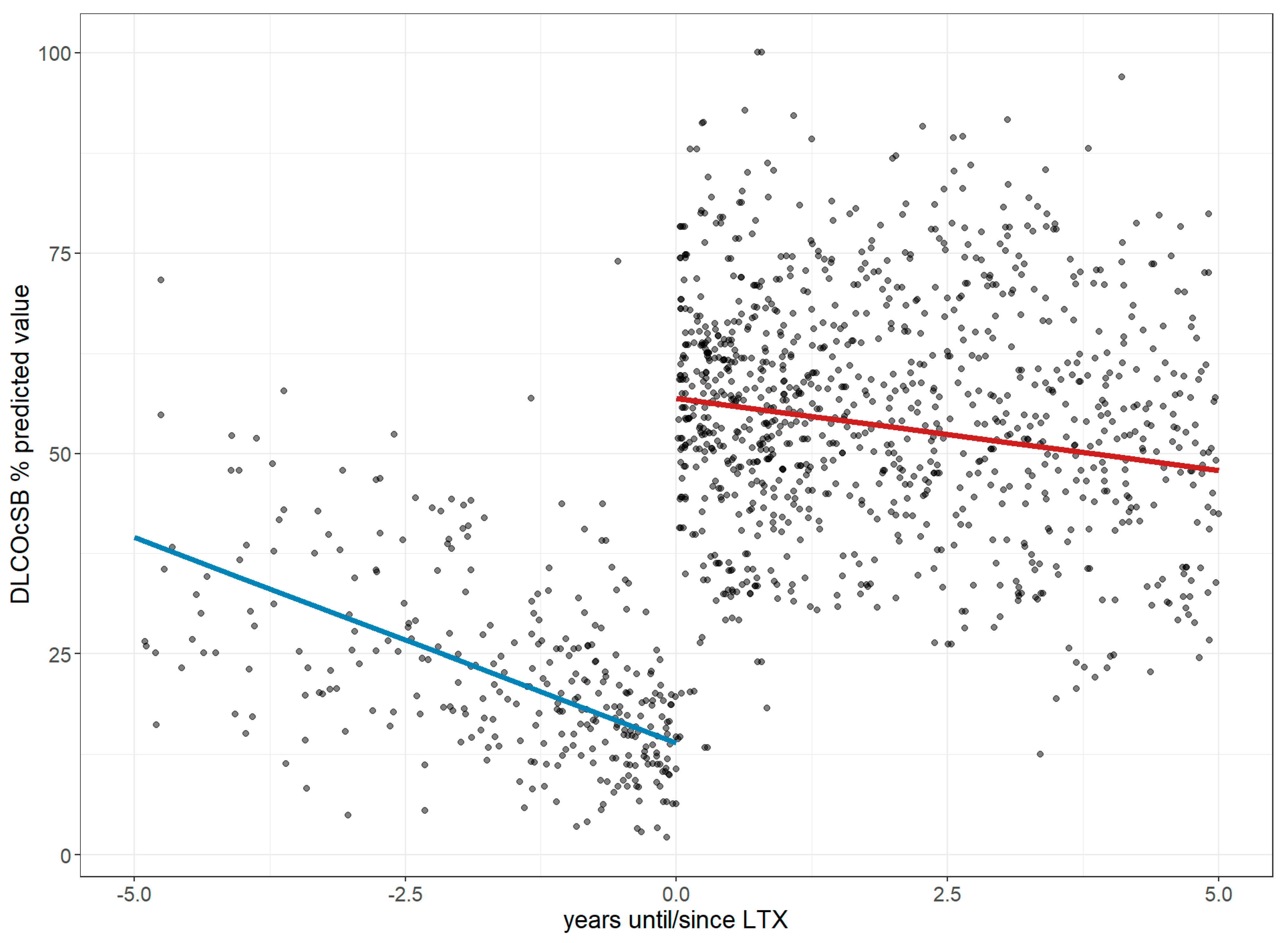
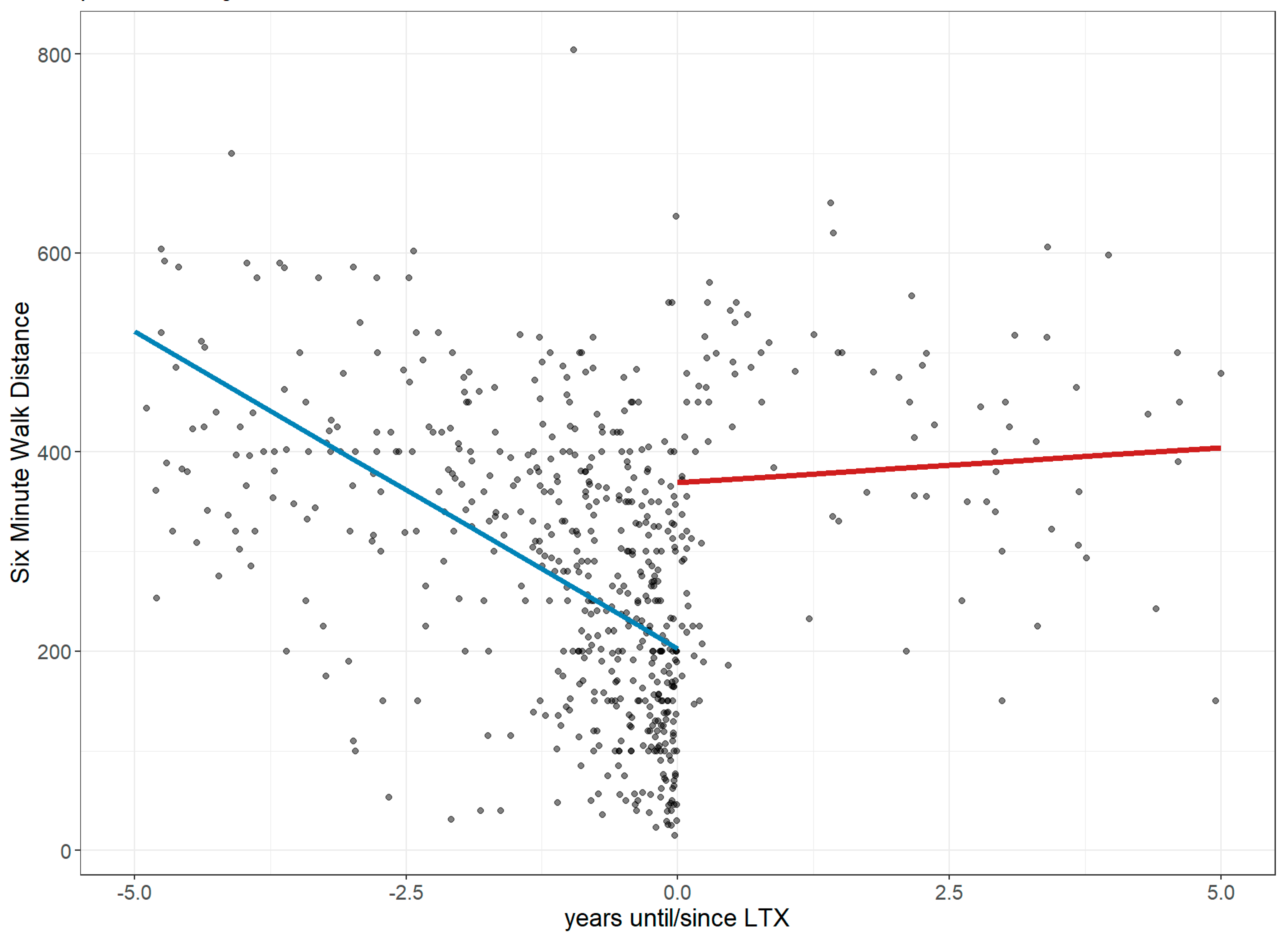
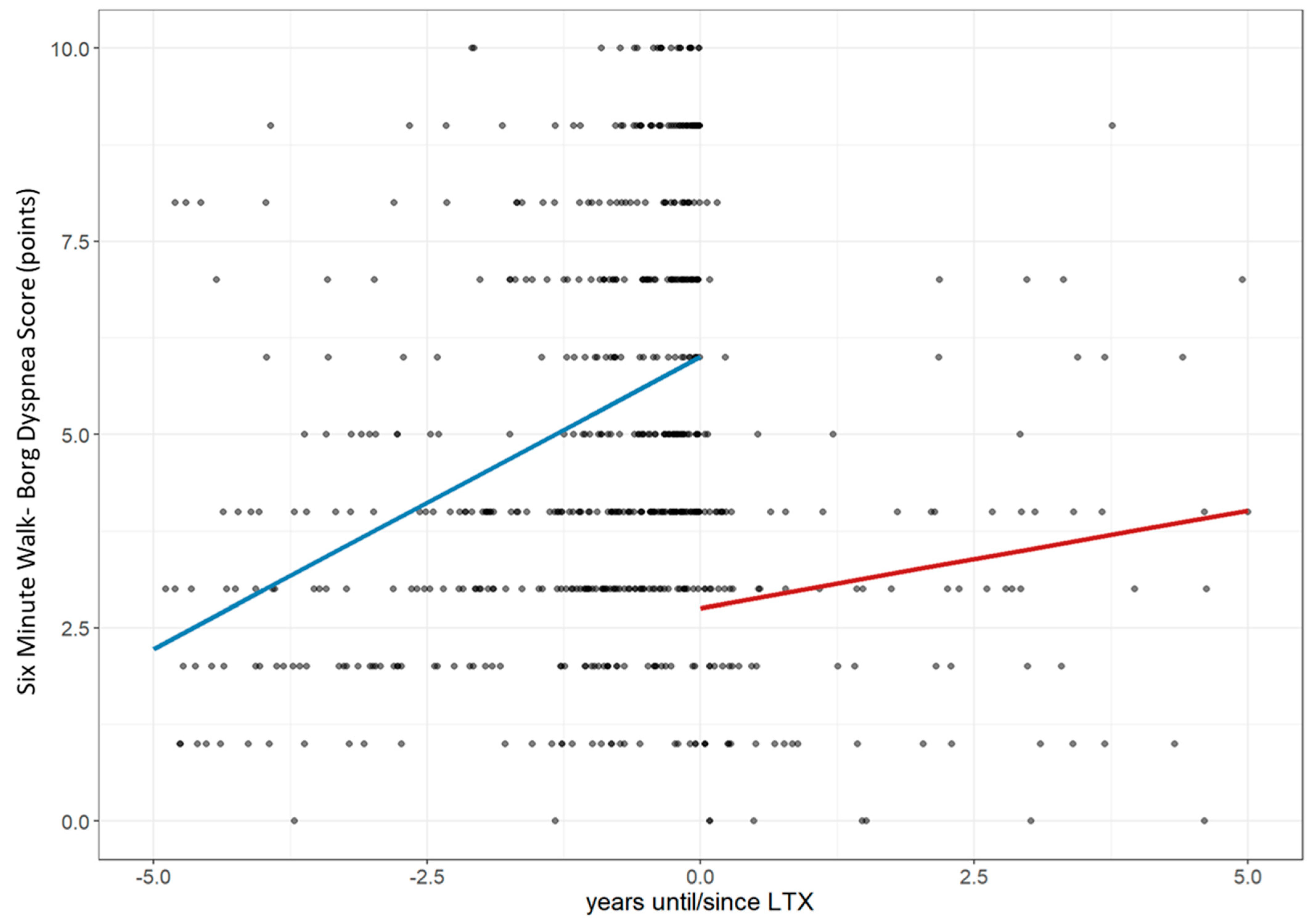
3.1. Lung Performance Improvement: Changes in FVC, Dlco, 6MWD, and Borg Score
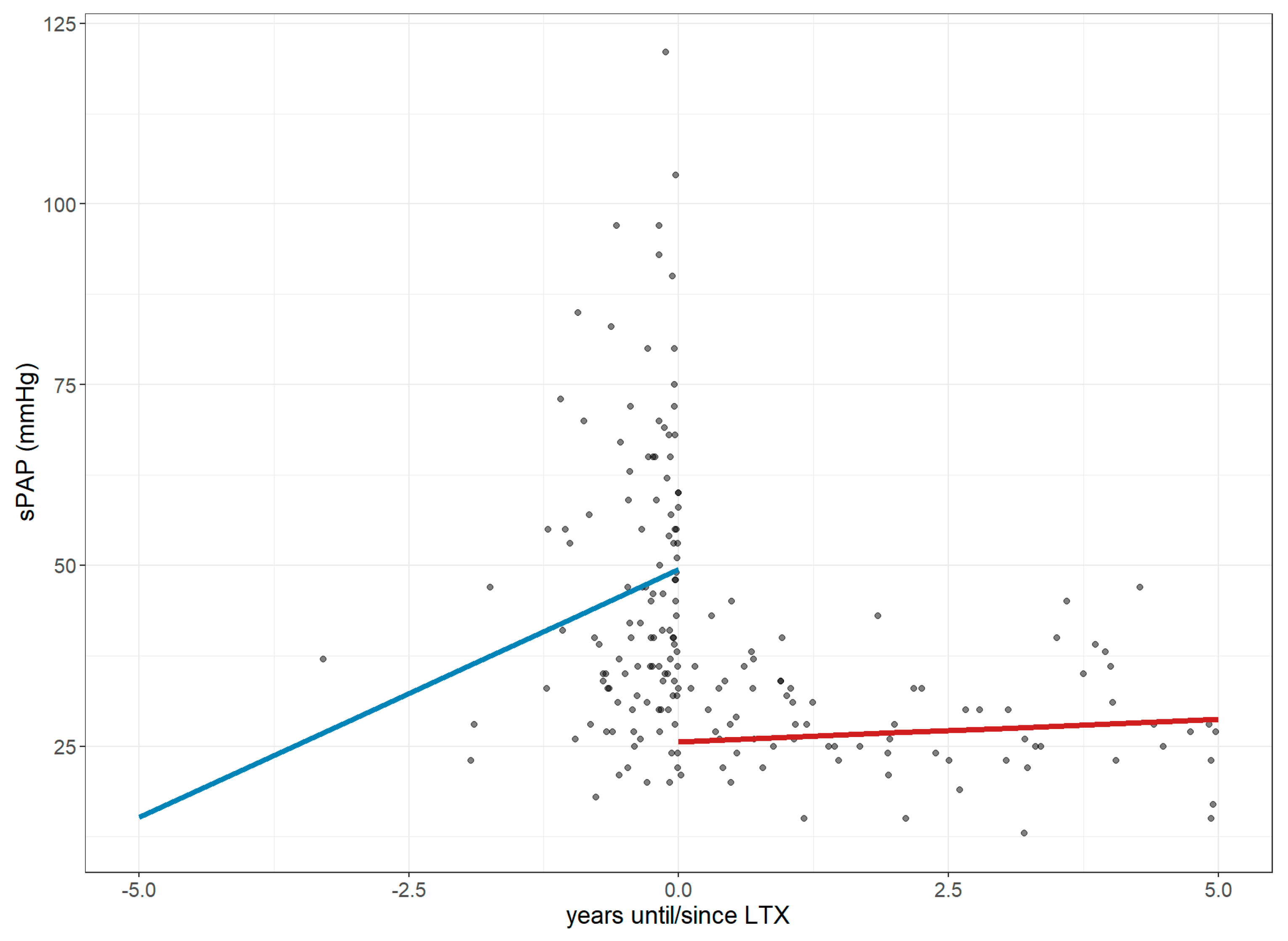
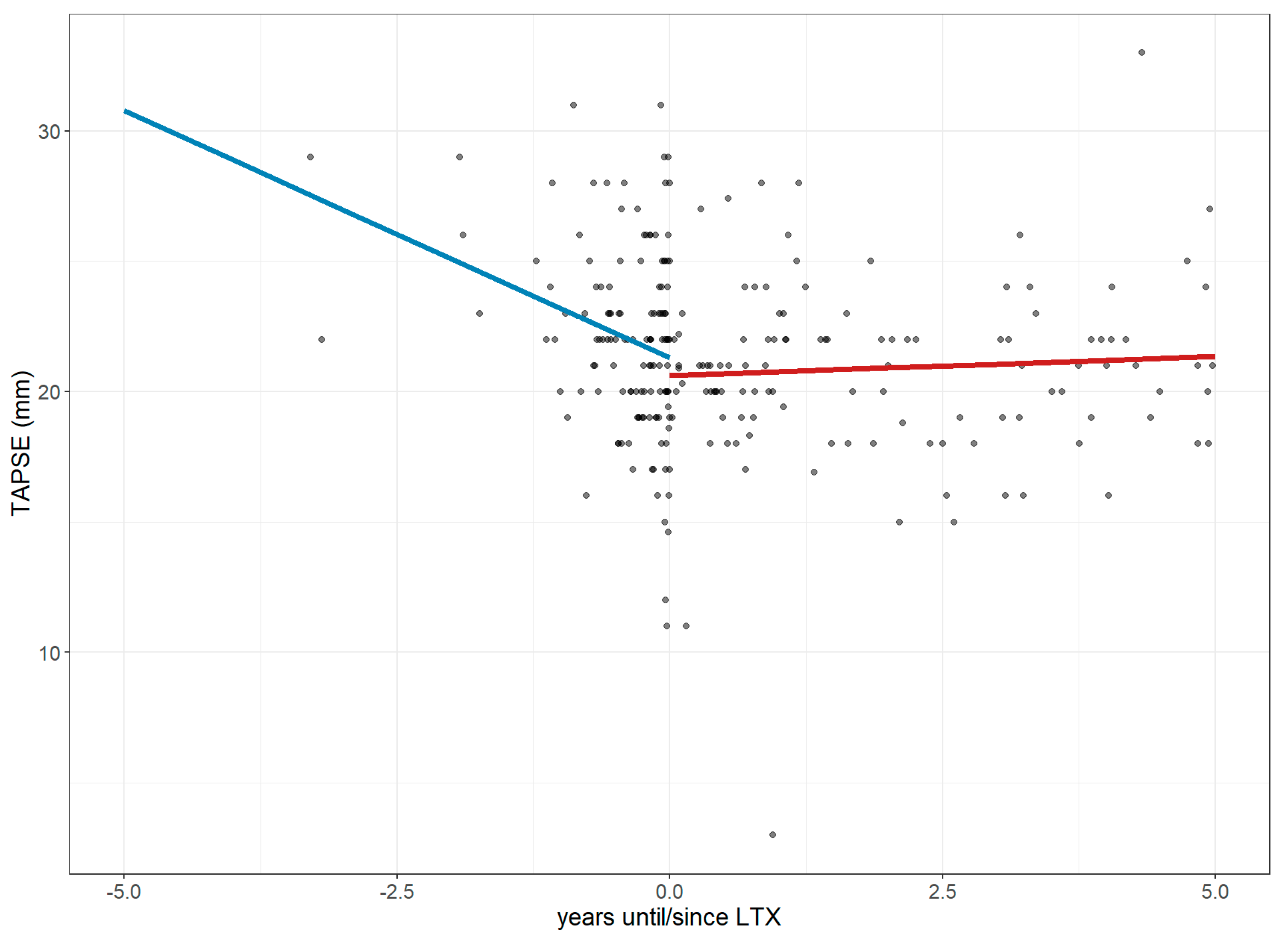
3.2. Changes in Right Ventricular Strain
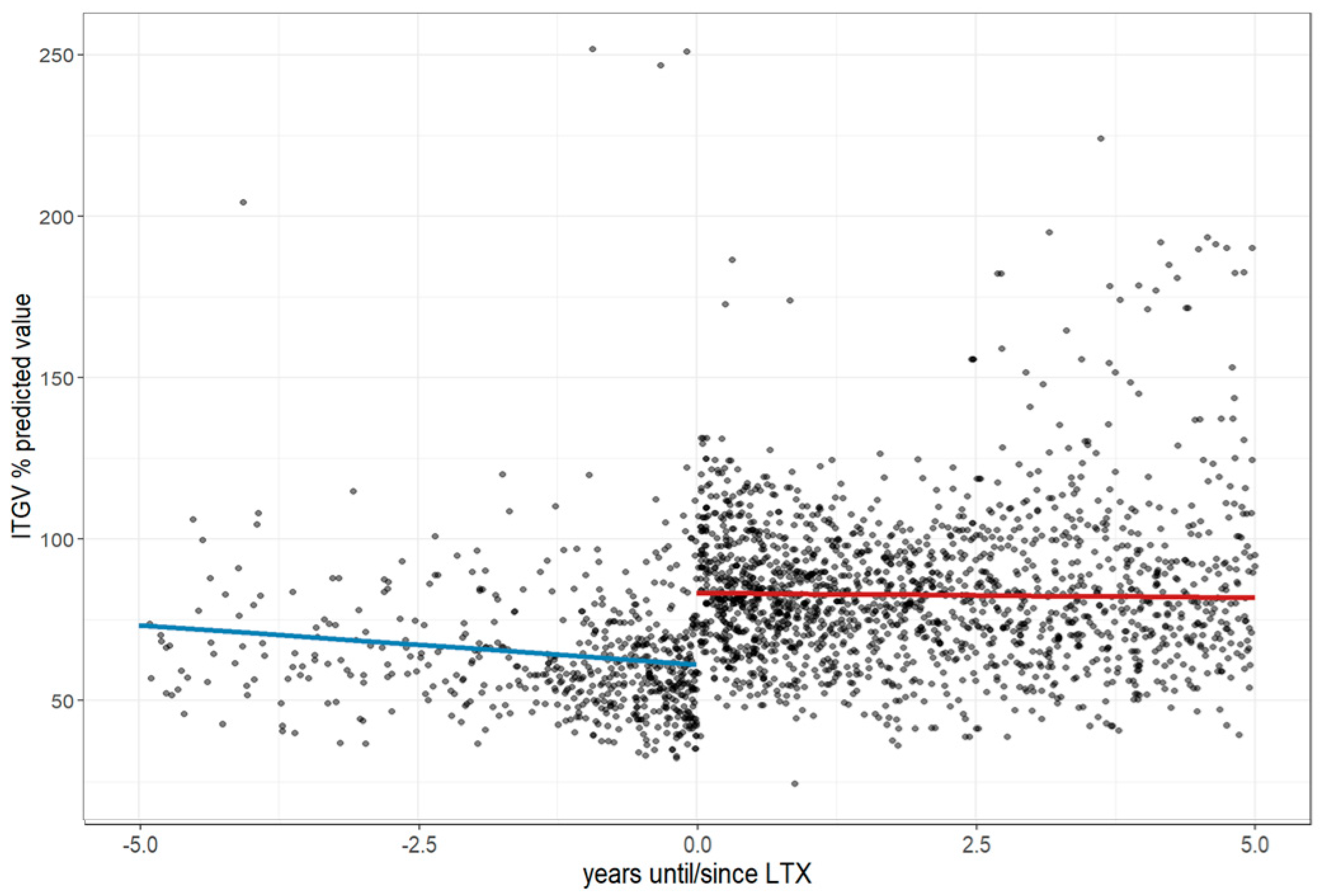
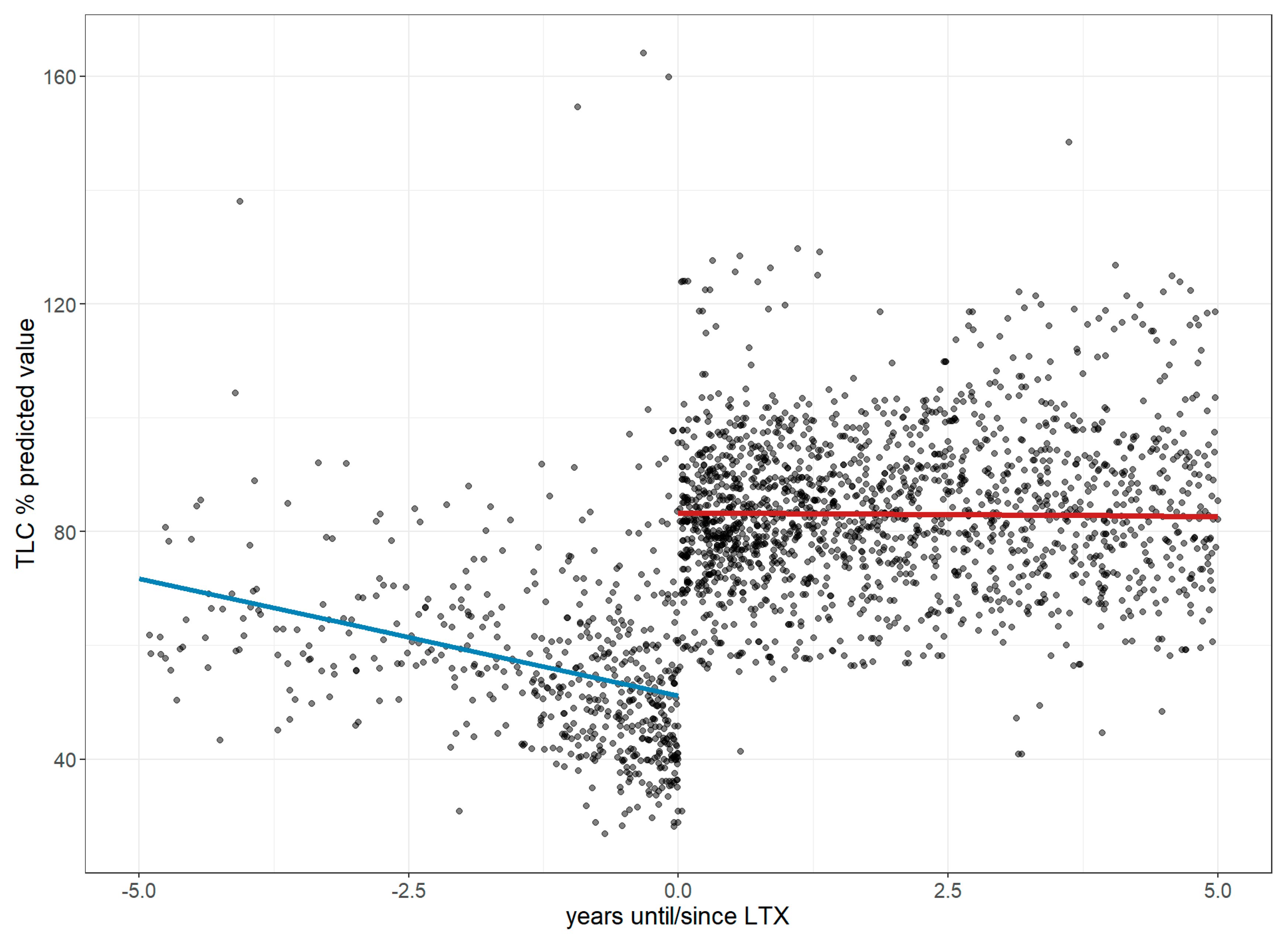
3.3. Further Changes in Pulmonary Function
3.4. Changes in Anthropometric Measures (Weight, Height, and BMI)
3.5. Changes in Laboratory Parameters
3.6. Changes in the CPS After Transplantation
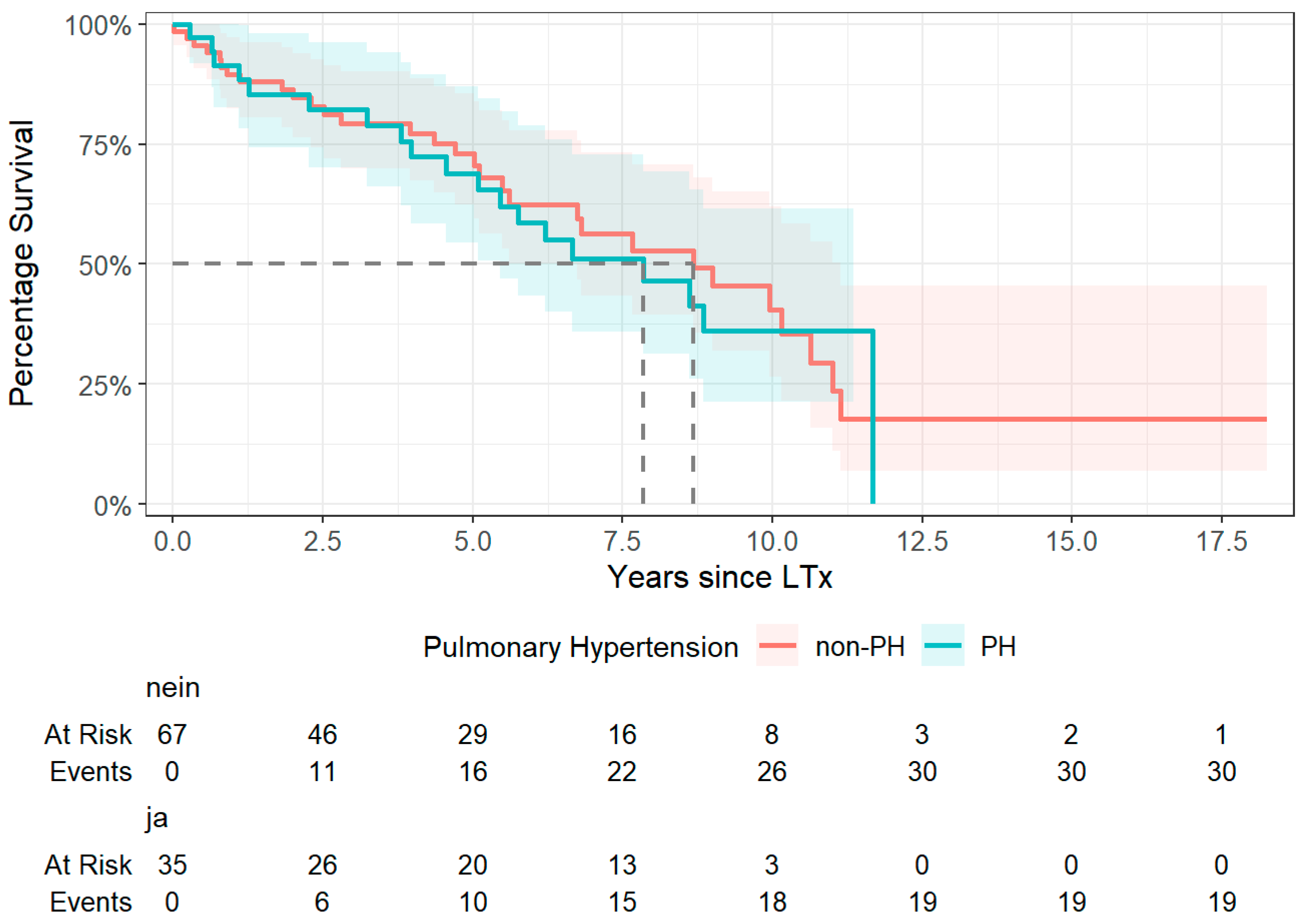
3.7. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behr, J.; Günther, A.; Bonella, F.; Dinkel, J.; Fink, L.; Geiser, T.; Geißler, K.; Gläser, S.; Handzhhiev, S.; Jonigk, D.; et al. S2K-Leitlinie zur Diagnostik der idiopathischen Lungenfibrose. Pneumologie 2020, 74, 263–293. [Google Scholar] [CrossRef]
- Dartsch, R.C.; Fink, L.; Breithecker, A.; Markart, P.; Tello, S.; Seeger, W.; Günther, A. Chronisch-fibrosierende Lungenerkrankungen: Die idiopathische pulmonale Fibrose im Spiegel ihrer Differenzialdiagnosen. Internist 2019, 60, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.K.; Cottin, V.; Dhar, R.; Danoff, S.; Flaherty, K.R.; Brown, K.K.; Mohan, A.; Renzoni, E.; Mohan, M.; Udwadia, Z.; et al. Progressive pulmonary fibrosis: An expert group consensus statement. Eur. Respir. J. 2023, 61, 2103187. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.W.; Ryerson, C.J.; Guler, S.A. Progression of fibrosing interstitial lung disease. Respir. Res. 2020, 21, 32. [Google Scholar] [CrossRef]
- Tello, S.; Krauss, E.; Günther, A.; Mahavadi, P. Medikamentöse Therapie der Lungenfibrose: Gestern, heute und morgen. Atemwegs- und Lungenkrankh. 2024, 50, 556–570. [Google Scholar] [CrossRef]
- De Oliveira, N.C.; Julliard, W.; Osaki, S.; Maloney, J.D.; Cornwell, R.D.; Sonetti, D.A.; Meyer, K.C. Lung transplantation for high-risk patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2016, 33, 235–241. Available online: https://www.test.mattioli1885journals.com/index.php/sarcoidosis/article/view/4742 (accessed on 19 August 2025).
- Paterniti, M.O.; Bi, Y.; Rekić, D.; Wang, Y.; Karimi-Shah, B.A.; Chowdhury, B.A. Acute Exacerbation and Decline in Forced Vital Capacity Are Associated with Increased Mortality in Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2017, 14, 1395–1402. [Google Scholar] [CrossRef]
- Brown, A.W.; Kaya, H.; Nathan, S.D. Lung transplantation in IIP: A review. Respirology 2016, 21, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Arjuna, A.; Olson, M.T.; Walia, R. Current trends in candidate selection, contraindications, and indications for lung transplantation. J. Thorac. Dis. 2021, 13, 6514–6527. [Google Scholar] [CrossRef]
- Leard, L.E.; Holm, A.M.; Valapour, M.; Glanville, A.R.; Attawar, S.; Aversa, M.; Campos, S.V.; Christon, L.M.; Cypel, M.; Dellgren, G.; et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2021, 40, 1349–1379. [Google Scholar] [CrossRef]
- Deri, O.; Ovadia, D.; Huszti, E.; Peled, M.; Saute, M.; Hod, T.; Onn, A.; Seluk, L.; Furie, N.; Shafran, I.; et al. Referral rates and barriers to lung transplantation based on pulmonary function criteria in interstitial lung diseases: A retrospective cohort study. Ther. Adv. Respir. Dis. 2024, 18, 17534666231221750. [Google Scholar] [CrossRef]
- Kapnadak, S.G.; Raghu, G. Lung transplantation for interstitial lung disease. Eur. Respir. Rev. 2021, 30, 210017. [Google Scholar] [CrossRef]
- Michel, S.G.; Hagl, C.; Kauke, T.; Kneidinger, N.; Schneider, C. Lungentransplantation: Aktueller Stand und Entwicklungen. Die Chir. 2024, 95, 108–114. [Google Scholar] [CrossRef]
- Tolentino, J.; Stoltzfus, J.; Harris, R.; Mazza, A.; Foltz, D.; Deringer, P.; Sakran, J.; Menak, R.; Mira, A.-E.; Nguyen, M.; et al. Comorbidity-polypharmacy score predicts readmissions and in-hospital mortality: A six-hospital health network experience. J. Basic Clin. Pharm. 2017, in press. [Google Scholar]
- Günther, A. eurILDreg—Portal of Medical Data Models. 2024. Available online: https://medical-data-models.org/46015 (accessed on 2 September 2025).
- Guenther, A.; Krauss, E.; Tello, S.; Wagner, J.; Paul, B.; Kuhn, S.; Maurer, O.; Heinemann, S.; Costabel, U.; Barbero, M.A.N.; et al. The European IPF registry (eurIPFreg): Baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 141. [Google Scholar] [CrossRef]
- Krauss, E.; Tello, S.; Naumann, J.; Wobisch, S.; Ruppert, C.; Kuhn, S.; Mahavadi, P.; Majeed, R.W.; Bonniaud, P.; Molina-Molina, M.; et al. Protocol and research program of the European registry and biobank for interstitial lung diseases (eurILDreg). BMC Pulm. Med. 2024, 24, 572. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Shaver, C.M.; Steele, M.P.; Robbins, I.M. Decline in DLCO Precedes Decline in FEV1 or FVC After Lung Transplantation. J. Heart Lung Transplant. 2017, 36, S402–S403. [Google Scholar] [CrossRef]
- Alshimali, H.; Coppolino, A.; Keshk, M.A.; Young, J.S.; Itoh, A.; Goldberg, H.J.; Sharma, N.S.; Mallidi, H.R. Heart recovery and reverse remodeling following lung transplant in pulmonary artery hypertension. Cardiothorac. Surg. 2022, 30, 20. [Google Scholar] [CrossRef]
- Hayes, D.; Higgins, R.S.; Black, S.M.; Wehr, A.M.; Lehman, A.M.; Kirkby, S.; Whitson, B.A. Effect of pulmonary hypertension on survival in patients with idiopathic pulmonary fibrosis after lung transplantation: An analysis of the United Network of Organ Sharing registry. J. Heart Lung Transplant. 2015, 34, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Jardel, S.; Reynaud, Q.; Durieu, I. Long-term extrapulmonary comorbidities after lung transplantation in cystic fibrosis: Update of specificities. Clin. Transplant. 2018, 32, e13269. [Google Scholar] [CrossRef]
- Malouf, M.A.; Milliken, S.; Glanville, A.R. Successful management of profound thrombocytopenia after lung and heart-lung transplantation. J. Heart Lung Transplant. 2000, 19, 612–614. [Google Scholar] [CrossRef]
- Silhan, L.L.; Shah, P.D.; Chambers, D.C.; Snyder, L.D.; Riise, G.C.; Wagner, C.L.; Hellström-Lindberg, E.; Orens, J.B.; Mewton, J.F.; Danoff, S.K.; et al. Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. Eur. Respir. J. 2014, 44, 178–187. [Google Scholar] [CrossRef]
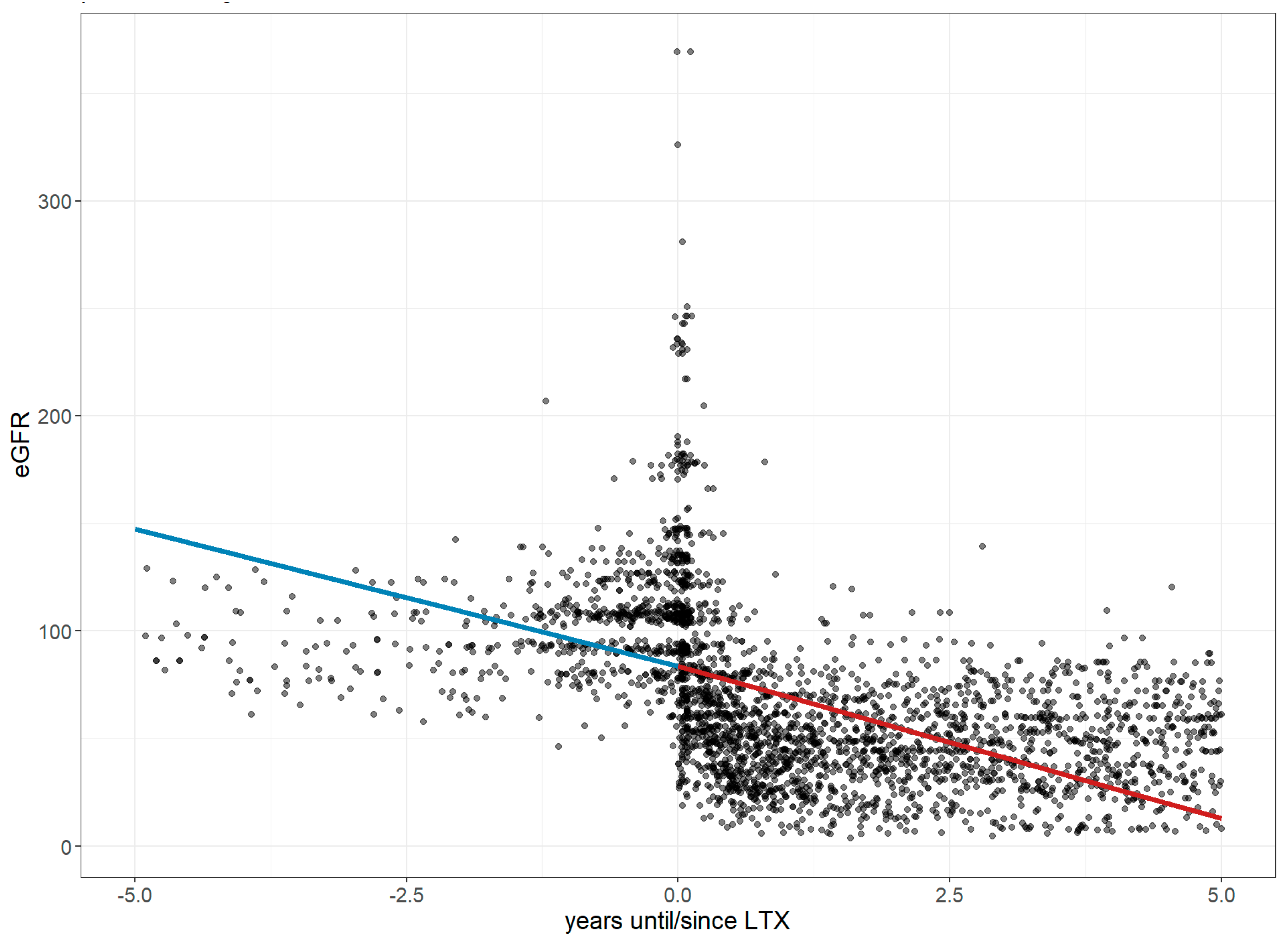
- Hornum, M.; Houlind, M.B.; Iversen, E.; Porrini, E.; Luis-Lima, S.; Oturai, P.; Iversen, M.; Bredahl, P.; Carlsen, J.; Møller, C.H.; et al. Estimating Renal Function Following Lung Transplantation. J. Clin. Med. 2022, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Heid, C.; Khoury, M.; Liu, C.; Maaraoui, K.; Kalsbeek, A.; Hackmann, A.; Wait, M.; Ring, W.S.; Huffman, L.C.; Peltz, M. Renal Decline Following Lung Transplant. J. Heart Lung Transplant. 2021, 40, S311. [Google Scholar] [CrossRef]
- Zapata, C.M.; Ibrahim, H.N. Kidney Disease after Heart and Lung Transplantation. Methodist Debakey Cardiovasc. J. 2022, 18, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Grins, E.; Wijk, J.; Bjursten, H.; Zeaiter, M.; Lindstedt, S.; Dellgren, G.; Ederoth, P.; Lannemyr, L. Acute kidney injury after lung transplantation, incidence, risk factors, and effects: A Swedish nationwide study. Acta Anaesthesiol. Scand. 2025, 69, e70014. [Google Scholar] [CrossRef]
- Lee, N.; Ying, H. Occurrence rate and risk factors for acute kidney injury after lung transplantation: A systematic review and meta-analysis. PeerJ 2025, 13, e18364. [Google Scholar] [CrossRef]




| Parameter | Pre-LTx Change per Year | Estimate Values at LTx | Post-LTx Change per Year | p-Value |
|---|---|---|---|---|
| Hematologic Parameters | ||||
| Erythrocyte count (×1012/L) | −0.38 | 3.91 | +0.09 | <0.001 |
| Leukocyte count (×109/L) | −0.75 | 7.81 | −0.34 | <0.001 |
| Thrombocyte count (×109/L) | +4.09 | 260.07 | −17.79 | <0.001 |
| Inflammatory Markers | ||||
| C-reactive protein (mg/L) | +3.33 | 19.16 | −1.13 | 0.002 |
| Liver Function Markers | ||||
| Total protein (g/L) | −5.49 | 63.86 | +0.45 | <0.001 |
| Albumin (g/L) | −2.10 | 37.37 | +1.19 | <0.001 |
| GGT (U/L) | +23.33 | 96.5 | +5.14 | <0.001 |
| GOT (U/L) | +0.88 | 25.05 | +1.97 | <0.001 |
| GPT (U/L) | −0.34 | 23.81 | +4.86 | 0.38 |
| LDH (U/L) | +24.87 | 320 | −12.53 | <0.001 |
| Total bilirubin (mg/dL) | +0.13 | 0.77 | +0.17 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhnert, S.; Wilke, L.; Sommerlad, J.; Tello, S.; Yogeswaran, A.; Husain-Syed, F.; Windhorst, A.; Guenther, A.; Hecker, M.; Krauss, E. Dual Impacts of Lung Transplantation on the Recovery and Comorbidity of Interstitial Lung Diseases: A Longitudinal Assessment of the Benefits and Burden. J. Clin. Med. 2025, 14, 6420. https://doi.org/10.3390/jcm14186420
Kuhnert S, Wilke L, Sommerlad J, Tello S, Yogeswaran A, Husain-Syed F, Windhorst A, Guenther A, Hecker M, Krauss E. Dual Impacts of Lung Transplantation on the Recovery and Comorbidity of Interstitial Lung Diseases: A Longitudinal Assessment of the Benefits and Burden. Journal of Clinical Medicine. 2025; 14(18):6420. https://doi.org/10.3390/jcm14186420
Chicago/Turabian StyleKuhnert, Stefan, Luise Wilke, Janine Sommerlad, Silke Tello, Athiththan Yogeswaran, Faeq Husain-Syed, Anita Windhorst, Andreas Guenther, Matthias Hecker, and Ekaterina Krauss. 2025. "Dual Impacts of Lung Transplantation on the Recovery and Comorbidity of Interstitial Lung Diseases: A Longitudinal Assessment of the Benefits and Burden" Journal of Clinical Medicine 14, no. 18: 6420. https://doi.org/10.3390/jcm14186420
APA StyleKuhnert, S., Wilke, L., Sommerlad, J., Tello, S., Yogeswaran, A., Husain-Syed, F., Windhorst, A., Guenther, A., Hecker, M., & Krauss, E. (2025). Dual Impacts of Lung Transplantation on the Recovery and Comorbidity of Interstitial Lung Diseases: A Longitudinal Assessment of the Benefits and Burden. Journal of Clinical Medicine, 14(18), 6420. https://doi.org/10.3390/jcm14186420






