Artificial Intelligence in Nuclear Cardiology
Abstract
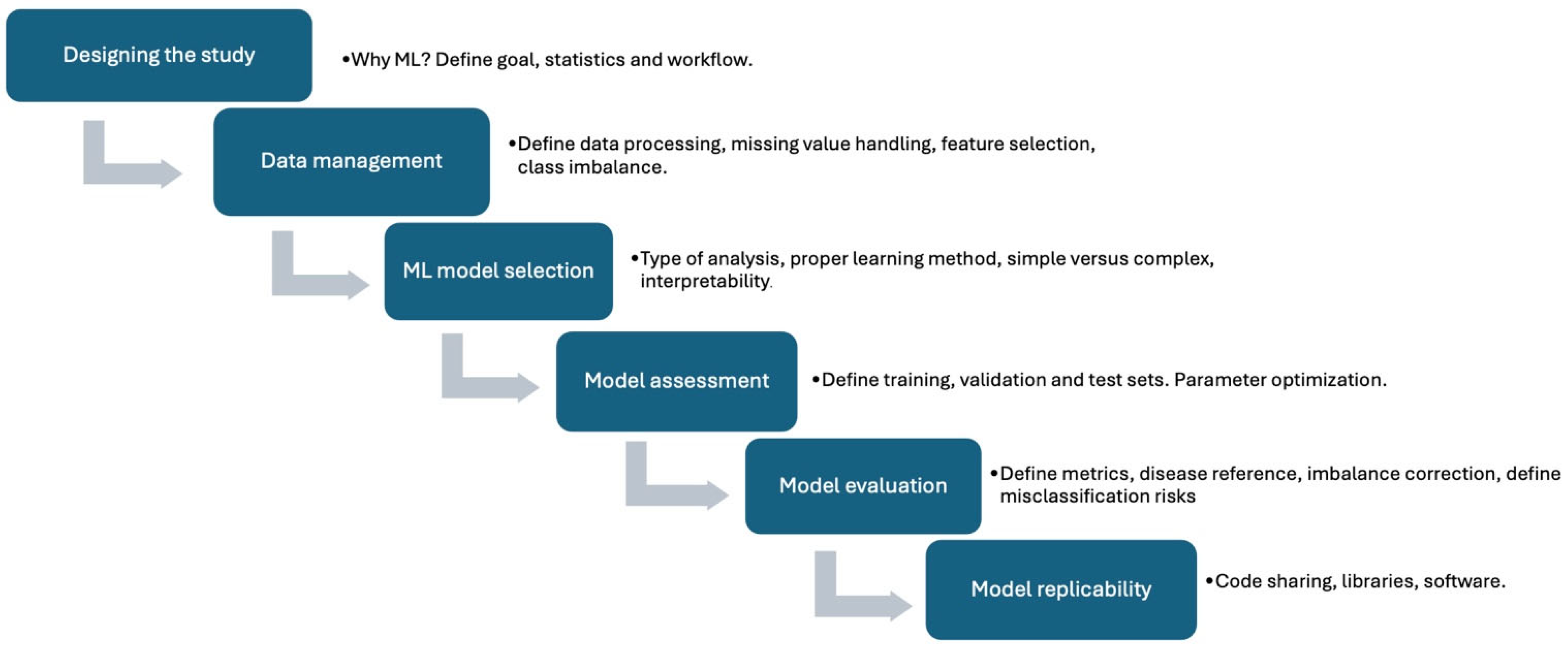
1. Introduction: Artificial Intelligence in Medicine
2. AI in Nuclear Cardiology
2.1. General Considerations
2.2. Historical Perspective
2.3. ML and DL: Diagnosis of CAD
2.4. ML and DL: Prognosis of CAD
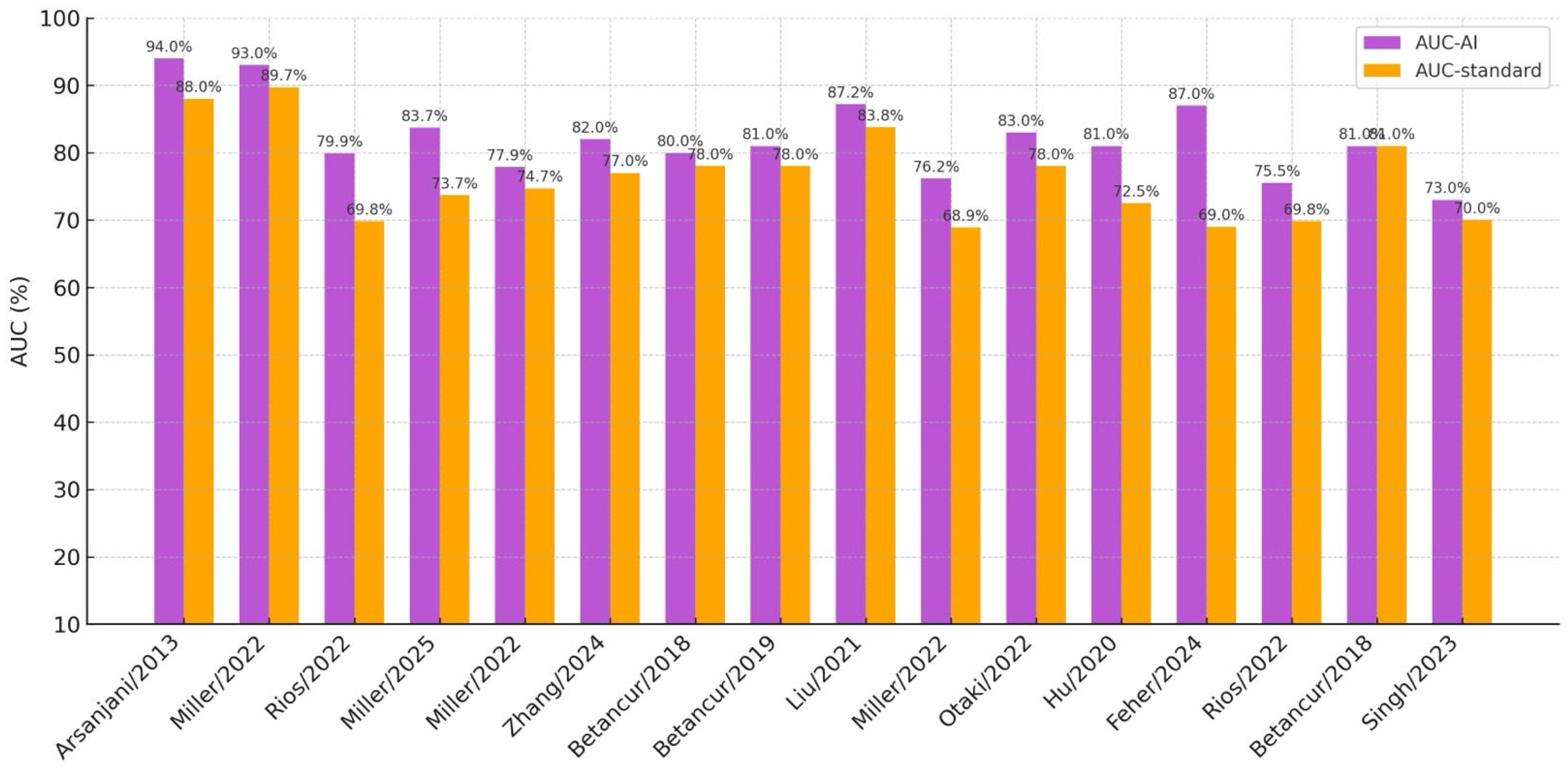
| Ref. | Main Author/Year | AI Method | Input Data Type | Dataset Size | Accuracy | AUC | REFINE | Study Purpose | Reference Standard |
|---|---|---|---|---|---|---|---|---|---|
| [26] | Arsanjani/2013 | LogitBoost | SPECT MPI * | 1181 | 87.3% | 0.94 | no | Diagnostic | TPD and expert reading |
| [27] | Berkaya/2020 | DL-based and knowledge- based models. | SPECT MPI | 192 | 94% (DL-based); 93% (K-based) | n.r. | no | Diagnostic | Expert reading |
| [28] | Apostolopoulos/2021 | CNN + RF | SPECT MPI * | 566 | 78,44% | 0.7926 | no | Diagnostic | ICA + expert reading |
| [29] | de Souza Filho/2021 | ML ensemble (AB, GB, RF, XGB) | SPECT MPI | 1007 | 93.8% RF | 0.853 RF | no | Diagnostic | n.r. |
| [30] | Miller/2022 | Grad-CAM | SPECT MPI * | 828 training, 511 test | n.r. | 0.93 | yes | Diagnostic | uTPD and sTPD |
| [31] | Rios/2022 | XGBoost, RF | SPECT MPI * | 20,179 | n.r. | 0.799 | yes | Prognostic | Stress TPD and expert reading |
| [35] | Miller/2025 | TPD-DL and SSS-DL | SPECT MPI | 555 | n.r. | 0.837 | yes | Diagnostic | Stress TPD and SSS |
| [36] | Miller/2022 | CAD-DL | SPECT MPI * | 240 patients | n.r. | 0.779 | no | Diagnostic | Expert reading and stress TPD |
| [37] | Zhang/2024 | DL | SPECT MPI | 1038 | 88.7% | 0.82 | no | Diagnostic | Expert reading |
| [40] | Betancur/2018 | CNN | SPECT MPI | 1638 | n.r. | 0.80 | yes | Diagnostic | Stress TPD |
| [41] | Betancur/2019 | CNN | SPECT MPI | 1160 | n.r. | 0.81 | yes | Diagnostic | cTPD |
| [42] | Liu/2021 | CNN | SPECT MPI | 37,243 | 82.7% | 0.872 | no | Diagnostic | Quantitative perfusion defect size |
| [43] | Miller/2022 | XGBoost | SPECT MPI * | 20,418 train, 9019 test | n.r. | 0.762 | yes | Prognostic | Clinical CAD consortium |
| [44] | Otaki/2022 | CAD-DL | SPECT MPI * | 3578 | n.r. | 0.83 | yes | Diagnostic | Stress TPD and expert reading |
| [45] | Hu/2020 | LogitBoost (ensemble ML) | SPECT MPI * | 1980 | n.r. | 0.81 | yes | Prognostic | Stress TPD and ischemic TPD |
| [46] | Feher/2024 | XGBoost | SPECT MPI * | 4766 train, 2912 test | n.r. | 0.87 | yes | Prognostic | Stress TPD and stress LVEF |
| [47] | Rios/2022 | Multiple ML | SPECT MPI * | 20,414 train, 2984 test | n.r. | 0.755 | yes | Prognostic | Stress TPD and expert reading |
| [48] | Betancur/2018 | Boosted ensemble | SPECT MPI * | 2619 | n.r. | 0.81 | no | Prognostic | Stress TPD and expert reading |
| [49] | Singh/2023 | HARD MACE-DL | SPECT MPI * | 20,418 train, 9019 test | n.r. | 0.73 | yes | Prognostic | Logistic regression model and stress TPD |
| [50] | Pieszko/2023 | Time-to-event DL | SPECT MPI *s | 20,418 train, 13,988 test | n.r. | 0.76 for ACS, 0.78 all-cause death | yes | Prognostic | n.r. |
| [53] | Juarez-Orozco/2020 | LogitBoost (ensemble ML) | 13N-PET * | 1234 | n.r. | 0.72 (ischemia), 0.71 (MACE) | no | Diagnostic and Prognostic | Logistic regression and SCORE risk model |
| [54] | Berman/2024 | Multiple ML | 82Rb-PET | 3245 | n.r. | 0.95 | no | Diagnostic | Standard LR |
| [55] | Juarez-Orozco/2020 | ResNet50 | 13N-PET | 1185 | n.r. | 0.90 | no | Prognostic | Integrated model |
| [56] | Singh/2022 | Grad-CAM | 82Rb-PET | 4735 | n.r. | 0.82 | no | Prognostic | Ischemia, MFR, logistic regression |
| [57] | Kwiecinski/2022 | XGBoost | 18F-NaF PET * | 293 | n.r. | 0.85 | no | Prognostic | Quantitative plaque analysis |
2.5. ML and DL: AI Applications Beyond MPI
3. Final Remarks
3.1. The Current Status
3.2. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ATTR | Transthyretin Amyloidosis |
| AUC | Area Under Curve |
| CAD | Coronary Artery Disease |
| CNN | Convolutional Neural Network |
| CT | Computed Tomography |
| CZT | Cadmium-Zinc-Telluride |
| DL | Deep Learning |
| MACE | Major Adverse Cardiac Event |
| ML | Machine Learning |
| MPI | Myocardial Perfusion Imaging |
| MRI | Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| SDS | Summed Difference Score |
| SPECT | Single-Photon Emission Computed Tomography |
| SSS | Summed Stress Score |
| TPD | Total Perfusion Defect |
References
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Jiang, F.; Jiang, Y.; Zhi, H.; Dong, Y.; Li, H.; Ma, S.; Wang, Y.; Dong, Q.; Shen, H.; Wang, Y. Artificial intelligence in healthcare: Past, present and future. Stroke Vasc. Neurol. 2017, 2, 230–243. [Google Scholar] [CrossRef]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Kufel, J.; Bargieł-Łączek, K.; Kocot, S.; Koźlik, M.; Bartnikowska, W.; Janik, M.; Czogalik, Ł.; Dudek, P.; Magiera, M.; Lis, A.; et al. What Is Machine Learning, Artificial Neural Networks and Deep Learning?—Examples of Practical Applications in Medicine. Diagnostics 2023, 13, 2582. [Google Scholar] [CrossRef]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: New York, NY, USA, 2006. [Google Scholar]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef]
- Sokolova, M.; Lapalme, G. A systematic analysis of performance measures for classification tasks. Inf. Process Manag. 2009, 45, 427–437. [Google Scholar] [CrossRef]
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.L. Machine Learning for Medical Imaging. Radiographics 2017, 37, 505–515. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Irvin, J.; Ball, R.L.; Zhu, K.; Yang, B.; Mehta, H.; Duan, T.; Ding, D.; Bagul, A.; Langlotz, C.P.; et al. Deep learning for chest radiograph diagnosis: A retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med. 2018, 15, e1002686. [Google Scholar] [CrossRef]
- Krittanawong, C.; Johnson, K.W.; Rosenson, R.S.; Wang, Z.; Aydar, M.; Baber, U.; Min, J.K.; Tang, W.H.W.; Halperin, J.L.; Narayan, S.M. Deep learning for cardiovascular medicine: A practical primer. Eur. Heart J. 2019, 40, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; He, B.; Ghorbani, A.; Yuan, N.; Ebinger, J.; Langlotz, C.P.; Heidenreich, P.A.; Harrington, R.A.; Liang, D.H.; Ashley, E.A.; et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature 2020, 580, 252–256. [Google Scholar] [CrossRef] [PubMed]
- van Assen, M.; Muscogiuri, G.; Caruso, D.; Lee, S.J.; Laghi, A.; De Cecco, C.N. Artificial intelligence in cardiac radiology. Radiol. Med. 2020, 125, 186–1199. [Google Scholar] [CrossRef] [PubMed]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory ECG using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef]
- Ribeiro, A.H.; Ribeiro, M.H.; Paixão, G.M.M.; Oliveira, D.M.; Gomes, P.R.; Canazart, J.A.; Ferreira, M.P.S.; Andersson, C.R.; Macfarlane, P.W.; Meira, W.; et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat. Commun. 2020, 11, 1760. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Shrestha, S.; Berthon, B.; Messas, E.; Donal, E.; Tison, G.H.; Min, J.K.; D’hooge, J.; Voigt, J.U.; Dudley, J.; et al. Proposed Requirements for cardiovascular Imaging-Related machine learning Evaluation (PRIME): A checklist: Reviewed by the American College of Cardiology Healthcare Innovation Council. JACC Cardiovasc. Imaging 2020, 13, 2017–2035. [Google Scholar] [CrossRef]
- Slart, R.H.J.A.; Williams, M.C.; Juarez-Orozco, L.E.; Rischpler, C.; Dweck, M.R.; Glaudemans, A.W.J.M.; Gimelli, A.; Georgoulias, P.; Gheysens, O.; Gaemperli, O.; et al. Position paper of the EACVI and EANM on artificial intelligence applications in multimodality cardiovascular imaging using SPECT/CT, PET/CT, and cardiac CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1399–1413. [Google Scholar] [CrossRef]
- Fujita, H.; Katafuchi, T.; Uehara, T.; Nishimura, T. Application of artificial neural network to computer-aided diagnosis of coronary artery disease in myocardial SPECT bull’s-eye images. J. Nucl. Med. 1992, 33, 272–276. [Google Scholar]
- Khorsand, A.; Haddad, M.; Graf, S.; Moertl, D.; Sochor, H.; Porenta, G. Automated assessment of dipyridamole 201Tl myocardial SPECT perfusion scintigraphy by case-based reasoning. J. Nucl. Med. 2001, 42, 189–193. [Google Scholar] [PubMed]
- Garcia, E.V.; Cooke, C.D.; Folks, R.D.; Santana, C.A.; Krawczynska, E.G.; De Braal, L.; Ezquerra, N.F. Diagnostic performance of an expert system for the interpretation of myocardial perfusion SPECT studies. J. Nucl. Med. 2001, 42, 1185–1191. [Google Scholar]
- Garcia, E.V.; Klein, J.L.; Moncayo, V.; Cooke, C.D.; Del’aUne, C.; Folks, R.; Moreiras, L.V.; Esteves, F. Diagnostic performance of an artificial intelligence-driven cardiac-structured reporting system for myocardial perfusion SPECT imaging. J. Nucl. Cardiol. 2020, 27, 1652–1664. [Google Scholar] [CrossRef]
- Arsanjani, R.; Xu, Y.; Dey, D.; Vahistha, V.; Shalev, A.; Nakanishi, R.; Hayes, S.; Fish, M.; Berman, D.; Germano, G.; et al. Improved accuracy of myocardial perfusion SPECT for detection of coronary artery disease by machine learning in a large population. J. Nucl. Cardiol. 2013, 20, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Kaplan Berkaya, S.; Ak Sivrikoz, I.; Gunal, S. Classification models for SPECT myocardial perfusion imaging. Comput. Biol. Med. 2020, 123, 103893. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, I.D.; Apostolopoulos, D.I.; Spyridonidis, T.I.; Papathanasiou, N.D.; Panayiotakis, G.S. Multi-input deep learning approach for cardiovascular disease diagnosis using myocardial perfusion imaging and clinical data. Phys. Med. 2021, 84, 168–177. [Google Scholar] [CrossRef]
- de Souza Filho, E.M.; Fernandes, F.A.; Wiefels, C.; de Carvalho, L.N.D.; dos Santos, T.F.; dos Santos, A.A.S.M.D.; Mesquita, E.T.; Seixas, F.L.; Chow, B.J.W.; Mesquita, C.T.; et al. Machine learning algorithms to distinguish myocardial perfusion SPECT polar maps. Front. Cardiovasc. Med. 2021, 11, 741667. [Google Scholar] [CrossRef]
- Miller, R.J.H.; Singh, A.; Otaki, Y.; Tamarappoo, B.K.; Kavanagh, P.; Parekh, T.; Hu, L.-H.; Gransar, H.; Sharir, T.; Einstein, A.J.; et al. Mitigating bias in deep learning for diagnosis of coronary artery disease from myocardial perfusion SPECT images. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 387–397. [Google Scholar] [CrossRef]
- Rios, R.; Miller, R.J.H.; Manral, N.; Sharir, T.; Einstein, A.J.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.A.; Sinusas, A.J.; Miller, E.J.; et al. Handling missing values in machine learning to predict patient-specific risk of adverse cardiac events: Insights from REFINE SPECT registry. Comput. Biol. Med. 2022, 145, 105449. [Google Scholar] [CrossRef]
- Nakajima, K.; Maruyama, K. Nuclear cardiology data analyzed using machine learning. Ann. Nucl. Cardiol. 2022, 8, 80–85. [Google Scholar] [CrossRef]
- Chiba, A.; Kudo, T.; Ideguchi, R.; Altay, M.; Koga, S.; Yonekura, T.; Tsuneto, A.; Morikawa, M.; Ikeda, S.; Kawano, H.; et al. Usefulness of an artificial neural network for a beginner to achieve similar interpretations to an expert when examining myocardial perfusion images. Int. J. Cardiovasc. Imaging 2021, 37, 2337–2343. [Google Scholar] [CrossRef]
- Kiso, K.; Nakajima, K.; Nimura, Y.; Nishimura, T. A novel algorithm developed using machine learning and a J-ACCESS database can estimate defect scores from myocardial perfusion single-photon emission tomography images. Ann. Nucl. Med. 2024, 38, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.H.; Kavanagh, P.; Lemley, M.; Liang, J.X.; Sharir, T.; Einstein, A.J.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.A.; Sinusas, A.J.; et al. Artificial intelligence-enhanced perfusion scoring improves the diagnostic accuracy of myocardial perfusion imaging. J. Nucl. Med. 2025, 66, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.H.; Kuronuma, K.; Singh, A.; Otaki, Y.; Hayes, S.; Chareonthaitawee, P.; Kavanagh, P.; Parekh, T.; Tamarappoo, B.K.; Sharir, T.; et al. Explainable Deep Learning Improves Physician Interpretation of Myocardial Perfusion Imaging. J. Nucl. Med. 2022, 63, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bos, E.; Clarkin, O.; Wilson, T.; Small, G.R.; Wells, R.G.; Lu, L.; Chow, B.J. Interpretation of SPECT wall motion with deep learning. J. Nucl. Cardiol. 2024, 37, 101881. [Google Scholar] [CrossRef]
- Slomka, P.J.; Betancur, J.; Liang, J.X.; Otaki, Y.; Hu, L.-H.; Sharir, T.; Dorbala, S.; Di Carli, M.; Fish, M.B.; Ruddy, T.D.; et al. Rationale and design of the REgistry of Fast Myocardial Perfusion Imaging with NExt generation SPECT (REFINE SPECT). J. Nucl. Cardiol. 2020, 27, 1010–1021. [Google Scholar] [CrossRef]
- Miller, R.J.H.; Lemley, M.; Shanbhag, A.; Ramirez, G.; Liang, J.X.; Builoff, V.; Kavanagh, P.; Sharir, T.; Hauser, M.T.; Ruddy, T.D.; et al. The updated Registry of fast myocardial perfusion imaging with next-generation SPECT (REFINE SPECT 2.0). J. Nucl. Med. 2024, 65, 1795–1801. [Google Scholar] [CrossRef]
- Betancur, J.; Commandeur, F.; Motlagh, M.; Sharir, T.; Einstein, A.J.; Bokhari, S.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.; Sinusas, A.J.; et al. Deep learning for prediction of obstructive disease from fast myocardial perfusion SPECT: A multicenter study. JACC Cardiovasc. Imaging 2018, 11, 1654–1663. [Google Scholar] [CrossRef]
- Betancur, J.; Hu, L.H.; Commandeur, F.; Sharir, T.; Einstein, A.J.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.A.; Sinusas, A.J.; Miller, E.J.; et al. Deep learning analysis of upright-supine high-efficiency SPECT myocardial perfusion imaging for prediction of obstructive coronary artery disease: A multicenter study. J. Nucl. Med. 2019, 60, 664–670. [Google Scholar] [CrossRef]
- Liu, H.; Wu, J.; Miller, E.J.; Liu, C.; Liu, Y.; Liu, Y.-H. Diagnostic accuracy of stress-only myocardial perfusion SPECT improved by deep learning. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2793–2800. [Google Scholar] [CrossRef]
- Miller, R.J.H.; Hauser, M.T.; Sharir, T.; Einstein, A.J.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.A.; Sinusas, A.J.; Miller, E.J.; Bateman, T.M.; et al. Machine learning to predict abnormal myocardial perfusion from pre-test features. J. Nucl. Cardiol. 2022, 29, 2393–2403. [Google Scholar] [CrossRef]
- Otaki, Y.; Singh, A.; Kavanagh, P.; Miller, R.J.; Parekh, T.; Tamarappoo, B.K.; Sharir, T.; Einstein, A.J.; Fish, M.B.; Ruddy, T.D.; et al. Clinical deployment of explainable artificial intelligence of SPECT for diagnosis of coronary artery disease. JACC Cardiovasc. Imaging 2022, 15, 1091–1102. [Google Scholar] [CrossRef]
- Hu, L.H.; Betancur, J.; Sharir, T.; Einstein, A.J.; Bokhari, S.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.A.; Sinusas, A.J.; Miller, E.J.; et al. Machine learning predicts per-vessel early coronary revascularization after fast myocardial perfusion SPECT: Results from multicentre REFINE SPECT registry. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 549–559. [Google Scholar] [CrossRef]
- Feher, A.; Bednarski, B.; Miller, R.J.; Shanbhag, A.; Lemley, M.; Miras, L.; Sinusas, A.J.; Miller, E.J.; Slomka, P.J. Artificial intelligence predicts hospitalization for acute heart failure exacerbation in patients undergoing myocardial perfusion imaging. J. Nucl. Med. 2024, 65, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Rios, R.; Miller, R.J.H.; Hu, L.H.; Otaki, Y.; Singh, A.; Diniz, M.; Sharir, T.; Einstein, A.J.; Fish, M.B.; Ruddy, T.D.; et al. Determining a minimum set of variables for machine learning cardiovascular event prediction: Results from REFINE SPECT registry. Cardiovasc. Res. 2022, 118, 2152–2164. [Google Scholar] [CrossRef] [PubMed]
- Betancur, J.; Otaki, Y.; Motwani, M.; Fish, M.B.; Lemley, M.; Dey, D.; Gransar, H.; Tamarappoo, B.; Germano, G.; Sharir, T.; et al. Prognostic value of combined clinical and myocardial perfusion imaging data using machine learning. JACC Cardiovasc. Imaging 2018, 11, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Miller, R.J.H.; Otaki, Y.; Kavanagh, P.; Hauser, M.T.; Tzolos, E.; Kwiecinski, J.; Van Kriekinge, S.; Wei, C.-C.; Sharir, T.; et al. Direct risk assesment from myocardial perfusion imaging using explainable deep learning. JACC Cardiovasc. Imaging 2023, 16, 209–220. [Google Scholar] [CrossRef]
- Pieszko, K.; Shanbhag, A.D.; Singh, A.; Hauser, M.T.; Miller, R.J.H.; Liang, J.X.; Motwani, M.; Kwieciński, J.; Sharir, T.; Einstein, A.J.; et al. Time and event-specific deep learning for personalized risk assessment after cardiac perfusion imaging. NPJ Digit. Med. 2023, 6, 78. [Google Scholar] [CrossRef]
- Williams, M.C.; Bednarski, B.P.; Pieszko, K.; Miller, R.J.H.; Kwiecinski, J.; Shanbhag, A.; Liang, J.X.; Huang, C.; Sharir, T.; Dorbala, S.; et al. Unsupervised learning to characterize patients with known coronary artery disease undergoing myocardial perfusion imaging. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2656–2668. [Google Scholar] [CrossRef]
- Miller, R.J.H.; Bednarski, B.P.; Pieszko, K.; Kwiecinski, J.; Williams, M.C.; Shanbhag, A.; Liang, J.X.; Huang, C.; Sharir, T.; Hauser, M.T.; et al. Clinical phenotypes among patients with normal cardiac perfusion using unsupervised learning: A retrospective observational study. eBioMedicine 2024, 99, 104930. [Google Scholar] [CrossRef]
- Juarez-Orozco, L.E.; Knol, R.J.J.; Sanchez-Catasus, C.A.; Martinez-Manzanera, O.; van der Zant, F.M.; Knuuti, J. Machine learning in the integration of simple variables for identifying patients with myocardial ischemia. J. Nucl. Cardiol. 2020, 27, 147–155. [Google Scholar] [CrossRef]
- Berman, D.; Hunter, C.; Hossain, A.; Yao, J.; Workman, E.; Guan, S.; Strickhart, L.; Beanlands, R.; Slater, D.; Dekemp, R.A. Machine and deep learning models for accurate detection of ischemia and scar with myocardial blood flow positron emission tomography imaging. J. Nucl. Cardiol. 2024, 32, 101797. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Orozco, L.E.; Martinez-Manzanera, O.; van der Zant, F.M.; Knol, R.J.J.; Knuuti, J. Deep learning in quantitative PET myocardial perfusion imaging: A study on cardiovascular event prediction. JACC Cardiovasc. Imaging 2020, 13, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kwiecinski, J.; Miller, R.J.H.; Otaki, Y.; Kavanagh, P.B.; Van Kriekinge, S.D.; Parekh, T.; Gransar, H.; Pieszko, K.; Killekar, A.; et al. Deep learning for explainable estimation of mortality risk from myocardial positron emission tomography images. Circ. Cardiovasc. Imaging 2022, 15, e014526, Erratum in Circ. Cardiovasc. Imaging 2022, 15, e000078. [Google Scholar] [PubMed]
- Kwiecinski, J.; Tzolos, E.; Meah, M.N.; Cadet, S.; Adamson, P.D.; Grodecki, K.; Joshi, N.V.; Moss, A.J.; Williams, M.C.; van Beek, E.J.R.; et al. Machine learning with 18F-sodium fluoride PET and quantitative plaque analysis on CT angiography for the future risk of myocardial infarction. J. Nucl. Med. 2022, 63, 158–165. [Google Scholar] [CrossRef]
- Delbarre, M.A.; Girardon, F.; Roquette, L.; Blanc-Durand, P.; Hubaut, M.-A.; Hachulla, É.; Semah, F.; Huglo, D.; Garcelon, N.; Marchal, E.; et al. Deep learning on bone scintigraphy to detect abnormal cardiac uptake at risk of cardiac amyloidosis. JACC Cardiovasc. Imaging 2023, 16, 1085–1095. [Google Scholar] [CrossRef]
- Halme, H.L.; Ihalainen, T.; Suomalainen, O.; Loimaala, A.; Mätzke, S.; Uusitalo, V.; Sipilä, O.; Hippeläinen, E. Convolutional neural networks for detection of transthyretin amyloidosis in 2D scintigraphy images. EJNMMI Res. 2022, 12, 27. [Google Scholar] [CrossRef]
- Salimi, Y.; Shiri, I.; Mansouri, Z.; Sanaat, A.; Hajianfar, G.; Hervier, E.; Bitarafan, A.; Caobelli, F.; Hundertmark, M.; Mainta, I.; et al. Artificial intelligence-based cardiac transthyretin amyloidosis detection and scoring in scintigraphy imaging: Multi-tracer, multi-scanner, and multi-center development and evaluation study. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 2513–2528. [Google Scholar] [CrossRef]
- Spielvogel, C.P.; Haberl, D.; Mascherbauer, K.; Ning, J.; Kluge, K.; Traub-Weidinger, T.; Davies, R.H.; Pierce, I.; Patel, K.; Nakuz, T.; et al. Diagnosis and prognosis of abnormal cardiac scintigraphy uptake suggestive of cardiac amyloidosis using artificial intelligence: A retrospective, international, multicentre, cross-tracer development and validation study. Lancet Digit. Health 2024, 6, e251–e260. [Google Scholar] [CrossRef]
- Miller, R.J.H.; Shanbhag, A.; Michalowska, A.M.; Kavanagh, P.; Liang, J.X.; Builoff, V.; Fine, N.M.; Dey, D.; Berman, D.S.; Slomka, P.J. Deep learning-enabled quantification of 99mTc-Pyrophosphate SPECT/CT for cardiac amyloidosis. J. Nucl. Med. 2024, 65, 1144–1150. [Google Scholar] [CrossRef]
- Togo, R.; Hirata, K.; Manabe, O.; Ohira, H.; Tsujino, I.; Magota, K.; Ogawa, T.; Haseyama, M.; Shiga, T. Cardiac sarcoidosis classification with deep convolutional neural network-based features using polar maps. Comput. Biol. Med. 2019, 104, 81–86. [Google Scholar] [CrossRef]
- Mudgal, K.S.; Das, N. The ethical adoption of artificial intelligence in radiology. BJR Open 2020, 2, 20190020. [Google Scholar] [CrossRef]
- Geis, J.R.; Brady, A.P.; Wu, C.C.; Spencer, J.; Ranschaert, E.; Jaremko, J.L.; Langer, S.G.; Kitts, A.B.; Birch, J.; Shields, W.F.; et al. Ethics of Artificial Intelligence in Radiology: Summary of the Joint European and North American Multisociety Statement. Radiology 2019, 293, 436–440. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciagrà, R.; Valente, S.; Dominietto, M. Artificial Intelligence in Nuclear Cardiology. J. Clin. Med. 2025, 14, 6416. https://doi.org/10.3390/jcm14186416
Sciagrà R, Valente S, Dominietto M. Artificial Intelligence in Nuclear Cardiology. Journal of Clinical Medicine. 2025; 14(18):6416. https://doi.org/10.3390/jcm14186416
Chicago/Turabian StyleSciagrà, Roberto, Samuele Valente, and Marco Dominietto. 2025. "Artificial Intelligence in Nuclear Cardiology" Journal of Clinical Medicine 14, no. 18: 6416. https://doi.org/10.3390/jcm14186416
APA StyleSciagrà, R., Valente, S., & Dominietto, M. (2025). Artificial Intelligence in Nuclear Cardiology. Journal of Clinical Medicine, 14(18), 6416. https://doi.org/10.3390/jcm14186416





