MRI-Based Evaluation of PRP Therapy in Knee Osteoarthritis: WORMS and Synovial Changes at 6 Months
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
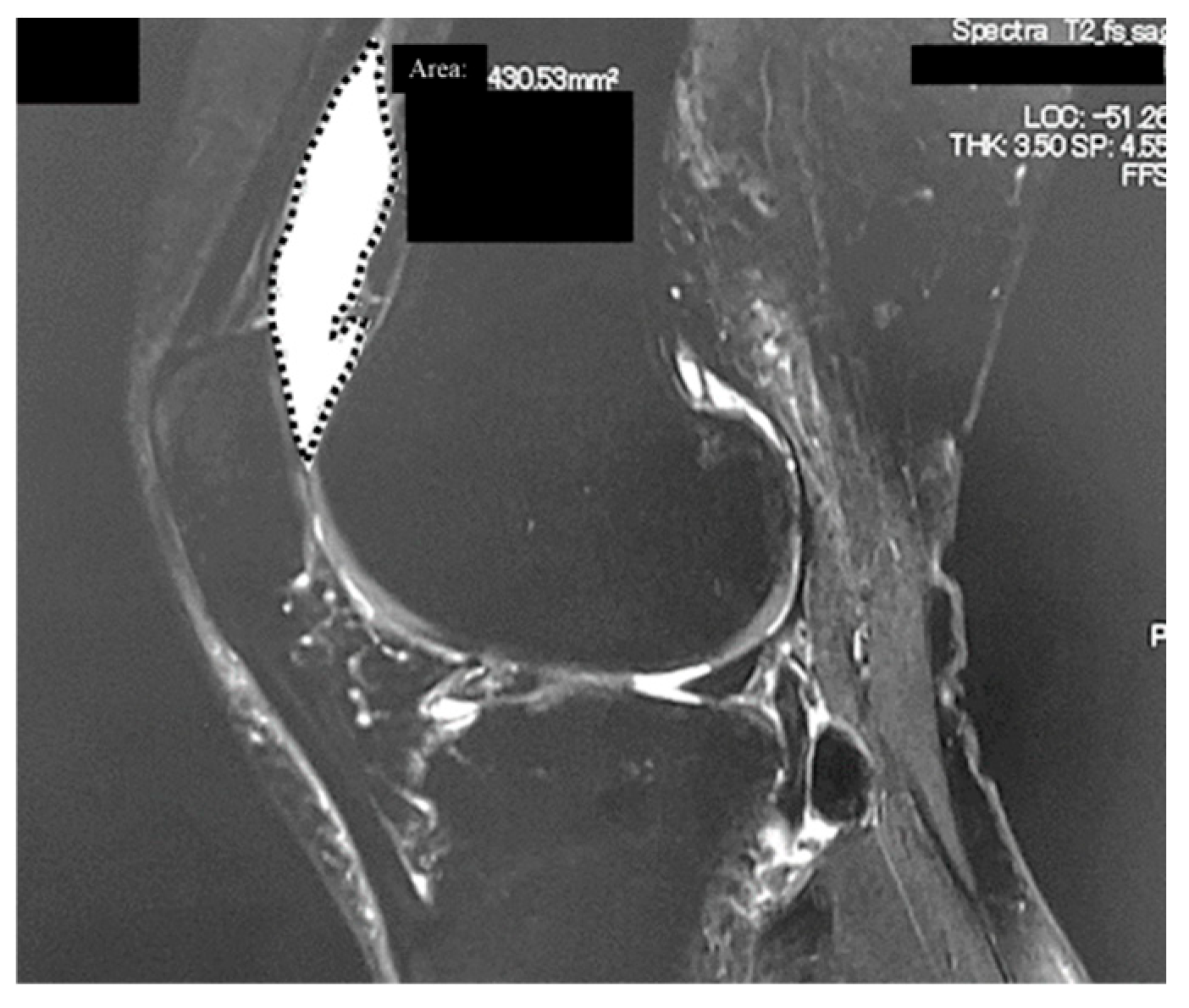
2.2. Radiological Analyses
2.3. Clinical Outcomes
2.4. PRP Preparation and Injection
2.5. Statistical Analyses
3. Results
3.1. Efficacy of PRP Therapy
3.2. MRI Analysis
3.3. Association Between PROMs and MRI Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRP | platelet-rich plasma |
| MRI | magnetic resonance imaging |
| PROMs | patient-reported outcome measures |
| WORMS | whole-organ MRI score |
| MFTJ | medial femorotibial joint |
| LFTJ | lateral femorotibial joint |
| PFJ | patellofemoral joints |
| OA | osteoarthritis |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| KL | Kellgren–Lawrence |
| BMA | bone marrow abnormality |
| VAS | visual analog scale |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| OMERACT | Outcome Measures in Rheumatology responder criteria |
| OARSI | Osteoarthritis Research Society International |
| CI | confidence interval |
| OR | odds ratio |
| ADL | Activities of daily living |
| QOL | quality of life |
| RCT | randomized controlled trial |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1 beta |
Appendix A
| Mean ± SD | p Value | |
|---|---|---|
| Sex (male/female) | R: 28/107 N: 27/59 | 0.08 |
| KL grade | R: KL2 = 37, KL3 = 38, KL4 = 60 N:KL2 = 17,KL3 = 20, KL4 = 49 | 0.07 |
| Age (years) | R: 68.9 ± 9.9 N: 67.6 ± 8.9 | 0.31 |
| Body mass index (kg/m2) | R: 25.2 ± 4.5 N: 24.8 ± 3.5 | 0.80 |
| VAS | R: 62.3 ± 24.0 N: 51.9 ± 26.2 | <0.01 |
| KOOS—pain | R: 50.3 ± 17.7 N: 62.3 ± 16.9 | <0.01 |
| KOOS—symptoms | R: 50.0 ± 19.0 N: 55.4 ± 19.1 | 0.04 |
| KOOS—activities of daily living | R: 62.6 ± 19.1 N: 71.9 ± 15.5 | <0.01 |
| KOOS—sports and recreation function | R: 24.4 ± 20.2 N: 36.1 ± 22.8 | <0.01 |
| KOOS—knee-related quality of life | R: 21.3 ± 15.7 N: 31.8 ± 18.3 | <0.01 |
| MTFJ WORMS cartilage score | R: 17.6 ± 8.9 N: 18.3 ± 8.0 | 0.91 |
| LFTJ WORMS cartilage score | R: 4.3 ± 5.8 N: 5.1 ± 7.1 | 0.66 |
| PFJ WORMS cartilage score | R: 7.0 ± 6.1 N: 7.0 ± 6.1 | 0.95 |
| WORMS bone marrow abnormality score | R: 4.5 ± 4.9 N: 5.2 ± 4.6 | 0.71 |
| Area of synovial fluid (cm2) | R: 1.9 ± 1.4 N: 2.3 ± 2.3 | 0.16 |
References
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Bray, C.C.; Walker, C.M.; Spence, D.D. Orthobiologics in pediatric sports medicine. Orthop. Clin. N. Am. 2017, 48, 333–342. [Google Scholar] [CrossRef]
- Southworth, T.M.; Naveen, N.B.; Nwachukwu, B.U.; Cole, B.J.; Frank, R.M. Orthobiologics for focal articular cartilage defects. Clin. Sports Med. 2019, 38, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, E.; Tetsworth, K.; Glatt, V. Is platelet-rich plasma effective for the treatment of knee osteoarthritis? A systematic review and meta-analysis of level 1 and 2 randomized controlled trials. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 955–967. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Roffi, A.; Di Matteo, B.; Merli, M.L.; Marcacci, M. Platelet-rich plasma: Why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2459–2474. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, M.; Meng, H.Y.; Wang, A.Y.; Guo, Q.Y.; Wang, Y.; Peng, J. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthr. Cartil. 2013, 21, 1627–1637. [Google Scholar] [CrossRef]
- Murray, M.M.; Spindler, K.P.; Abreu, E.; Muller, J.A.; Nedder, A.; Kelly, M.; Frino, J.; Zurakowski, D.; Valenza, M.; Snyder, B.D.; et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J. Orthop. Res. 2007, 25, 81–91. [Google Scholar] [CrossRef]
- Boffa, A.; Salerno, M.; Merli, G.; De Girolamo, L.; Laver, L.; Magalon, J.; Sánchez, M.; Tischer, T.; Filardo, G. Platelet-rich plasma injections induce disease-modifying effects in the treatment of osteoarthritis in animal models. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 4100–4121. [Google Scholar] [CrossRef]
- Peterfy, C.G.; Guermazi, A.; Zaim, S.; Tirman, P.F.; Miaux, Y.; White, D.; Kothari, M.; Lu, Y.; Fye, K.; Zhao, S.; et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr. Cartil. 2004, 12, 177–190. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; van der Heijde, D.; Altman, R.D.; Anderson, J.J.; Bellamy, N.; Hochberg, M.; Simon, L.; Strand, V.; Woodworth, T.; Dougados, M. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr. Cartil. 2004, 12, 389–399. [Google Scholar] [CrossRef]
- DeLong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-rich plasma: The PAW classification system. Arthroscopy 2012, 28, 998–1009. [Google Scholar] [CrossRef]
- Campbell, K.A.; Saltzman, B.M.; Mascarenhas, R.; Khair, M.M.; Verma, N.N.; Bach, B.R.; Cole, B.J. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy 2015, 31, 2213–2221. [Google Scholar] [CrossRef]
- Meheux, C.J.; McCulloch, P.C.; Lintner, D.M.; Varner, K.E.; Harris, J.D. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy 2016, 32, 495–505. [Google Scholar] [CrossRef]
- Saita, Y. Corrigendum to “Platelet-rich plasma therapy for knee osteoarthritis: Insights from real-world clinical data in Japan” [Regen Ther 29 (2025) 427–434]. Regen. Ther. 2025, 29, 540. [Google Scholar] [CrossRef]
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Duong, V.; Eyles, J.; Kasza, J.; Wang, Y.; Cicuttini, F.; Buchbinder, R.; Forbes, A.; et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: The RESTORE randomized clinical trial. JAMA 2021, 326, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Buendía-López, D.; Medina-Quirós, M.; Fernández-Villacañas Marín, M. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: A randomized controlled trial. J. Orthop. Traumatol. 2018, 19, 3. [Google Scholar] [CrossRef]
- de Girolamo, L.; Filardo, G.; Laver, L. Disease-modifying effects of orthobiologics in the treatment of knee osteoarthritis: The lesson learned from preclinical research models. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5286–5290. [Google Scholar] [CrossRef] [PubMed]
- Raeissadat, S.A.; Ghorbani, E.; Sanei Taheri, M.; Soleimani, R.; Rayegani, S.M.; Babaee, M.; Payami, S. MRI changes after platelet rich plasma injection in knee osteoarthritis (randomized clinical trial). J. Pain Res. 2020, 13, 65–73. [Google Scholar] [CrossRef]
- Dong, C.; Zhao, C.; Wang, F. Clinical benefit of high tibial osteotomy combined with the intervention of platelet-rich plasma for severe knee osteoarthritis. J. Orthop. Surg. Res. 2022, 17, 405. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Oka, T.; Suzuki, K.; Tanaka, K.; Okuyama, K.; Kitagawa, T. Effectiveness of combined regenerative medicine and exercise therapy for patients with knee osteoarthritis: A scoping review. Front. Rehabil. Sci. 2025, 6, 1612615. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.J.; Miller, R.E.; Malfait, A.M. The genesis of pain in osteoarthritis: Inflammation as a mediator of osteoarthritis pain. Clin. Geriatr. Med. 2022, 38, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Duan, W.; Yu, Z.; Tao, T.; Xu, J.; Ma, Q.; Zhao, L.; Guo, J.J. Intra-articular injections of platelet-rich plasma decrease pain and improve functional outcomes than sham saline in patients with knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, H.; Hirose, J.; Okamoto, N.; Okada, T.; Oka, K.; Taniwaki, T.; Nakamura, E.; Yamashita, Y.; Mizuta, H. Evaluation of the relationship between T1ρ and T2 values and patella cartilage degeneration in patients of the same age group. Eur. J. Radiol. 2015, 84, 463–468. [Google Scholar] [CrossRef]
- Nishioka, H.; Hirose, J.; Nakamura, E.; Oniki, Y.; Takada, K.; Yamashita, Y.; Mizuta, H. T1ρ and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J. Magn. Reson. Imaging 2012, 35, 147–155. [Google Scholar] [CrossRef]
| Severity of Knee OA (KL Grade 2/3/4) | 54/58/109 |
| Sex (male/female) | 55/166 |
| Age (years) | 68.4 ± 9.5 |
| Body mass index (kg/m2) | 25.0 ± 4.2 |
| Pretreatment | 6 Months After PRP | p-Value (Wilcoxon Test) | |
|---|---|---|---|
| VAS | 58.6 ± 25.2 | 35.0 ± 25.4 | <0.0001 |
| KOOS—pain | 55.0 ± 18.4 | 67.6 ± 16.9 | <0.0001 |
| KOOS—symptoms | 52.3 ± 19.2 | 67.1 ± 20.0 | <0.0001 |
| KOOS—activities of daily living | 66.3 ± 18.4 | 77.3 ± 17.2 | <0.0001 |
| KOOS—sports and recreation function | 29.2 ± 22.0 | 42.9 ± 28.2 | <0.0001 |
| KOOS—knee-related quality of life | 25.4 ± 17.6 | 45.5 ± 21.8 | <0.0001 |
| KL Grade | Pre Treatment | 6 Months after PRP | p-Value (Wilcoxon Test) | |
|---|---|---|---|---|
| WORMS cartilage score (MFTJ) | KL2 | 8.7 ± 7.0 | 8.3 ± 7.5 | 0.09 |
| KL3 | 15.9 ± 7.3 | 16.1 ± 7.6 | 0.60 | |
| KL4 | 23.5 ± 4.6 | 23.8 ± 4.5 | 0.12 | |
| Total (KL2~4) | 17.9 ± 8.6 | 18.0 ± 8.9 | 0.79 | |
| WORMS cartilage score (LFTJ) | KL2 | 0.6 ± 1.4 | 0.5 ± 1.2 | 0.17 |
| KL3 | 3.7 ± 5.4 | 3.3 ± 5.0 | 0.053 | |
| KL4 | 7.1 ± 7.1 | 6.7 ± 7.2 | 0.009 | |
| Total (KL2~4) | 4.7 ± 6.3 | 4.3 ± 6.2 | <0.001 | |
| WORMS cartilage score (PFJ) | KL2 | 3.4 ± 5.1 | 3.0 ± 4.8 | 0.02 |
| KL3 | 7.0 ± 5.5 | 6.9 ± 5.7 | 0.65 | |
| KL4 | 8.8 ± 6.1 | 8.6 ± 5.7 | 0.21 | |
| Total (KL2~4) | 7.0 ± 6.1 | 6.8 ± 5.9 | 0.048 | |
| WORMS cartilage score (total) | KL2 | 12.7 ± 11.4 | 11.8 ± 11.4 | 0.059 |
| KL3 | 26.3 ± 13.5 | 26.1 ± 13.0 | 0.28 | |
| KL4 | 39.6 ± 13.3 | 39.2 ± 12.8 | 0.36 | |
| Total (KL2~4) | 29.5 ± 16.9 | 29.0 ± 16.8 | 0.021 | |
| WORMS bone marrow abnormality score | KL2 | 2.3 ± 3.8 | 2.5 ± 4.3 | 0.29 |
| KL3 | 3.8 ± 4.0 | 3.6 ± 3.6 | 0.32 | |
| KL4 | 6.5 ± 4.9 | 6.5 ± 5.2 | 0.94 | |
| Total (KL2~4) | 4.8 ± 4.7 | 4.7 ± 4.9 | 0.87 | |
| Area of synovial fluid (cm2) | KL2 | 1.6 ± 1.3 | 1.2 ± 1.2 | 0.008 |
| KL3 | 2.2 ± 1.7 | 1.8 ± 1.6 | 0.17 | |
| KL4 | 2.3 ± 2.0 | 2.0 ± 1.7 | 0.009 | |
| Total (KL2~4) | 2.1 ± 1.8 | 1.7 ± 1.6 | <0.001 |
| KL Grade | Baseline | At 6 Months | p-Value | |
|---|---|---|---|---|
| WORMS cartilage score (MFTJ) | KL2 | 3.3 ± 3.3 | 3.6 ± 3.6 | 0.02 |
| KL3 | 14.0 ± 5.1 | 14.4 ± 5.3 | 0.50 | |
| KL4 | 19.2 ± 3.3 | 19.4 ± 2.6 | 0.62 | |
| Total (KL2~4) | 8.1 ± 7.5 | 8.4 ± 7.6 | 0.051 | |
| WORMS cartilage score (LFTJ) | KL2 | 2.4 ± 2.8 | 2.3 ± 3.1 | 0.81 |
| KL3 | 3.2 ± 1.0 | 3.3 ± 1.2 | 0.36 | |
| KL4 | 11.8 ± 7.5 | 11.8 ± 8.0 | 1.00 | |
| Total (KL2~4) | 4.1 ± 5.0 | 4.1 ± 5.2 | 0.88 | |
| WORMS cartilage score (PFJ) | KL2 | 2.4 ± 4.6 | 2.7 ± 4.7 | 0.056 |
| KL3 | 4.2 ± 4.8 | 4.2 ± 5.0 | 1.00 | |
| KL4 | 7.4 ± 4.5 | 7.6 ± 4.7 | 0.37 | |
| Total (KL2~4) | 3.6 ± 4.8 | 3.8 ± 5.0 | 0.056 | |
| WORMS cartilage score (total) | KL2 | 8.1 ± 6.1 | 8.6 ± 6.5 | 0.03 |
| KL3 | 21.3 ± 6.5 | 21.9 ± 6.8 | 0.36 | |
| KL4 | 38.4 ± 9.2 | 38.9 ± 9.3 | 0.58 | |
| Total (KL2~4) | 15.8 ± 13.2 | 16.3 ± 13.4 | 0.01 | |
| WORMS bone marrow abnormality score | KL2 | 0.2 ± 0.5 | 0.3 ± 0.8 | 0.08 |
| KL3 | 0.6 ± 0.8 | 0.5 ± 0.8 | 0.36 | |
| KL4 | 0.4 ± 0.5 | 0.4 ± 0.5 | 0.99 | |
| Total (KL2~4) | 0.3 ± 0.6 | 0.4 ± 0.7 | 0.42 | |
| Area of synovial fluid (cm2) | KL2 | 1.0 ± 0.9 | 1.0 ± 0.9 | 0.62 |
| KL3 | 0.7 ± 0.5 | 0.7 ± 0.7 | 1.00 | |
| KL4 | 0.9 ± 0.8 | 1.9 ± 1.7 | 0.37 | |
| Total (KL2~4) | 0.9 ± 0.8 | 1.1 ± 1.0 | 0.08 |
| Magnetic Resonance Imaging Findings | Correlation with Improvement in VAS | |
|---|---|---|
| r (95% CI) | p-Value | |
| WORMS cartilage score (MFTJ) | 0.21 (0.08–0.34) | 0.001 |
| WORMS cartilage score (LFTJ) | 0.13 (−0.004–0.26) | 0.051 |
| WORMS cartilage score (PFJ) | 0.16 (0.03–0.29) | 0.01 |
| WORMS bone marrow abnormality score | 0.15 (0.01–0.28) | 0.02 |
| Area of synovial fluid | 0.08 (−0.05–0.21) | 0.23 |
| Responders | n | Pretreatment | 6 Months after PRP | p-Value (Wilcoxon Test) | ||||
|---|---|---|---|---|---|---|---|---|
| WORMS cartilage score (MFTJ) | 135 | 17.6 | ± | 8.9 | 17.7 | ± | 9.2 | 0.72 |
| WORMS cartilage score (LFTJ) | 135 | 4.3 | ± | 5.8 | 3.9 | ± | 5.6 | <0.001 |
| WORMS cartilage score (PFJ) | 135 | 7.0 | ± | 6.1 | 6.6 | ± | 5.8 | 0.001 |
| WORMS cartilage score (total) | 135 | 28.9 | ± | 17.0 | 28.1 | ± | 16.7 | 0.001 |
| WORMS bone marrow abnormality score | 135 | 4.5 | ± | 4.9 | 4.6 | ± | 5.0 | 0.51 |
| Area of synovial fluid (cm2) | 135 | 1.9 | ± | 1.4 | 1.7 | ± | 1.4 | 0.052 |
| Non-responders | n | Pretreatment | 6 months after PRP | p-value (Wilcoxon test) | ||||
| WORMS cartilage score (MFTJ) | 86 | 18.3 | ± | 8.0 | 18.4 | ± | 8.3 | 0.91 |
| WORMS cartilage score (LFTJ) | 86 | 5.1 | ± | 7.1 | 4.9 | ± | 7.1 | 0.21 |
| WORMS cartilage score (PFJ) | 86 | 7.0 | ± | 6.1 | 7.1 | ± | 6.1 | 0.65 |
| WORMS cartilage score (total) | 86 | 30.5 | ± | 16.9 | 30.5 | ± | 16.9 | 0.91 |
| WORMS bone marrow abnormality score | 86 | 5.2 | ± | 4.6 | 5.0 | ± | 4.8 | 0.33 |
| Area of synovial fluid (cm2) | 86 | 2.3 | ± | 2.3 | 1.8 | ± | 1.8 | <0.001 |
| Improvement in PROMs | Correlation with Improvement in Area of Synovial Fluid (cm2) | |
|---|---|---|
| r (95% CI) | p-Value | |
| ΔVAS | 0.001 (−0.13–0.13) | 0.98 |
| ΔKOOS–pain | 0.16 (0.03–0.29) | 0.017 |
| ΔKOOS—symptom | 0.16 (0.04–0.294) | 0.012 |
| ΔKOOS—ADL | 0.12 (−0.01–0.25) | 0.074 |
| ΔKOOS—sports | 0.18 (0.05–0.31) | 0.007 |
| ΔKOOS—QOL | 0.15 (0.02–0.28) | 0.024 |
| Independent Variables (Difference Between Pre- and Post-PRP) | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| WORMS cartilage score (MFTJ) | 1.03 (0.92–1.16) | 0.62 | 1.12 (0.91–1.38) | 0.26 |
| WORMS cartilage score (LFTJ) | 1.03 (0.86–1.24) | 0.75 | 1.19 (0.87–1.64) | 0.27 |
| WORMS cartilage score (PFJ) | 1.27 (1.07–1.52) | 0.007 | 1.45 (1.09–1.94) | 0.01 |
| WORMS cartilage score (total) | 1.05 (0.97–1.13) | 0.22 | 0.86 (0.69–1.07) | 0.17 |
| WORMS bone marrow abnormality score | 0.98 (0.88–1.10) | 0.79 | 0.98 (0.88–1.11) | 0.84 |
| Area of synovial fluid | 0.99 (0.99–1.00) | 0.06 | 0.99 (0.99–1.00) | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakayama, T.; Saita, Y.; Uchino, S.; Kobayashi, Y.; Nishio, H.; Fukusato, S.; Momoi, Y.; Ikeda, H.; Kaneko, K.; Ishijima, M. MRI-Based Evaluation of PRP Therapy in Knee Osteoarthritis: WORMS and Synovial Changes at 6 Months. J. Clin. Med. 2025, 14, 6408. https://doi.org/10.3390/jcm14186408
Wakayama T, Saita Y, Uchino S, Kobayashi Y, Nishio H, Fukusato S, Momoi Y, Ikeda H, Kaneko K, Ishijima M. MRI-Based Evaluation of PRP Therapy in Knee Osteoarthritis: WORMS and Synovial Changes at 6 Months. Journal of Clinical Medicine. 2025; 14(18):6408. https://doi.org/10.3390/jcm14186408
Chicago/Turabian StyleWakayama, Takanori, Yoshitomo Saita, Sayuri Uchino, Yohei Kobayashi, Hirofumi Nishio, Shin Fukusato, Yasumasa Momoi, Hiroshi Ikeda, Kazuo Kaneko, and Muneaki Ishijima. 2025. "MRI-Based Evaluation of PRP Therapy in Knee Osteoarthritis: WORMS and Synovial Changes at 6 Months" Journal of Clinical Medicine 14, no. 18: 6408. https://doi.org/10.3390/jcm14186408
APA StyleWakayama, T., Saita, Y., Uchino, S., Kobayashi, Y., Nishio, H., Fukusato, S., Momoi, Y., Ikeda, H., Kaneko, K., & Ishijima, M. (2025). MRI-Based Evaluation of PRP Therapy in Knee Osteoarthritis: WORMS and Synovial Changes at 6 Months. Journal of Clinical Medicine, 14(18), 6408. https://doi.org/10.3390/jcm14186408







