Serum Osmolality and Stroke Mortality in the ICU: A U-Shaped Risk Pattern and Its Clinical Implications
Abstract
1. Introduction
2. Materials
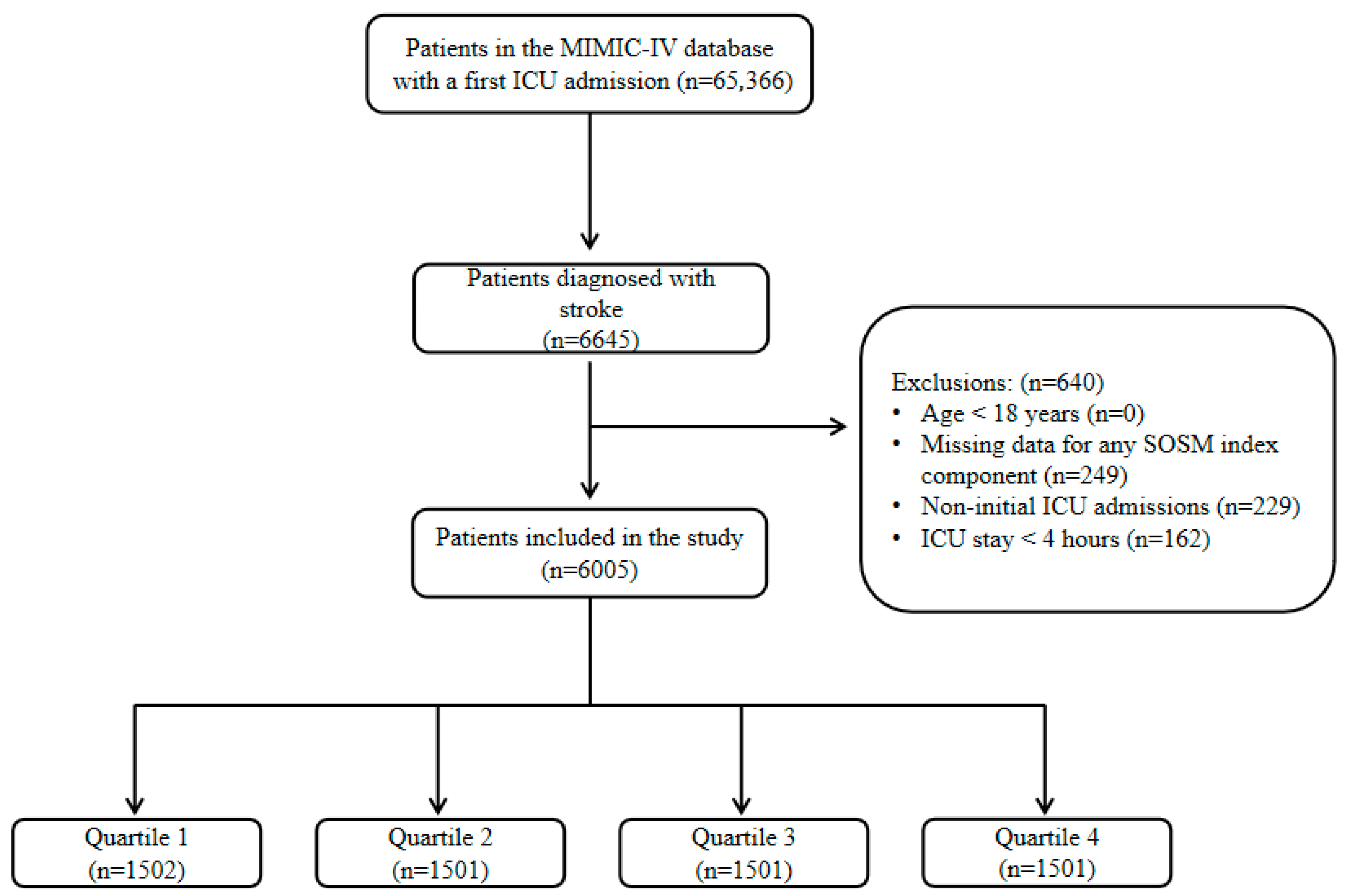
2.1. Study Population
2.2. Data Extraction
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Population
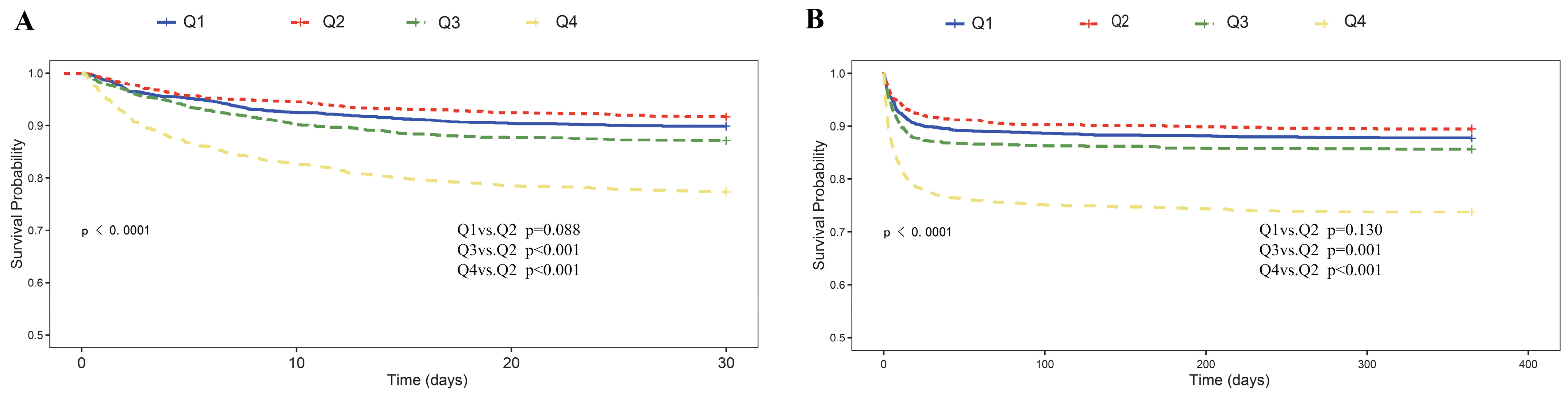
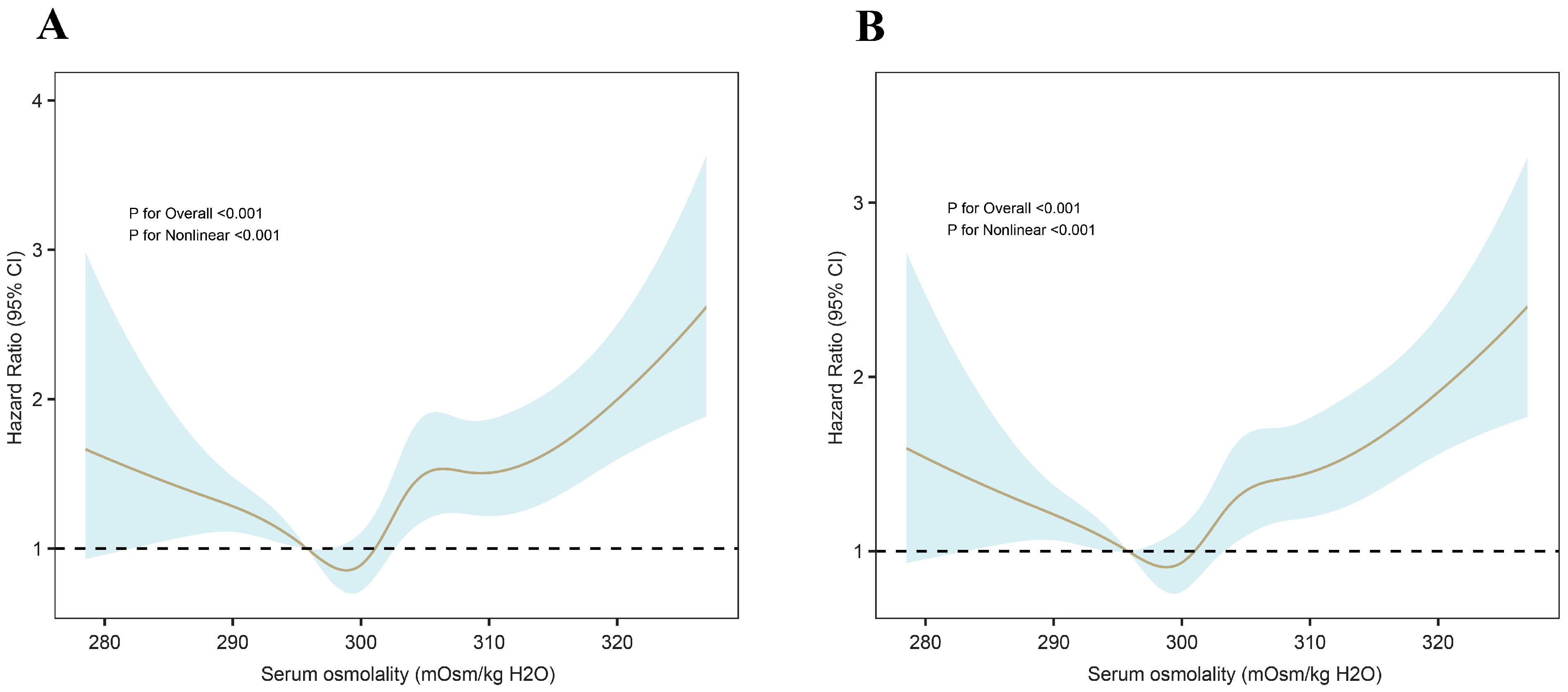
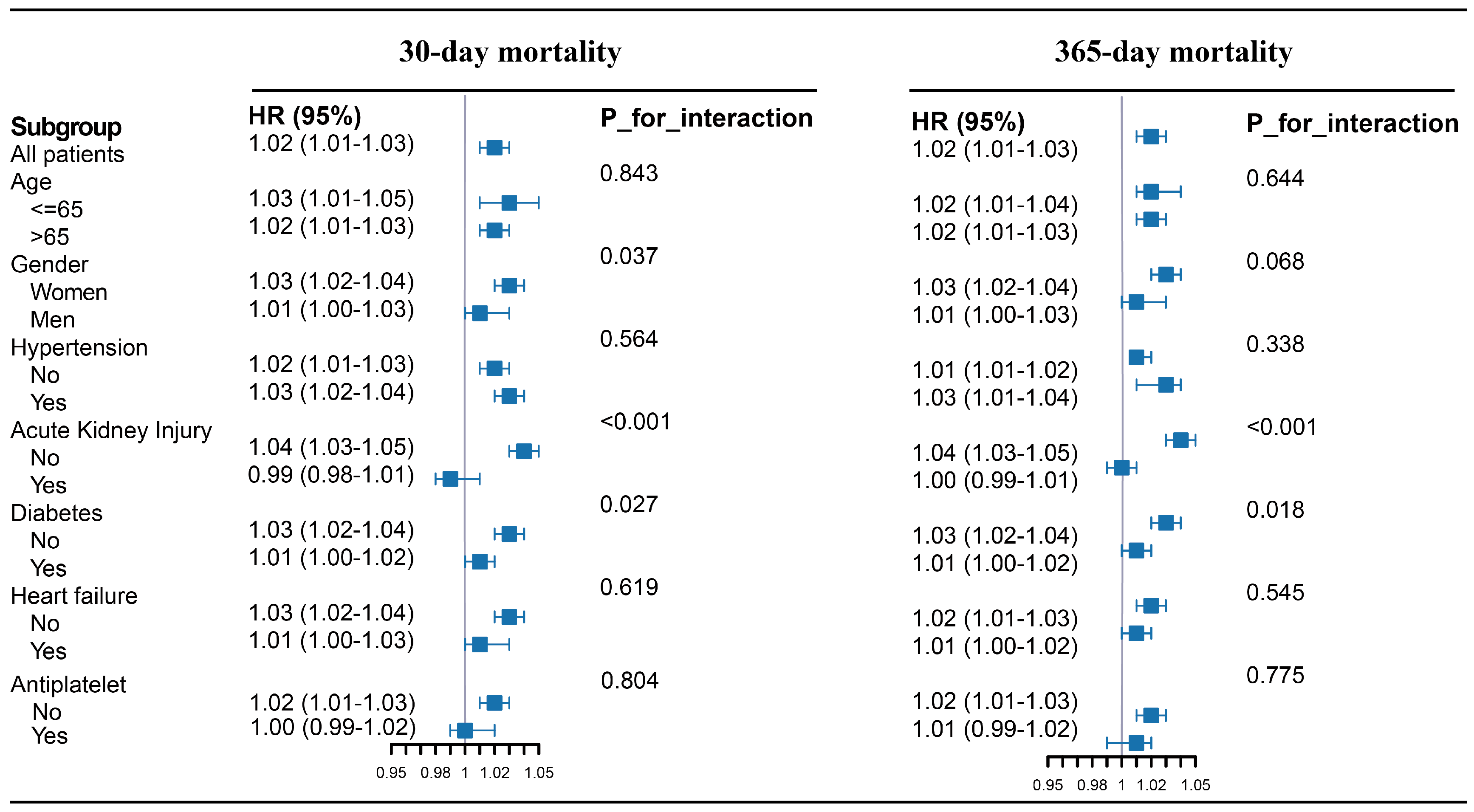
3.2. Primary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilkens, N.A.; Casolla, B.; Leung, T.W.; de Leeuw, F.-E. Stroke. Lancet 2024, 403, 2820–2836. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; F Grupper, M.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Rasouli, M. Basic concepts and practical equations on osmolality: Biochemical approach. Clin. Biochem. 2016, 49, 936–941. [Google Scholar] [CrossRef]
- Najem, O.; Shah, M.M.; Zubair, M.; De Jesus, O. Serum Osmolality. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK567764/ (accessed on 16 August 2025).
- Büyükkaragöz, B.; Bakkaloğlu, S.A. Serum osmolality and hyperosmolar states. Pediatr. Nephrol. 2023, 38, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Frith, J. New horizons in the diagnosis and management of dehydration. Age Ageing 2023, 52, afad193. [Google Scholar] [CrossRef]
- Gui, L.; Cao, H.; Zheng, M.; Pan, Y.; Ning, C.; Cheng, M. The J-shaped relationship between serum osmolality and all-cause mortality in critically ill patients with myocardial infarction: A retrospective cohort study. Front. Endocrinol. 2025, 16, 1542403. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Sha, Q. Association between serum osmolality and risk of in-hospital mortality in patients with intracerebral hemorrhage. Front. Neurol. 2024, 15, 1410569. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Deng, Y.; Cheng, Y.; Hao, Z.; Wu, S.; Liu, M. Association between Plasma Osmolality and Case Fatality within 1 Year after Severe Acute Ischemic Stroke. Yonsei Med. J. 2021, 62, 600–607. [Google Scholar] [CrossRef]
- Çelik, D.; Yildiz, M.; Çifci, A. Serum osmolarity does not predict mortality in patients with respiratory failure. Medicine 2022, 101, e28840. [Google Scholar] [CrossRef]
- Johnson, A.E.W.; Bulgarelli, L.; Shen, L.; Gayles, A.; Shammout, A.; Horng, S.; Pollard, T.J.; Hao, S.; Moody, B.; Gow, B.; et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 2023, 10, 1. [Google Scholar] [CrossRef]
- Khajuria, A.; Krahn, J. Osmolality revisited—Deriving and validating the best formula for calculated osmolality. Clin. Biochem. 2005, 38, 514–519. [Google Scholar] [CrossRef]
- Rasouli, M.; Kalantari, K.R. Comparison of methods for calculating serum osmolality: Multivariate linear regression analysis. Clin. Chem. Lab. Med. 2005, 43, 635–640. [Google Scholar] [CrossRef]
- Deißler, L.; Wirth, R.; Frilling, B.; Janneck, M.; Rösler, A. Hydration Status Assessment in Older Patients. Dtsch. Arzteblatt Int. 2023, 120, 663–669. [Google Scholar] [CrossRef]
- Dasgupta, A.; Wahed, A. Chapter 5—Water homeostasis electrolytes and acid-base balance. In Clinical Chemistry, Immunology and Laboratory Quality Control, 2nd ed.; Dasgupta, A., Wahed, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 87–103. Available online: https://www.sciencedirect.com/science/article/pii/B9780128159606000248 (accessed on 12 July 2025).
- Kaya, H.; Yücel, O.; Ege, M.R.; Zorlu, A.; Yücel, H.; Güneş, H.; Ekmekçi, A.; Yılmaz, M.B. Plasma osmolality predicts mortality in patients with heart failure with reduced ejection fraction. Kardiol. Pol. 2017, 75, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Li, C.; Wang, T.; Liu, H.; Geng, J.; Gong, A. Serum osmolality was non-linearly associated with the risk of all-cause and cardiovascular mortality in patients with diabetes. BMJ Open Diabetes Res. Care 2023, 11, e003826. [Google Scholar] [CrossRef] [PubMed]
- Rowat, A.; Graham, C.; Dennis, M. Dehydration in hospital-admitted stroke patients: Detection, frequency, and association. Stroke 2012, 43, 857–859. [Google Scholar] [CrossRef]
- Asplund, K.; Israelsson, K.; Schampi, I. Haemodilution for acute ischaemic stroke. Cochrane Database Syst. Rev. 2000, 2, CD000103. [Google Scholar] [CrossRef]
- Bahouth, M.N.; Bahrainwala, Z.; Hillis, A.E.; Gottesman, R.F. Dehydration Status is Associated With More Severe Hemispatial Neglect After Stroke. Neurologist 2016, 21, 101–105. [Google Scholar] [CrossRef]
- Swerdel, J.N.; Janevic, T.M.; Kostis, W.J.; Faiz, A.; Cosgrove, N.M.; Kostis, J.B.; Myocardial Infarction Data Acquisition System (MIDAS 27) Study Group. Association Between Dehydration and Short-Term Risk of Ischemic Stroke in Patients with Atrial Fibrillation. Transl. Stroke Res. 2017, 8, 122–130. [Google Scholar] [CrossRef]
- Kanbay, M.; Yilmaz, S.; Dincer, N.; Ortiz, A.; Sag, A.A.; Covic, A.; Sánchez-Lozada, L.G.; Lanaspa, M.A.; Cherney, D.Z.I.; Johnson, R.J.; et al. Antidiuretic Hormone and Serum Osmolarity Physiology and Related Outcomes: What Is Old, What Is New, and What Is Unknown? J. Clin. Endocrinol. Metab. 2019, 104, 5406–5420. [Google Scholar] [CrossRef]
- Iba, T.; Helms, J.; Maier, C.L.; Levi, M.; Scarlatescu, E.; Levy, J.H. The role of thromboinflammation in acute kidney injury among patients with septic coagulopathy. J. Thromb. Haemost. JTH 2024, 22, 1530–1540. [Google Scholar] [CrossRef]
- Vilay, A.M.; Churchwell, M.D.; Mueller, B.A. Clinical review: Drug metabolism and nonrenal clearance in acute kidney injury. Crit. Care 2008, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Mi, D.; Wang, P.; Yang, B.; Pu, Y.; Yang, Z.; Liu, L. Correlation of hyperglycemia with mortality after acute ischemic stroke. Ther. Adv. Neurol. Disord. 2018, 11, 1756285617731686. [Google Scholar] [CrossRef]
- Tran, K.H.; Akhtar, N.; Ali, A.; Joseph, S.; Morgan, D.; Babu, B.; Uy, R.T.; Shuaib, A. Impact of stroke severity on aspiration pneumonia risks in the medical ward versus the stroke unit: A 10-year retrospective cohort study. BMJ Open 2025, 15, e093328. [Google Scholar] [CrossRef]
- Bond, V.E.; Doeltgen, S.; Kleinig, T.; Murray, J. Dysphagia-related acute stroke complications: A retrospective observational cohort study. J. Stroke Cerebrovasc. Dis. 2023, 32, 107123. [Google Scholar] [CrossRef] [PubMed]
- Katanga, J.; Nkandala, I.; Ngimbwa, J.; Mwamba, L.A.; Paul, I.K.; Berling, S.; Xavier, G.; Basinda, M.K.; Kagoye, S.; Mahawish, K.; et al. The burden of hyponatremia and 30-day outcomes among adults admitted with stroke at a large tertiary teaching hospital in Northwestern Tanzania. Front. Stroke 2025, 4, 1546358. [Google Scholar] [CrossRef]
- Khan, A.; Khan, Z.; Khan, S.; Ullah, A.; Ayub, G.; Tariq, M.N. Frequency of Hyponatremia and Its Impact on Prognosis in Ischemic Stroke. Cureus 2023, 15, e40317. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-J.; Dewi, S.R.K.; Hsu, W.-T.; Hsu, T.-Y.; Liao, S.-F.; Chan, L.; Lin, M.-C. Exploring Relationships of Heart Rate Variability, Neurological Function, and Clinical Factors with Mortality and Behavioral Functional Outcome in Patients with Ischemic Stroke. Diagnostics 2024, 14, 1304. [Google Scholar] [CrossRef]
- Lerner, D.P.; Shepherd, S.A.; Batra, A. Hyponatremia in the Neurologically Ill Patient: A Review. Neurohospitalist 2020, 10, 208–216. [Google Scholar] [CrossRef]
- Gankam Kengne, F.; Decaux, G. Hyponatremia and the Brain. Kidney Int. Rep. 2017, 3, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Vicente, E.; Guisado-Alonso, D.; Delgado-Mederos, R.; Camps-Renom, P.; Prats-Sánchez, L.; Martínez-Domeño, A.; Martí-Fàbregas, J. Frequency, Risk Factors, and Prognosis of Dehydration in Acute Stroke. Front. Neurol. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Nagin, D.S.; Jones, B.L.; Elmer, J. Recent Advances in Group-Based Trajectory Modeling for Clinical Research. Annu. Rev. Clin. Psychol. 2024, 20, 285–305. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall (N = 6005) | Q1 (N = 1502) | Q2 (N = 1501) | Q3 (N = 1501) | Q4 (N = 1501) | p-Value |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age (years) | 74.00 (63.00–83.00) | 72.00 (61.00–82.00) | 72.00 (62.00–82.00) | 74.00 (64.00–82.00) | 77.00 (67.00–85.00) | <0.001 |
| Weight (kg) | 76.90 (64.70–90.00) | 75.30 (63.00–87.85) | 77.20 (65.50–89.50) | 77.20 (65.50–91.25) | 77.10 (65.00–92.20) | <0.001 |
| Sex: men (n, %) | 3206.00 (53.39%) | 777.00 (51.73%) | 826.00 (55.03%) | 792.00 (52.76%) | 811.00 (54.03%) | <0.001 |
| RACE (n, %) | <0.001 | |||||
| BLACK | 632.00 (10.52%) | 136.00 (9.05%) | 133.00 (8.86%) | 150.00 (9.99%) | 213.00 (14.19%) | |
| WHITE | 4247.00 (70.72%) | 1088.00 (72.44%) | 1089.00 (72.55%) | 1069.00 (71.22%) | 1001.00 (66.69%) | |
| OTHER | 1126.00 (18.75%) | 278.00 (18.51%) | 279.00 (18.59%) | 282.00 (18.79%) | 287.00 (19.12%) | |
| Clinical score | ||||||
| GCS (score) | 15.00 (13.00–15.00) | 14.00 (13.00–15.00) | 14.00 (13.00–15.00) | 15.00 (13.00–15.00) | 14.00 (13.00–15.00) | 0.091 |
| SOFA (score) | 4.00 (2.00–6.00) | 3.00 (2.00–5.00) | 3.00 (2.00–5.00) | 3.00 (2.00–5.00) | 5.00 (3.00–7.00) | <0.001 |
| APSIII (score) | 39.00 (29.00–51.00) | 34.00 (26.00–46.00) | 35.00 (27.00–45.00) | 38.00 (30.00–49.00) | 48.00 (38.00–63.00) | <0.001 |
| SAPSII (score) | 35.00 (28.00–43.00) | 32.00 (25.00–40.00) | 32.00 (26.00–40.00) | 35.00 (28.00–42.00) | 40.00 (33.00–50.00) | <0.001 |
| OASIS (score) | 30.00 (25.00–36.00) | 29.00 (24.00–35.00) | 29.00 (24.00–35.00) | 30.00 (25.00–36.00) | 33.00 (27.00–39.00) | <0.001 |
| Commorbidities | ||||||
| Heart failure (n, %) | 1851.00 (30.82%) | 349.00 (23.24%) | 375.00 (24.98%) | 477.00 (31.78%) | 650.00 (43.30%) | <0.001 |
| Diabetes (n, %) | 2082.00 (34.67%) | 382.00 (25.43%) | 436.00 (29.05%) | 554.00 (36.91%) | 710.00 (47.30%) | <0.001 |
| COPD (n, %) | 931.00 (15.50%) | 229.00 (15.25%) | 215.00 (14.32%) | 232.00 (15.46%) | 255.00 (16.99%) | 0.241 |
| Arterial fibrillation (n, %) | 1131.00 (18.83%) | 282.00 (18.77%) | 250.00 (16.66%) | 271.00 (18.05%) | 328.00 (21.85%) | 0.003 |
| Hypertension (n, %) | 3078.00 (51.26%) | 855.00 (56.92%) | 873.00 (58.16%) | 784.00 (52.23%) | 566.00 (37.71%) | <0.001 |
| Acute Kidney Injury (n, %) | 1674.00 (27.88%) | 250.00 (16.64%) | 278.00 (18.52%) | 406.00 (27.05%) | 740.00 (49.30%) | <0.001 |
| Pneumonia (n, %) | 1141.00 (19.00%) | 253.00 (16.84%) | 245.00 (16.32%) | 244.00 (16.26%) | 399.00 (26.58%) | <0.001 |
| Ischemic Heart Disease (n, %) | 2499.00 (41.62%) | 573.00 (38.15%) | 599.00 (39.91%) | 616.00 (41.04%) | 711.00 (47.37%) | <0.001 |
| Laboratory tests | ||||||
| WBC (K/μL) | 10.40 (7.70–13.78) | 10.32 (7.75–13.48) | 10.20 (7.60–13.58) | 10.30 (7.60–13.54) | 10.80 (7.85–14.70) | 0.004 |
| RBC (m/μL) | 3.65 (3.17–4.15) | 3.61 (3.18–4.09) | 3.78 (3.29–4.23) | 3.74 (3.25–4.23) | 3.46 (3.00–4.00) | <0.001 |
| Platelet (K/μL) | 193.50 (148.00–251.00) | 198.67 (149.00–264.00) | 197.00 (150.00–250.00) | 190.80 (150.00–244.75) | 190.00 (142.00–245.33) | <0.001 |
| Hemoglobin (g/dL) | 10.95 (9.47–12.45) | 10.95 (9.50–12.40) | 11.35 (9.87–12.70) | 11.20 (9.70–12.60) | 10.30 (8.85–11.93) | <0.001 |
| Scr (mg/dL) | 1.00 (0.75–1.40) | 0.80 (0.65–1.00) | 0.90 (0.70–1.20) | 1.00 (0.80–1.30) | 1.43 (1.00–2.24) | <0.001 |
| BUN (mg/dL) | 19.00 (14.00–29.25) | 14.00 (10.67–19.00) | 16.67 (13.00–22.50) | 20.00 (15.50–27.67) | 34.33 (23.00–49.67) | <0.001 |
| PT (s) | 14.00 (12.40–15.93) | 13.85 (12.30–15.93) | 13.80 (12.30–15.93) | 13.70 (12.25–15.93) | 14.60 (12.75–16.78) | <0.001 |
| PTT (s) | 31.80 (27.55–37.24) | 31.90 (27.70–37.24) | 31.37 (27.60–37.24) | 31.30 (27.20–37.24) | 32.75 (27.70–38.40) | 0.002 |
| Glucose (mg/dL) | 127.00 (105.67–157.00) | 116.00 (100.50–137.33) | 122.00 (104.00–147.67) | 129.50 (108.00–161.00) | 145.00 (115.00–191.00) | <0.001 |
| Sodium (mmol/L) | 139.00 (136.67–141.00) | 136.00 (134.00–137.00) | 139.00 (137.20–140.00) | 140.50 (138.80–142.00) | 142.00 (139.50–144.00) | <0.001 |
| Potassium (mmol/L) | 4.10 (3.77–4.45) | 4.03 (3.70–4.35) | 4.05 (3.77–4.37) | 4.10 (3.80–4.40) | 4.20 (3.80–4.65) | <0.001 |
| Medications | ||||||
| Antiplatelet (n, %) | 3419.00 (56.94%) | 857.00 (57.06%) | 889.00 (59.23%) | 848.00 (56.50%) | 825.00 (54.96%) | 0.126 |
| Statin (n, %) | 1306.00 (21.75%) | 319.00 (21.24%) | 349.00 (23.25%) | 320.00 (21.32%) | 318.00 (21.19%) | 0.447 |
| IV-tPA (n, %) | 298.00 (4.96%) | 84.00 (5.59%) | 76.00 (5.06%) | 58.00 (3.86%) | 80.00 (5.33%) | 0.135 |
| Clinical outcomes | ||||||
| 30-day mortality (n, %) | 810.00 (13.49%) | 152.00 (10.12%) | 125.00 (8.33%) | 193.00 (12.86%) | 340.00 (22.65%) | <0.001 |
| 365-day mortality (n, %) | 951.00 (15.84%) | 184.00 (12.25%) | 158.00 (10.53%) | 215.00 (14.32%) | 394.00 (26.25%) | <0.001 |
| ICU stay (day) | 2.04 (1.14–4.02) | 1.94 (1.13–3.84) | 1.99 (1.12–3.59) | 2.02 (1.10–4.08) | 2.23 (1.24–4.95) | <0.001 |
| Hospital stay (day) | 7.11 (4.04–12.65) | 7.33 (4.27–12.46) | 6.72 (3.97–11.89) | 6.90 (3.96–11.95) | 7.76 (4.03–13.95) | <0.001 |
| Outcomes | 30-Day Mortality | 365-Day Mortality | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Threshold (W) | 297.244 | 297.201 | ||
| <W | 0.968 (0.946–0.991) | 0.006 | 0.971 (0.950–0.992) | 0.007 |
| >W | 1.034 (1.024–1.044) | <0.001 | 1.032 (1.023–1.041) | <0.001 |
| Log-likelihood ratio test | <0.001 | <0.001 | ||
| Categories | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | P for Trend | HR (95%CI) | p-Value | P for Trend | HR (95%CI) | p-Value | P for Trend | |
| 30-day mortality | |||||||||
| Continuous variable | 1.05 (1.04–1.05) | <0.001 | 1.04 (1.04–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 | |||
| per unit | |||||||||
| Quartile | <0.001 | <0.001 | <0.001 | ||||||
| Q1 (N = 1502) | 1.23 (0.97–1.56) | 0.088 | 1.23 (0.97–1.56) | 0.09 | 1.30 (1.03–1.65) | 0.031 | |||
| Q2 (N = 1501) | Ref | ||||||||
| Q3 (N = 1501) | 1.59 (1.27–1.99) | <0.001 | 1.54 (1.23–1.93) | <0.001 | 1.54 (1.23–1.93) | <0.001 | |||
| Q4 (N = 1501) | 2.98 (2.43–3.66) | <0.001 | 2.79 (2.27–3.43) | <0.001 | 1.83 (1.48–2.27) | <0.001 | |||
| 365-day mortality | |||||||||
| Continuous variable | 1.05 (1.04–1.05) | <0.001 | 1.04 (1.03–1.05) | <0.001 | 1.02 (1.01–1.03) | <0.001 | |||
| per unit | |||||||||
| Quartile | <0.001 | <0.001 | <0.001 | ||||||
| Q1 (N = 1502) | 1.18 (0.95–1.46) | 0.13 | 1.17 (0.95–1.45) | 0.142 | 1.22 (0.99–1.51) | 0.068 | |||
| Q2 (N = 1501) | Ref | ||||||||
| Q3 (N = 1501) | 1.40 (1.14–1.72) | 0.001 | 1.37 (1.11–1.68) | 0.003 | 1.35 (1.10–1.66) | 0.004 | |||
| Q4 (N = 1501) | 2.77 (2.30–3.33) | <0.001 | 2.60 (2.158–3.13) | <0.001 | 1.71 (1.41–2.08) | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Wei, W. Serum Osmolality and Stroke Mortality in the ICU: A U-Shaped Risk Pattern and Its Clinical Implications. J. Clin. Med. 2025, 14, 6406. https://doi.org/10.3390/jcm14186406
Li G, Wei W. Serum Osmolality and Stroke Mortality in the ICU: A U-Shaped Risk Pattern and Its Clinical Implications. Journal of Clinical Medicine. 2025; 14(18):6406. https://doi.org/10.3390/jcm14186406
Chicago/Turabian StyleLi, Ge, and Wenshi Wei. 2025. "Serum Osmolality and Stroke Mortality in the ICU: A U-Shaped Risk Pattern and Its Clinical Implications" Journal of Clinical Medicine 14, no. 18: 6406. https://doi.org/10.3390/jcm14186406
APA StyleLi, G., & Wei, W. (2025). Serum Osmolality and Stroke Mortality in the ICU: A U-Shaped Risk Pattern and Its Clinical Implications. Journal of Clinical Medicine, 14(18), 6406. https://doi.org/10.3390/jcm14186406






