Wearable Sensors for the Assessment of Functional Outcome Following Reverse Shoulder Arthroplasty: A Systematic Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
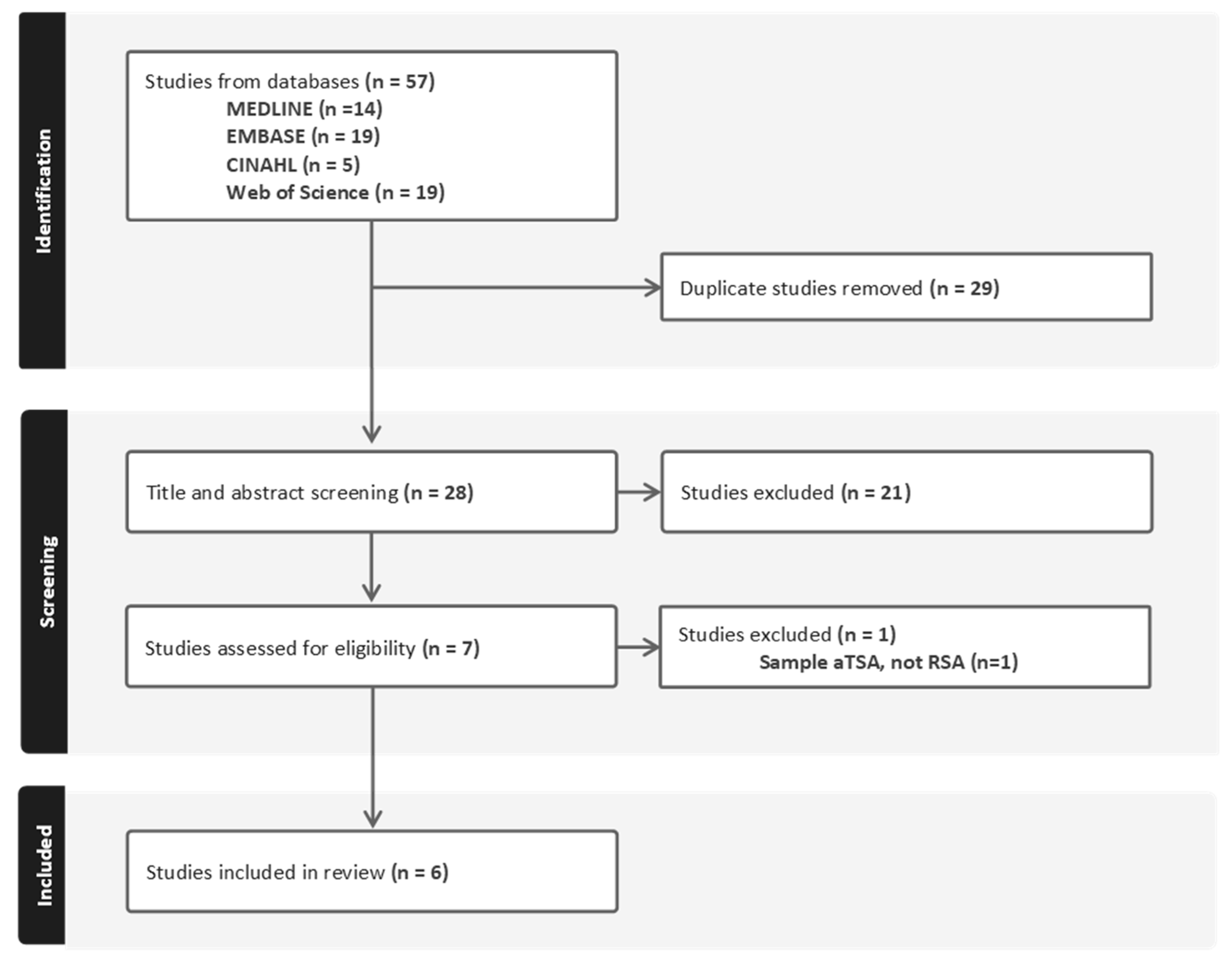
2.2. Study Selection
2.3. Data Extraction, Synthesis, and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, P.N.; Gill, D.R.; McAuliffe, M.J.; McDougall, C.; Stoney, J.D.; Vertullo, C.J.; Wall, C.J.; Corfield, S.; Page, R.; Cuthbert, A.R.; et al. Hip, Knee and Shoulder Arthroplasty: 2023 Annual Report; Australian Orthopaedic Association: Sydney, Australia, 2023. [Google Scholar]
- Garcia, G.H.; Taylor, S.A.; DePalma, B.J.; Mahony, G.T.; Grawe, B.M.; Nguyen, J.; Dines, J.S.; Dines, D.M.; Warren, R.F.; Craig, E.V.; et al. Patient Activity Levels After Reverse Total Shoulder Arthroplasty: What Are Patients Doing? Am. J. Sports Med. 2015, 43, 2816–2821. [Google Scholar] [CrossRef] [PubMed]
- MacInnes, S.J.; Mackie, K.E.; Titchener, A.; Gibbons, R.; Wang, A.W. Activity following reverse total shoulder arthroplasty: What should surgeons be advising? Shoulder Elb. 2019, 11, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Simovitch, R.W.; Gerard, B.K.; Brees, J.A.; Fullick, R.; Kearse, J.C. Outcomes of reverse total shoulder arthroplasty in a senior athletic population. J. Shoulder Elb. Surg. 2015, 24, 1481–1485. [Google Scholar] [CrossRef]
- Roche, C.; Kumar, V.; Overman, S.; Simovitch, R.; Flurin, P.-H.; Wright, T.; Routman, H.; Teredesai, A.; Zuckerman, J. Validation of a machine learning–derived clinical metric to quantify outcomes after total shoulder arthroplasty. J. Shoulder Elb. Surg. 2021, 30, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Schoch, B.S.; King, J.J.; Fan, W.; Flurin, P.-H.; Wright, T.W.; Zuckerman, J.D.; Roche, C.P. Characteristics of anatomic and reverse total shoulder arthroplasty patients who achieve ceiling scores with 3 common patient-reported outcome measures. J. Shoulder Elb. Surg. 2022, 31, 1647–1657. [Google Scholar] [CrossRef]
- Eckhard, L.; Munir, S.; Wood, D.; Talbot, S.; Brighton, R.; Walter, B.; Baré, J. The ceiling effects of patient reported outcome measures for total knee arthroplasty. Orthop. Traumatol. Surg. Res. 2021, 107, 102758. [Google Scholar] [CrossRef]
- Small, S.R.; Bullock, G.S.; Khalid, S.; Barker, K.; Trivella, M.; Price, A.J. Current clinical utilisation of wearable motion sensors for the assessment of outcome following knee arthroplasty: A scoping review. BMJ Open 2019, 9, e033832. [Google Scholar] [CrossRef]
- Christensen, J.C.; Blackburn, B.E.; Anderson, L.A.; Gililland, J.M.; Peters, C.L.; Archibeck, M.J.; Pelt, C.E. Recovery Curve for Patient Reported Outcomes and Objective Physical Activity After Primary Total Knee Arthroplasty-A Multicenter Study Using Wearable Technology. J. Arthroplast. 2023, 38, S94–S102. [Google Scholar] [CrossRef]
- Christensen, J.C.; Blackburn, B.E.; Kapron, C.R.; Pelt, C.E.; Peters, C.L.; Archibeck, M.J.; Anderson, L.A.; Gililland, J.M. Unicompartmental Knee Arthroplasty Patients Recover More like Total Hip Patients than Total Knee Patients: A Prospective Longitudinal Study. J. Arthroplast. 2025, 40, S77–S83. [Google Scholar] [CrossRef]
- The Joanna Briggs Institute. The Joanna Briggs Institute Reviewer’s Manual: Methodology for JBI Scoping Reviews. Available online: https://www.joannabriggs.org (accessed on 22 August 2024).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Langohr, G.D.G.; Haverstock, J.P.; Johnson, J.A.; Athwal, G.S. Comparing daily shoulder motion and frequency after anatomic and reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2018, 27, 325–332. [Google Scholar] [CrossRef]
- Morgan, C.; Hargreaves, M.; Williams, M.; Hoyt, R.E.; Snider, D.H.; Callanan, M.; Nelson, A.; Brabston, E.W.; Momaya, A.M.; Ponce, B.A.; et al. The use of actigraphy to objectively define motion and function before and after shoulder arthroplasty. J. Orthop. 2024, 56, 6–11. [Google Scholar] [CrossRef]
- Edwards, P.K.; Ebert, J.R.; Morrow, M.M.; Goodwin, B.M.; Ackland, T.; Wang, A. Accelerometry evaluation of shoulder movement and its association with patient-reported and clinical outcomes following reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2020, 29, 2308–2318. [Google Scholar] [CrossRef]
- Hurd, W.J.; Morrow, M.M.; Miller, E.J.; Adams, R.A.; Sperling, J.W.; Kaufman, K.R. Patient-Reported and Objectively Measured Function Before and After Reverse Shoulder Arthroplasty. J. Geriatr. Phys. Ther. 2018, 41, 126–133. [Google Scholar] [CrossRef]
- Chapman, R.M.; Torchia, M.T.; Bell, J.-E.; Van Citters, D.W. Using inertial measurement units to quantify shoulder elevation after reverse total shoulder arthroplasty: A pilot study comparing goniometric measures captured clinically to inertial measures captured ‘in-the-wild’. Semin. Arthroplast. JSES 2023, 33, 85–93. [Google Scholar] [CrossRef]
- Van de Kleut, M.L.; Bloomfield, R.A.; Teeter, M.G.; Athwal, G.S. Monitoring daily shoulder activity before and after reverse total shoulder arthroplasty using inertial measurement units. J. Shoulder Elb. Surg. 2021, 30, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Hurd, W.J.; Morrow, M.M.; Kaufman, K.R. Tri-axial accelerometer analysis techniques for evaluating functional use of the extremities. J. Electromyogr. Kinesiol. 2013, 23, 924–929. [Google Scholar] [CrossRef]
- Hilden, P.; Schwartz, J.E.; Pascual, C.; Diaz, K.M.; Goldsmith, J. How many days are needed? Measurement reliability of wearable device data to assess physical activity. PLoS ONE 2023, 18, e0282162. [Google Scholar] [CrossRef] [PubMed]
- Coley, B.; Jolles, B.M.; Farron, A.; Aminian, K. Arm position during daily activity. Gait Posture 2008, 28, 581–587. [Google Scholar] [CrossRef]
- Fary, C.; Cholewa, J.; Abshagen, S.; Van Andel, D.; Ren, A.; Anderson, M.B.; Tripuraneni, K. Stepping Beyond Counts in Recovery of Total Hip Arthroplasty: A Prospective Study on Passively Collected Gait Metrics. Sensors 2023, 23, 6538. [Google Scholar] [CrossRef] [PubMed]
- Fary, C.; Cholewa, J.; Abshagen, S.; Van Andel, D.; Ren, A.; Anderson, M.B.; Tripuraneni, K.R. Stepping beyond Counts in Recovery of Total Knee Arthroplasty: A Prospective Study on Passively Collected Gait Metrics. Sensors 2023, 23, 5588. [Google Scholar] [CrossRef] [PubMed]
- Yocum, D.; Elashoff, B.; Verta, P.; Armock, G.; Yergler, J. Patient reported outcomes do not correlate to functional knee recovery and range of motion in total knee arthroplasty. J. Orthop. 2023, 43, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Lawinger, E.; Uhl, T.L.; Abel, M.; Kamineni, S. Assessment of Accelerometers for Measuring Upper-Extremity Physical Activity. J. Sport Rehabil. 2015, 24, 236–243. [Google Scholar] [CrossRef] [PubMed]
| # | Search Terms |
|---|---|
| 1 | Arthroplasty, Replacement, Shoulder/ |
| 2 | (shoulder arthroplast* OR shoulder replace*).mp |
| 3 | Wearable electronic device/ |
| 4 | Wearable*.ti,ab. |
| 5 | (IMU* OR inertial measurement unit* OR inertial sens*).mp |
| 6 | (smart watch* OR fitbit* OR apple watch* OR activity monitor* OR activity tracker* OR garmin* OR activpal OR pedometer OR actigraph*).mp |
| 7 | (accelerometer* OR gyroscope*).mp |
| 8 | 1 OR 2 |
| 9 | 3 OR 4 OR 5 OR 6 OR 7 |
| 10 | 8 AND 9 |
| Study | Year | Study Design | Sample Size | Sample Demographics | Follow-Up Time Points | Setting | Implant Type(s) |
|---|---|---|---|---|---|---|---|
| Chapman et al. [17] | 2023 | Prospective pilot study | RSA (n = 10); control (n = 10) | RSA: Mean age: 82.0 y ± 5.0; 1 M/9 F Control: Mean age: 69.0 y ± 20.0; 4 M/6 F | Preoperative, 3, 12, 24 months | Free-living | Trabecular Metal Reverse Shoulder System (Zimmer Biomet) |
| Edwards et al. [15] | 2020 | Prospective non-randomized | RSA (n = 36) | Mean age: 73.9 y (range: 56–84); 61% F | 3, 6 and 12 months | Free-living | Equinoxe Reverse Shoulder Design (Exactech) |
| Hurd et al. [16] | 2018 | Prospective cohort study | RSA (n = 14) | Mean age 73.0 y ± 6.0; 7 M/7 F | Preoperative, 2 and 12 months | Free-living | Not reported |
| Langohr et al. [13] | 2018 | Basic science study | RSA (n = 20); aTSA (n = 16) | Mean age 73.0 y ± 10.0 *,† | >12 months | Free-living | Not specified |
| Morgan et al. [14] | 2024 | Prospective cohort study | RSA (n = 28); aTSA (n = 36) | Mean age: 68.7 y (range: 38–86); 60% M * | Preoperative and 24 weeks | Free-living | Biomet (n = 40), Tornier Flex (n = 21) and Simpliciti (n = 2) |
| Van de Kleut et al. [18] | 2021 | Prospective case series | RSA (n = 33) | Mean age 71.8 y ± 8.0; 58% M | Preoperative, 3 and 12 months | Free-living | Aequalis Ascend Flex (Wright Medical-Tornier Group |
| Study | Sensor Type | Axes | Components Used | Sampling Rate | Sensor Placement | No. of Sensors | Session Length | Wear Time |
|---|---|---|---|---|---|---|---|---|
| Chapman et al. [17] | APDM IMU | 3 | Accelerometer, gyroscope, magnetometer | Not specified | Unilateral humerus (operated limb only), and sternum | 2 | >7 days | >8 h/day |
| Edwards et al. [15] | ActiGraph GT9X Link | 3 | Accelerometer | 100 Hz | Bilateral humeri, bilateral wrists | 4 | ≤3 days | ≥10 h/day |
| Hurd et al. [16] | ActiGraph GT3X+ | 3 | Accelerometer | 100 Hz | Unilateral humerus and wrist (operated limb only) | 2 | ≤3 days | ≥10 h/day |
| Langohr et al. [13] | YEI Technology IMU | 3 | Accelerometer, gyroscope, compass | Not specified | Sternum, bilateral humeri, bilateral wrists via compression shirts | 5 | <24 h | Full waking hours |
| Morgan et al. [14] | ActiGraph GT9X Link | 3 | Accelerometer, gyroscope, magnetometer | 100 Hz | Unilateral humerus (operated limb only) | 1 | <24 h | Full waking hours |
| Van de Kleut et al. [18] | 3-Space Data Logger; Yost Labs | 3 | Accelerometer, gyroscope, compass | 10 Hz | Unilateral humerus (operated limb only), and sternum | 2 | <24 h | Full waking hours |
| Study | Outcome Category | Outcome Variables and Metrics Reported |
|---|---|---|
| Chapman et al. [17] | Motion | Average degrees elevation (weekly); maximum degrees elevation (weekly); per cent time in 15° bins <90°; per cent time in 45° bins over >90°. |
| Edwards et al. [15] | Activity | Mean activity values (calculated from the vector magnitude per 15 s epoch), limb symmetry index, magnitude ratio. |
| Hurd et al. [16] | Activity | Mean activity values (calculated from the vector magnitude per 60 s epoch), per cent time inactive or in low-intensity or high-intensity activity. |
| Langohr et al. [13] | Motion | Per cent time spent in elevation ranges (e.g., <60°, 60–80°, >80°, >100°); per cent time in elevation planes (forward flexion = 0°); motion frequency (e.g., elevations) per hour; estimated annual cycles of the shoulder extrapolated from daily data. |
| Morgan et al. [14] | Activity | Mean activity count (counts/sec) per axis; mean vector magnitude per 1 s epoch; per cent time spent in sedentary, light, moderate, vigorous, and very vigorous activity. |
| Van de Kleut et al. [18] | Motion | Elevation events per hour; elevation events per hour under >90°; per cent time spent >90° elevation; per cent time spent in low-, moderate-, and high-intensity activity per day. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwards, P.K.; Ebert, J.R.; Blakeney, W.G.; Bauer, S.; Wang, A.W. Wearable Sensors for the Assessment of Functional Outcome Following Reverse Shoulder Arthroplasty: A Systematic Scoping Review. J. Clin. Med. 2025, 14, 6401. https://doi.org/10.3390/jcm14186401
Edwards PK, Ebert JR, Blakeney WG, Bauer S, Wang AW. Wearable Sensors for the Assessment of Functional Outcome Following Reverse Shoulder Arthroplasty: A Systematic Scoping Review. Journal of Clinical Medicine. 2025; 14(18):6401. https://doi.org/10.3390/jcm14186401
Chicago/Turabian StyleEdwards, Peter K., Jay R. Ebert, William G. Blakeney, Stefan Bauer, and Allan W. Wang. 2025. "Wearable Sensors for the Assessment of Functional Outcome Following Reverse Shoulder Arthroplasty: A Systematic Scoping Review" Journal of Clinical Medicine 14, no. 18: 6401. https://doi.org/10.3390/jcm14186401
APA StyleEdwards, P. K., Ebert, J. R., Blakeney, W. G., Bauer, S., & Wang, A. W. (2025). Wearable Sensors for the Assessment of Functional Outcome Following Reverse Shoulder Arthroplasty: A Systematic Scoping Review. Journal of Clinical Medicine, 14(18), 6401. https://doi.org/10.3390/jcm14186401