Predictive Value of Classical and Emerging Autoantibodies for Cardiac Dysfunction in Systemic Sclerosis: Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Protocol, Registration and Criteria
- Enrolled adult patients diagnosed with SSc based on American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria [18];
- Reported cardiac involvement (e.g., myocardial fibrosis, arrhythmias, conduction abnormalities, left ventricular (LV) dysfunction);
- Assessed classical or emerging autoantibodies (e.g., anti-centromere antibodies [ACA], anti-topoisomerase I antibodies [anti-Scl-70], anti-RNA polymerase III [anti-RNAP III], anti-U3 ribonucleoprotein [anti-U3 RNP], anti-heart antibodies [AHA], anti-intercalated disk antibodies [AIDA]);
- Used imaging (e.g., cardiac magnetic resonance [CMR], echocardiography) or biomarker-based methods (e.g., N-terminal pro-brain natriuretic peptide [NT-proBNP], troponin) for cardiac assessment.
- Non-human studies, editorials, and conference abstracts.
2.2. Information Sources
- PubMed
- Web of Science
- Scopus
- Cochrane Library
2.3. Search Strategy and Study Selection
2.4. Data Collection Process
- Study design and setting;
- Sample size and demographics;
- Diagnostic criteria for SSc;
- Autoantibody types and titers;
- Cardiac outcome measures (e.g., fibrosis, LV dysfunction, arrhythmias);
- Imaging and biomarkers used;
- Main findings and statistical associations.
2.5. Risk of Bias Assessment
2.6. Synthesis of Results and Assessment of Evidence Quality
3. Results
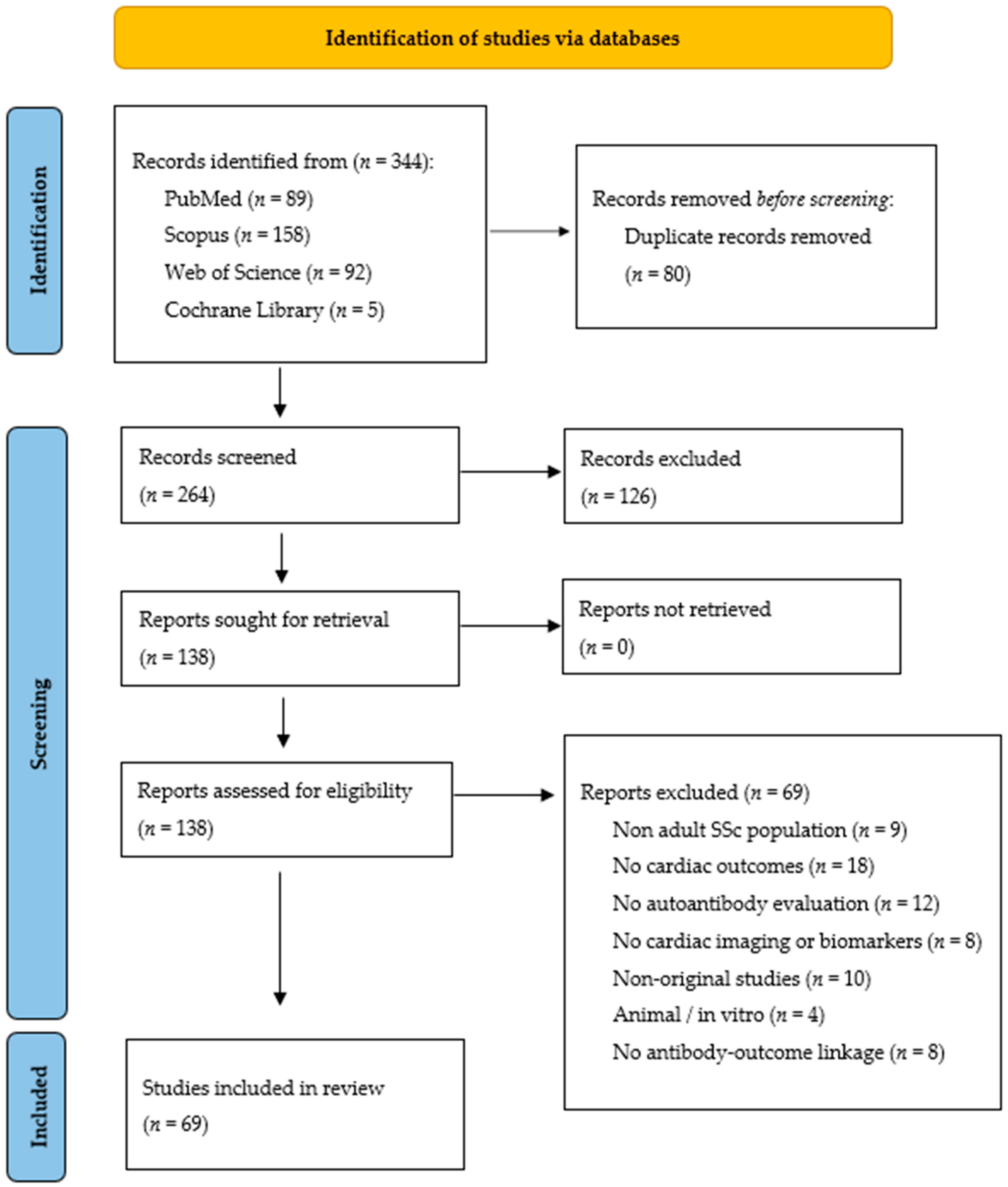
3.1. Study Selection
3.2. Study Characteristics and Quality Assessment
4. Pathogenesis of Primary Cardiac Involvement in SSc
5. Autoantibodies and Cardiac Manifestations in SSc
5.1. Anticentromere Antibodies
5.2. Anti-Topoisomerase I Antibodies
5.3. Emerging Autoantibodies and Cardiac Manifestations in SSc
5.4. Associations Between Autoantibody Profiles and Cardiac Outcomes
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACA | anti-centromere antibodies |
| ACR/EULAR | American College of Rheumatology/European League Against Rheumatism |
| AHA | anti-heart antibodies |
| AIDA | anti–intercalated disk antibodies |
| Anti-NOR90 | anti–nucleolar organizing region 90 antibodies |
| anti-Mi2 | antibodies targeting Mi-2 helicase |
| anti-PL-7 | anti–PL7 antibodies |
| anti-PL12 | anti–PL-12 antibodies |
| anti-PmScl | anti–Pm/Scl antibodies |
| anti-RNAP III | anti–RNA polymerase III |
| anti-Ro/SSA | anti–Ro/Sjögren’s-syndrome-related antigen A antibodies |
| anti-Scl-70 | anti–topoisomerase I antibodies |
| anti-U1 RNP | anti–U1 ribonucleoprotein antibodies |
| anti-U3 RNP | anti–U3 ribonucleoprotein |
| AV | atrioventricular |
| CMR | cardiac magnetic resonance |
| CPET | cardiopulmonary exercise testing |
| CTGF | connective tissue growth factor |
| CV | cardiovascular |
| dc | diffuse cutaneous |
| DLCO | diffusing capacity of the lungs for carbon monoxide |
| DNA | deoxyribonucleic acid |
| ECG | Electrocardiographic |
| EndMT | endothelial-to-mesenchymal transition |
| EUSTAR | European League Against Rheumatism Scleroderma Trials and Research |
| FRS | Framingham Risk Score |
| HFA | Heart Failure Association |
| IL | interleukin |
| ILD | interstitial lung disease |
| IRR | incidence rate ratio |
| lc | limited cutaneous |
| LGE | late gadolinium enhancement |
| LV | left ventricular |
| LVDD | left ventricular diastolic dysfunction |
| microRNA | micro-ribonucleic acid |
| NOS | Newcastle–Ottawa Scale |
| NT-proBNP | N-terminal pro–brain natriuretic peptide |
| PAH | pulmonary arterial hypertension |
| PDGF | platelet-derived growth factor |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PVCs | premature ventricular contractions |
| RNA | ribonucleic acid |
| RP | Raynaud’s phenomenon |
| RSS | Reynolds Risk Score |
| SCORE | Systematic Coronary Risk Evaluation |
| SSc | systemic sclerosis |
| SSc-pHI | SSc-primary cardiac involvement |
| TGF-β | transforming growth factor-beta |
| WSF | World Scleroderma Foundation |
References
- Kowal-Bielecka, O.; Bielecki, M.; Kowal, K. Recent Advances in the Diagnosis and Treatment of Systemic Sclerosis. Pol. Arch. Intern. Med. 2013, 123, 51–58. [Google Scholar] [CrossRef][Green Version]
- Ross, L.; Prior, D.; Proudman, S.; Vacca, A.; Baron, M.; Nikpour, M. Defining Primary Systemic Sclerosis Heart Involvement: A Scoping Literature Review. Semin. Arthritis Rheum. 2019, 48, 874–887. [Google Scholar] [CrossRef]
- Tyndall, A.J.; Bannert, B.; Vonk, M.; Airò, P.; Cozzi, F.; Carreira, P.E.; Bancel, D.F.; Allanore, Y.; Müller-Ladner, U.; Distler, O.; et al. Causes and Risk Factors for Death in Systemic Sclerosis: A Study from the EULAR Scleroderma Trials and Research (EUSTAR) Database. Ann. Rheum. Dis. 2010, 69, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Bairkdar, M.; Dong, Z.; Andell, P.; Hesselstrand, R.; Holmqvist, M. Arrhythmia in Patients with Systemic Sclerosis: Incidence, Risk Factors and Impact on Mortality in a Swedish Register-Based Study. RMD Open 2024, 10, e004532. [Google Scholar] [CrossRef]
- Narváez, J.; Lluch, J.; Ruiz-Majoral, A.; Sánchez-Corral, M.A.; Claver, E.; Nolla, J.M. Increased Prevalence of Moderate to Severe Mitral and Aortic Valve Dysfunction in Systemic Sclerosis: A Case-Control Study. J. Rheumatol. 2021, 48, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Joven, B.E.; Almodovar, R.; Carmona, L.; Carreira, P.E. Survival, Causes of Death, and Risk Factors Associated with Mortality in Spanish Systemic Sclerosis Patients: Results from a Single University Hospital. Semin. Arthritis Rheum. 2010, 39, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Muresan, L.; Petcu, A.; Pamfil, C.; Muresan, C.; Rinzis, M.; Mada, R.O.; Gusetu, G.N.; Pop, D.; Zdrenghea, D.; Rednic, S. Cardiovascular profiles of scleroderma patients with arrhythmias and conduction disorders. Acta Reum. Port. 2016, 41, 26–39. [Google Scholar]
- Varga, J.; Denton, C.P.; Wigley, F.M.; Allanore, Y.; Kuwana, M. (Eds.) Scleroderma: From Pathogenesis to Comprehensive Management, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Dinser, R.; Frerix, M.; Meier, F.M.; Klingel, K.; Rolf, A. Endocardial and Myocardial Involvement in Systemic Sclerosis—Is There a Relevant Inflammatory Component? Jt. Bone Spine 2013, 80, 320–323. [Google Scholar] [CrossRef]
- Györfi, A.H.; Filla, T.; Polzin, A.; Tascilar, K.; Buch, M.; Tröbs, M.; Matei, A.E.; Airo, P.; Balbir-Gurman, A.; Kuwert, F.; et al. Evaluation of Systemic Sclerosis Primary Heart Involvement and Chronic Heart Failure in the European Scleroderma Trials and Research Cohort. J. Am. Heart Assoc. 2025, 14, e036730. [Google Scholar] [CrossRef]
- Tzelepis, G.E.; Kelekis, N.L.; Plastiras, S.C.; Mitseas, P.; Economopoulos, N.; Kampolis, C.; Gialafos, E.J.; Moyssakis, I.; Moutsopoulos, H.M. Pattern and Distribution of Myocardial Fibrosis in Systemic Sclerosis: A Delayed Enhanced Magnetic Resonance Imaging Study. Arthritis Rheum. 2007, 56, 3827–3836. [Google Scholar] [CrossRef]
- Moysidou, G.S.; Dara, A.; Arvanitaki, A.; Skalkou, A.; Pagkopoulou, E.; Daoussis, D.; Kitas, G.D.; Dimitroulas, T. Understanding and Managing Cardiac Involvement in Systemic Sclerosis. Expert Rev. Clin. Immunol. 2023, 19, 293–304. [Google Scholar] [CrossRef]
- Nie, L.Y.; Wang, X.D.; Zhang, T.; Xue, J. Cardiac Complications in Systemic Sclerosis: Early Diagnosis and Treatment. Chin. Med. J. 2019, 132, 2865–2871. [Google Scholar] [CrossRef]
- Elhai, M.; Meune, C.; Boubaya, M.; Avouac, J.; Hachulla, E.; Balbir-Gurman, A.; Riemekasten, G.; Airò, P.; Joven, B.; Vettori, S.; et al. Mapping and Predicting Mortality from Systemic Sclerosis. Ann. Rheum. Dis. 2017, 76, 1897–1905. [Google Scholar] [CrossRef]
- Bruni, C.; Buch, M.H.; Furst, D.E.; De Luca, G.; Djokovic, A.; Dumitru, R.B.; Giollo, A.; Polovina, M.; Steelandt, A.; Bratis, K.; et al. Primary Systemic Sclerosis Heart Involvement: A Systematic Literature Review and Preliminary Data-Driven, Consensus-Based WSF/HFA Definition. J. Scleroderma Relat. Disord. 2022, 7, 24–32. [Google Scholar] [CrossRef]
- Oliveira, M.I.; Bragança, B.; Gomes, J.R.; Santos, M. Cardiac Involvement and Heart Failure Staging in Patients with Systemic Sclerosis without Pulmonary Arterial Hypertension. J. Clin. Med. 2025, 14, 2211. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 26 July 2025).
- Allanore, Y.; Meune, C.; Vonk, M.C.; Airo, P.; Hachulla, E.; Caramaschi, P.; Riemekasten, G.; Cozzi, F.; Beretta, L.; Derk, C.T.; et al. Prevalence and Factors Associated with Left Ventricular Dysfunction in the EULAR Scleroderma Trial and Research Group (EUSTAR) Database of Patients with Systemic Sclerosis. Ann. Rheum. Dis. 2010, 69, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Höppner, J.; Tabeling, C.; Casteleyn, V.; Kedor, C.; Windisch, W.; Burmester, G.R.; Huscher, D.; Siegert, E. Comprehensive Autoantibody Profiles in Systemic Sclerosis: Clinical Cluster Analysis. Front. Immunol. 2023, 13, 1045523. [Google Scholar] [CrossRef]
- Radwan, Y.A.; Kurmann, R.D.; Sandhu, A.S.; El-Am, E.A.; Crowson, C.S.; Matteson, E.L.; Osborn, T.G.; Warrington, K.J.; Mankad, R.; Makol, A. Systemic Sclerosis Portends Increased Risk of Conduction and Rhythm Abnormalities at Diagnosis and During Disease Course: A US Population-Based Cohort. J. Scleroderma Relat. Disord. 2021, 6, 277–285. [Google Scholar] [CrossRef]
- López Núñez, L.; Carrión-Barberà, I.; Molina, L.; Padró, I.; Ciria, M.; Salman-Monte, T.C.; Pros, A. Left Ventricular Dysfunction and Arrhythmias in Asymptomatic Patients with Systemic Sclerosis. Med. Clin. 2023, 160, 434–442. [Google Scholar] [CrossRef]
- Avouac, J.; Meune, C.; Chenevier-Gobeaux, C.; Borderie, D.; Lefevre, G.; Kahan, A.; Allanore, Y. Cardiac Biomarkers in Systemic Sclerosis: Contribution of High-Sensitivity Cardiac Troponin in Addition to N-Terminal Pro-Brain Natriuretic Peptide. Arthritis Care Res. 2015, 67, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, R.B.; Bissell, L.A.; Erhayiem, B.; Fent, G.; Kidambi, A.; Swoboda, P.; Abignano, G.; Donica, H.; Burska, A.; Greenwood, J.P.; et al. Predictors of Subclinical Systemic Sclerosis Primary Heart Involvement Characterised by Microvasculopathy and Myocardial Fibrosis. Rheumatology 2021, 60, 2934–2945. [Google Scholar] [CrossRef]
- Cusmà Piccione, M.; Zito, C.; Bagnato, G.; Oreto, G.; Di Bella, G.; Bagnato, G.; Carerj, S. Role of 2D Strain in the Early Identification of Left Ventricular Dysfunction and in the Risk Stratification of Systemic Sclerosis Patients. Cardiovasc. Ultrasound 2013, 11, 6. [Google Scholar] [CrossRef]
- Barison, A.; Gargani, L.; De Marchi, D.; Aquaro, G.D.; Guiducci, S.; Picano, E.; Cerinic, M.M.; Pingitore, A. Early Myocardial and Skeletal Muscle Interstitial Remodelling in Systemic Sclerosis: Insights from Extracellular Volume Quantification Using Cardiovascular Magnetic Resonance. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 74–80. [Google Scholar] [CrossRef]
- Hachulla, A.L.; Launay, D.; Gaxotte, V.; de Groote, P.; Lamblin, N.; Devos, P.; Hatron, P.Y.; Beregi, J.P.; Hachulla, E. Cardiac Magnetic Resonance Imaging in Systemic Sclerosis: A Cross-Sectional Observational Study of 52 Patients. Ann. Rheum. Dis. 2009, 68, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Krumm, P.; Mueller, K.A.; Klingel, K.; Kramer, U.; Horger, M.S.; Zitzelsberger, T.; Kandolf, R.; Gawaz, M.; Nikolaou, K.; Klumpp, B.D.; et al. Cardiovascular Magnetic Resonance Patterns of Biopsy Proven Cardiac Involvement in Systemic Sclerosis. J. Cardiovasc. Magn. Reson. 2016, 18, 70. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yokoe, I.; Hirano, M.; Nakamura, T.; Nakajima, Y.; Fontaine, K.R.; Giles, J.T.; Kobayashi, Y. Cardiac Magnetic Resonance Imaging with Pharmacological Stress Perfusion and Delayed Enhancement in Asymptomatic Patients with Systemic Sclerosis. J. Rheumatol. 2009, 36, 106–112. [Google Scholar] [CrossRef]
- Hui, M.; Zhou, J.; Zhang, L.; Duan, X.; Li, M.; Wang, Q.; Zhao, J.; Hou, Y.; Xu, D.; Zeng, X. Prevalence and Risk Factors for Left Ventricular Diastolic Dysfunction in Systemic Sclerosis: A Multi-Center Study of CRDC Cohort in China. Clin. Rheumatol. 2021, 40, 4589–4596. [Google Scholar] [CrossRef]
- Vacca, A.; Meune, C.; Gordon, J.; Chung, L.; Proudman, S.; Assassi, S.; Nikpour, M.; Rodriguez-Reyna, T.S.; Khanna, D.; Lafyatis, R.; et al. Cardiac Arrhythmias and Conduction Defects in Systemic Sclerosis. Rheumatology 2014, 53, 1172–1177. [Google Scholar] [CrossRef]
- D’Andrea, A.; Bellissimo, S.; Scotto di Uccio, F.; Vigorito, F.; Moscato, F.; Tozzi, N.; Di Donato, M.; Citro, R.; Stisi, S.; Scherillo, M. Associations of Right Ventricular Myocardial Function with Skin and Pulmonary Involvement in Asymptomatic Patients with Systemic Sclerosis. Ital. Heart J. 2004, 5, 831–839. [Google Scholar]
- Kahan, A.; Allanore, Y. Primary Myocardial Involvement in Systemic Sclerosis. Rheumatology 2006, 45 (Suppl. S4), iv14–iv17. [Google Scholar] [CrossRef]
- Flower, V.A.; Barratt, S.L.; Ward, S.; Pauling, J.D. The Role of Vascular Endothelial Growth Factor in Systemic Sclerosis. Curr. Rheumatol. Rev. 2019, 15, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Champion, H.C. The Heart in Scleroderma. Rheum. Dis. Clin. N. Am. 2008, 34, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Del Rosso, A.; Giacomelli, R.; Cipriani, P.; Conforti, M.L.; Guiducci, S.; Gay, R.E.; Michel, B.A.; Brühlmann, P.; Müller-Ladner, U.; et al. Angiogenic and Angiostatic Factors in Systemic Sclerosis: Increased Levels of Vascular Endothelial Growth Factor Are a Feature of the Earliest Disease Stages and Are Associated with the Absence of Fingertip Ulcers. Arthritis Res. 2002, 4, R11. [Google Scholar] [CrossRef] [PubMed]
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic Sclerosis. Nat. Rev. Dis. Primers 2015, 1, 15002. [Google Scholar] [CrossRef]
- Fuschiotti, P. Current Perspectives on the Immunopathogenesis of Systemic Sclerosis. Immunotargets Ther. 2016, 5, 21–35. [Google Scholar] [CrossRef]
- Dantas, A.T.; Gonçalves, S.M.; Pereira, M.C.; Gonçalves, R.S.; Marques, C.D.; Rego, M.J.; Pitta, I.d.R.; Duarte, A.L.; Pitta, M.G. Increased IL-35 Serum Levels in Systemic Sclerosis and Association with Pulmonary Interstitial Involvement. Clin. Rheumatol. 2015, 34, 1621–1625. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Pepe, A.; Nijveldt, R.; Ntusi, N.; Sierra-Galan, L.M.; Bratis, K.; Wei, J.; Mukherjee, M.; Markousis-Mavrogenis, G.; Gargani, L.; et al. Cardiovascular Magnetic Resonance in Autoimmune Rheumatic Diseases: A Clinical Consensus Document by the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e308–e322. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-Mesenchymal Transition Contributes to Cardiac Fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Di Benedetto, P.; Ruscitti, P.; Berardicurti, O.; Vomero, M.; Navarini, L.; Dolo, V.; Cipriani, P.; Giacomelli, R. Endothelial-to-Mesenchymal Transition in Systemic Sclerosis. Clin. Exp. Immunol. 2021, 205, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, H.; Zhao, M.; Lu, Q. Meta-Analysis of Differentially Expressed microRNAs in Systemic Sclerosis. Int. J. Rheum. Dis. 2020, 23, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana, I.; Vojinovic, T.; Airo’, P.; Fredi, M.; Ceribelli, A.; Pedretti, E.; Lazzaroni, M.G.; Garrafa, E.; Franceschini, F. Systemic Sclerosis-Specific Antibodies: Novel and Classical Biomarkers. Clin. Rev. Allergy Immunol. 2023, 64, 412–430. [Google Scholar] [CrossRef]
- Koenig, M.; Dieudé, M.; Senécal, J.L. Predictive Value of Antinuclear Autoantibodies: The Lessons of the Systemic Sclerosis Autoantibodies. Autoimmun. Rev. 2008, 7, 588–593. [Google Scholar] [CrossRef]
- Walker, U.A.; Tyndall, A.; Czirják, L.; Denton, C.; Farge-Bancel, D.; Kowal-Bielecka, O.; Müller-Ladner, U.; Bocelli-Tyndall, C.; Matucci-Cerinic, M. Clinical Risk Assessment of Organ Manifestations in Systemic Sclerosis: A Report from the EULAR Scleroderma Trials and Research Group Database. Ann. Rheum. Dis. 2007, 66, 754–763. [Google Scholar] [CrossRef]
- Gunn, J.; Pauling, J.D.; McHugh, N.J. Impact of Anti-Centromere Antibodies on Pulmonary Function Test Results in Patients with Systemic Sclerosis without Established or Suspected Pulmonary Disease. Clin. Rheumatol. 2014, 33, 869–871. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Müller, C.S.; Paiva, E.S.; Azevedo, V.F.; Radominski, S.C.; Lima Filho, J.H. Autoantibody Profile and Clinical Correlation in a Group of Patients with Systemic Sclerosis in Southern Brazil. Rev. Bras. Reumatol. 2011, 51, 314–318, 323–324. [Google Scholar] [PubMed][Green Version]
- Mani, P.; Gonzalez, D.; Chatterjee, S.; Faulx, M.D. Cardiovascular Complications of Systemic Sclerosis: What to Look For. Cleve. Clin. J. Med. 2019, 86, 685–695. [Google Scholar] [CrossRef]
- Coghlan, J.G.; Denton, C.P.; Grünig, E.; Bonderman, D.; Distler, O.; Khanna, D.; Müller-Ladner, U.; Pope, J.E.; Vonk, M.C.; Doelberg, M.; et al. Evidence-Based Detection of Pulmonary Arterial Hypertension in Systemic Sclerosis: The DETECT Study. Ann. Rheum. Dis. 2014, 73, 1340–1349. [Google Scholar] [CrossRef]
- Boutou, A.K.; Pitsiou, G.G.; Siakka, P.; Dimitroulas, T.; Paspala, A.; Sourla, E.; Chavouzis, N.; Garyfallos, A.; Argyropoulou, P.; Stanopoulos, I. Phenotyping Exercise Limitation in Systemic Sclerosis: The Use of Cardiopulmonary Exercise Testing. Respiration 2016, 91, 115–123. [Google Scholar] [CrossRef]
- Tan, E.M.; Rodnan, G.P.; Garcia, I.; Moroi, Y.; Fritzler, M.J.; Peebles, C. Diversity of Antinuclear Antibodies in Progressive Systemic Sclerosis: Anti-Centromere Antibody and Its Relationship to CREST Syndrome. Arthritis Rheum. 1980, 23, 617–625. [Google Scholar] [CrossRef]
- Boonstra, M.; Bakker, J.A.; Grummels, A.; Ninaber, M.K.; Ajmone Marsan, N.; Wortel, C.M.; Huizinga, T.W.J.; Jordan, S.; Hoffman-Vold, A.M.; Distler, O.; et al. Association of Anti-Topoisomerase I Antibodies of the IgM Isotype with Disease Progression in Anti-Topoisomerase I-Positive Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Guillevin, L.; Krieg, T.; Grummeyer, E.; Behra, H.; Riemekasten, G.; Rosenberg, D.; Silkey, M.; Zultak, M.; Matucci-Cerinic, M.; et al. Autoantibody Status and Disease Manifestations in Patients with Systemic Sclerosis and Digital Ulcers: Results from the DUO Registry. Rheumatology 2012, 51, 413–422. [Google Scholar] [CrossRef]
- Hromadka, M.; Baxa, J.; Seidlerova, J.; Miklik, R.; Rajdl, D.; Sudova, V.; Suchy, D.; Rokyta, R. Myocardial Involvement Detected Using Cardiac Magnetic Resonance Imaging in Patients with Systemic Sclerosis: A Prospective Observational Study. J. Clin. Med. 2021, 10, 5364. [Google Scholar] [CrossRef]
- Mousseaux, E.; Agoston-Coldea, L.; Marjanovic, Z.; Baudet, M.; Reverdito, G.; Bollache, E.; Kachenoura, N.; Messas, E.; Soulat, G.; Farge, D. Diastolic Function Assessment of Left and Right Ventricles by MRI in Systemic Sclerosis Patients. J. Magn. Reson. Imaging 2022, 56, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Bissell, L.A.; Md Yusof, M.Y.; Buch, M.H. Primary Myocardial Disease in Scleroderma—A Comprehensive Review of the Literature to Inform the UK Systemic Sclerosis Study Group Cardiac Working Group. Rheumatology 2017, 56, 882–895. [Google Scholar] [CrossRef]
- Schneider, A.; Jhawar, N.; Balistreri, L.; Chirila, R.; Berianu, F. A Rare Case of Cardiac Tamponade in a Patient with Anti-RNA Polymerase III Scleroderma. Cureus 2023, 15, e38533. [Google Scholar] [CrossRef]
- Sharif, R.; Fritzler, M.J.; Mayes, M.D.; Gonzalez, E.B.; McNearney, T.A.; Draeger, H.; Baron, M.; Furst, D.E.; Khanna, D.K.; del Junco, D.J.; et al. Anti-Fibrillarin Antibody in African American Patients with Systemic Sclerosis: Immunogenetics, Clinical Features, and Survival Analysis. J. Rheumatol. 2011, 38, 1622–1630. [Google Scholar] [CrossRef]
- Steen, V.D. Autoantibodies in Systemic Sclerosis. Semin. Arthritis Rheum. 2005, 35, 35–42. [Google Scholar] [CrossRef]
- Avouac, J.; Airò, P.; Meune, C.; Beretta, L.; Dieude, P.; Caramaschi, P.; Tiev, K.; Cappelli, S.; Diot, E.; Vacca, A.; et al. Prevalence of Pulmonary Hypertension in Systemic Sclerosis in European Caucasians and Metaanalysis of 5 Studies. J. Rheumatol. 2010, 37, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Hanke, K.; Dähnrich, C.; Brückner, C.S.; Huscher, D.; Becker, M.; Jansen, A.; Meyer, W.; Egerer, K.; Hiepe, F.; Burmester, G.R.; et al. Diagnostic Value of Anti-Topoisomerase I Antibodies in a Large Monocentric Cohort. Arthritis Res. Ther. 2009, 11, R28. [Google Scholar] [CrossRef]
- Jacobsen, S.; Halberg, P.; Ullman, S.; Van Venrooij, W.J.; Høier-Madsen, M.; Wiik, A.; Petersen, J. Clinical Features and Serum Antinuclear Antibodies in 230 Danish Patients with Systemic Sclerosis. Br. J. Rheumatol. 1998, 37, 39–45. [Google Scholar] [CrossRef]
- Rodriguez-Reyna, T.S.; Hinojosa-Azaola, A.; Martinez-Reyes, C.; Nuñez-Alvarez, C.A.; Torrico-Lavayen, R.; García-Hernández, J.L.; Cabiedes-Contreras, J. Distinctive Autoantibody Profile in Mexican Mestizo Systemic Sclerosis Patients. Autoimmunity 2011, 44, 576–584. [Google Scholar] [CrossRef]
- Ceribelli, A.; Cavazzana, I.; Franceschini, F.; Airò, P.; Tincani, A.; Cattaneo, R.; Pauley, B.A.; Chan, E.K.; Satoh, M. Anti-Th/To Are Common Antinucleolar Autoantibodies in Italian Patients with Scleroderma. J. Rheumatol. 2010, 37, 2071–2075. [Google Scholar] [CrossRef] [PubMed]
- Guédon, A.F.; Carrat, F.; Mouthon, L.; Launay, D.; Chaigne, B.; Pugnet, G.; Lega, J.C.; Hot, A.; Cottin, V.; Agard, C.; et al. Heart and Systemic Sclerosis—Findings from a National Cohort Study. Rheumatology 2024, 63, 3380–3389. [Google Scholar] [CrossRef] [PubMed]
- Jones, X.M.; Bottini, N.; Boin, F.; Marbán, E. Cardiac Involvement in Systemic Sclerosis: A Critical Review of Knowledge Gaps and Opportunities. J. Scleroderma Relat. Disord. 2025, 10, 91–100. [Google Scholar] [CrossRef]
- Kayser, C.; Fritzler, M.J. Autoantibodies in Systemic Sclerosis: Unanswered Questions. Front. Immunol. 2015, 6, 167. [Google Scholar] [CrossRef]
- Tennøe, A.H.; Murbræch, K.; Andreassen, J.C.; Fretheim, H.; Garen, T.; Gude, E.; Andreassen, A.; Aakhus, S.; Molberg, Ø.; Hoffmann-Vold, A.M. Left Ventricular Diastolic Dysfunction Predicts Mortality in Patients with Systemic Sclerosis. J. Am. Coll. Cardiol. 2018, 72, 1804–1813. [Google Scholar] [CrossRef]
- Kampolis, C.; Plastiras, S.; Vlachoyiannopoulos, P.; Moyssakis, I.; Tzelepis, G. The Presence of Anti-Centromere Antibodies May Predict Progression of Estimated Pulmonary Arterial Systolic Pressure in Systemic Sclerosis. Scand. J. Rheumatol. 2008, 37, 278–283. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; De Luca, G.; Baritussio, A.; Seguso, M.; Gallo, N.; Bison, E.; Cattini, M.G.; Pontara, E.; Gargani, L.; Pepe, A.; et al. Serum Organ-Specific Anti-Heart and Anti-Intercalated Disk Autoantibodies as New Autoimmune Markers of Cardiac Involvement in Systemic Sclerosis: Frequency, Clinical and Prognostic Correlates. Diagnostics 2021, 11, 2165. [Google Scholar] [CrossRef] [PubMed]
- Nadel, A.; Nadel, M.; Taborska, N.; Stępień, B.; Gajdecki, J.; Brzezińska, O.; Opinc-Rosiak, A.; Makowska, J.; Lewandowska-Polak, A. Heart Involvement in Patients with Systemic Sclerosis—What Have We Learned About It in the Last 5 Years. Rheumatol. Int. 2024, 44, 1823–1836. [Google Scholar] [CrossRef]
- Osgueritchian, R.; Mombeini, H.; Jani, V.P.; Hsu, S.; Hummers, L.K.; Wigley, F.M.; Mathai, S.C.; Shah, A.A.; Mukherjee, M. Myocardial Disease in Systemic Sclerosis: Recent Updates and Clinical Implications. Curr. Cardiol. Rep. 2025, 27, 3. [Google Scholar] [CrossRef]
- Bruni, C.; Buch, M.H.; Djokovic, A.; De Luca, G.; Dumitru, R.B.; Giollo, A.; Galetti, I.; Steelandt, A.; Bratis, K.; Suliman, Y.A.; et al. Consensus on the Assessment of Systemic Sclerosis-Associated Primary Heart Involvement: World Scleroderma Foundation/Heart Failure Association Guidance on Screening, Diagnosis, and Follow-Up Assessment. J. Scleroderma Relat. Disord. 2023, 8, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Au, K.; Singh, M.K.; Bodukam, V.; Bae, S.; Maranian, P.; Ogawa, R.; Spiegel, B.; McMahon, M.; Hahn, B.; Khanna, D. Atherosclerosis in Systemic Sclerosis: A Systematic Review and Meta-Analysis. Arthritis Rheum. 2011, 63, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Dorniak, K.; Gogulska, Z.; Viti, A.; Glińska, A.; Kulawiak-Gałȩska, D.; Fijałkowska, J.; Wojteczek, A.; Wojtowicz, D.; Sienkiewicz, K.; Hellmann, M.; et al. Cardiac Morpho-Functional Changes, Inflammation and Fibrosis in Systemic Sclerosis—A Pilot Study of a Tertiary Center Cohort. Diagnostics 2025, 15, 393. [Google Scholar] [CrossRef] [PubMed]
| Study (First Author, Year) | Reference No. | Study Design | NOS Score | Risk of Bias |
|---|---|---|---|---|
| Bairkdar et al., 2024 | [4] | Cohort | 9 | Low |
| Muresan et al., 2016 | [7] | Cohort | 7 | Low |
| Tzelepis et al., 2007 | [11] | Cohort | 8 | Low |
| Allanore et al., 2010 | [20] | Cohort | 9 | Low |
| Höppner et al., 2023 | [21] | Cross-sectional Observational | 9 | Low |
| Radwan et al., 2021 | [22] | Cohort | 8 | Low |
| Lopez Nunez et al., 2023 | [23] | Cross-sectional | 8 | Low |
| Avouac et al., 2015 | [24] | Cohort | 8 | Low |
| Dumitru et al., 2021 | [25] | Cohort | 8 | Low |
| Cusma Piccione et al., 2013 | [26] | Cohort | 7 | Low |
| Barison et al., 2015 | [27] | Cross-sectional observational | 7 | Low |
| Hachulla et al., 2009 | [28] | Cohort | 7 | Low |
| Krumm et al., 2016 | [29] | Cross-sectional cohort | 7 | Low |
| Kobayashi et al., 2009 | [30] | Cohort | 7 | Low |
| Hui et al., 2021 | [31] | Cohort | 7 | Low |
| Vacca et al., 2014 | [32] | Cohort | 7 | Low |
| D’Andrea et al., 2004 | [33] | Cohort | 6 | Moderate |
| Autoantibody | Associated Cardiac Manifestations | Clinical Utility |
|---|---|---|
| Anti-centromere (ACA) | Conduction abnormalities, arrhythmias (esp. in older adults); potential risk of PAH | Common marker for lcSSc; useful for PAH risk but limited in predicting fibrosis |
| Anti-topoisomerase I (anti-Scl-70) | Myocardial fibrosis, diastolic dysfunction, elevated NT-proBNP and troponin, arrhythmias | High-risk marker for early cardiac fibrosis and dysfunction; strong clinical relevance |
| Anti-RNA polymerase III | Pericardial effusion, arrhythmias, potential tamponade | Predictive for scleroderma renal crisis and cardiac effusion |
| Anti-U3 RNP (anti-fibrillarin) | Myocardial fibrosis, conduction system disease, pericarditis (especially in African-American patients) | Ethnic-specific marker (African-American); strong indicator of cardiac involvement |
| Anti-Ku/Anti-Histone | Increased risk of heart disease | Supportive marker; less specific |
| Anti-Th/To | Greater association with pericarditis compared to ACA | More specific than ACA for pericardial involvement |
| AHA/AIDA | Potential myocardial specificity; associated with cardiac fibrosis | Emerging biomarkers; require further validation |
| Anti-U1 RNP | Mild conduction system disease; may coexist with PAH and overlap syndromes | Overlap syndromes (e.g., MCTD); cardiac risk less well-defined |
| Anti-PmScl | Occasional myocardial involvement; more often linked with myositis-overlap | Useful in overlap syndrome diagnostics; less specific for cardiac disease |
| Anti-Ro/SSA | Occasionally pericardial effusion; mostly linked with systemic autoimmune overlap | Non-specific marker; may aid in overlap diagnosis |
| Anti-PL7/PL12 | Rarely cardiac involvement; more associated with inflammatory myopathy | More relevant in ILD and myositis; cardiac links unclear |
| Anti-NOR90 | Limited evidence for direct cardiac involvement | Rare; not clinically routine for cardiac screening |
| Anti-Mi-2 | Primarily associated with dermatomyositis; occasional myocardial fibrosis | Muscle-specific; used in differential diagnosis with myositis-cardiac overlap |
| Autoantibody | Strength of Evidence for Cardiac Involvement |
|---|---|
| Anti–Scl-70 | High |
| ACA | Moderate |
| Anti–RNAP III | Moderate |
| Anti–U3 RNP | Moderate |
| AHA/AIDA | Preliminary |
| Others (Ku, histone, Th/To, U1 RNP, PmScl, Ro/SSA, PL7/PL12, NOR90, Mi-2) | Low to minimal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radić, M.; Bečić, T.; Šimac, P.; Đogaš, H.; Jukić, I.; Fabijanić, D.; Radić, J. Predictive Value of Classical and Emerging Autoantibodies for Cardiac Dysfunction in Systemic Sclerosis: Systematic Review. J. Clin. Med. 2025, 14, 6383. https://doi.org/10.3390/jcm14186383
Radić M, Bečić T, Šimac P, Đogaš H, Jukić I, Fabijanić D, Radić J. Predictive Value of Classical and Emerging Autoantibodies for Cardiac Dysfunction in Systemic Sclerosis: Systematic Review. Journal of Clinical Medicine. 2025; 14(18):6383. https://doi.org/10.3390/jcm14186383
Chicago/Turabian StyleRadić, Mislav, Tina Bečić, Petra Šimac, Hana Đogaš, Ivana Jukić, Damir Fabijanić, and Josipa Radić. 2025. "Predictive Value of Classical and Emerging Autoantibodies for Cardiac Dysfunction in Systemic Sclerosis: Systematic Review" Journal of Clinical Medicine 14, no. 18: 6383. https://doi.org/10.3390/jcm14186383
APA StyleRadić, M., Bečić, T., Šimac, P., Đogaš, H., Jukić, I., Fabijanić, D., & Radić, J. (2025). Predictive Value of Classical and Emerging Autoantibodies for Cardiac Dysfunction in Systemic Sclerosis: Systematic Review. Journal of Clinical Medicine, 14(18), 6383. https://doi.org/10.3390/jcm14186383








