Optical Coherence Tomography- and Optical Coherence Tomography Angiography-Based Evaluation in Treatment-Naïve Non-Exudative Macular Neovascularization
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ophthalmic Examinations
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Monés, J.; Richard, G.; Bandello, F.; et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef]
- Regillo, C.; Singh, R.; Hamilton, R.; Gedif, K.; Best, C.; Koh, A.; Holz, F.G. Fluid control in neovascular age-related macular degeneration with brolucizumab: An analysis of the HAWK and HARRIER phase 3 trials. Ophthalmologica 2022, 245, 403–412. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Chang, T.S.; Bressler, N.M.; Fine, J.T.; Dolan, C.M.; Ward, J.; Klesert, T.R.; MARINA Study Group. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: Results of a randomized clinical trial. Arch. Ophthalmol. 2007, 125, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF Trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ohkubo, Y.; Sato, A.; Takezawa, M.; Fujino, Y.; Yanagi, Y.; Kawashima, H. Relationship between visual prognosis and delay of intravitreal injection of ranibizumab when treating age-related macular degeneration. Retina 2015, 35, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Q.; Motulsky, E.H.; Thulliez, M.; Shi, Y.; Lyu, C.; de Sisternes, L.; Durbin, M.K.; Feuer, W.; Wang, R.K.; et al. Two-year risk of exudation in eyes with nonexudative age-related macular degeneration and subclinical neovascularization detected with swept source optical coherence tomography angiography. Am. J. Ophthalmol. 2019, 208, 1–11. [Google Scholar] [CrossRef]
- Bailey, S.T.; Thaware, O.; Wang, J.; Hagag, A.M.; Zhang, X.; Flaxel, C.J.; Lauer, A.K.; Hwang, T.S.; Lin, P.; Huang, D.; et al. Detection of nonexudative choroidal neovascularization and progression to exudative choroidal neovascularization using OCT angiography. Ophthalmol. Retina 2019, 3, 629–636. [Google Scholar] [CrossRef]
- Palejwala, N.V.; Jia, Y.; Gao, S.S.; Liu, L.; Flaxel, C.J.; Hwang, T.S.; Lauer, A.K.; Wilson, D.J.; Huang, D.; Bailey, S.T. Detection of nonexudative choroidal neovascularization in age-related macular degeneration with optical coherence tomography angiography. Retina 2015, 35, 2204–2211. [Google Scholar] [CrossRef]
- Chen, L.; Messinger, J.D.; Sloan, K.R.; Swain, T.A.; Sugiura, Y.; Yannuzzi, L.A.; Curcio, C.A.; Freund, K.B. Nonexudative macular neovascularization supporting outer retina in age-related macular degeneration: A clinicopathologic correlation. Ophthalmology 2020, 127, 931–947. [Google Scholar] [CrossRef]
- Querques, G.; Srour, M.; Massamba, N.; Georges, A.; Ben Moussa, N.; Rafaeli, O.; Souied, E.H. Functional characterization and multimodal imaging of treatment-naive “quiescent” choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6886–6892. [Google Scholar] [CrossRef]
- Heiferman, M.J.; Fawzi, A.A. Progression of subclinical choroidal neovascularization in age-related macular degeneration. PLoS ONE 2019, 14, e0217805. [Google Scholar] [CrossRef]
- Yanagi, Y.; Mohla, A.; Lee, S.Y.; Mathur, R.; Chan, C.M.; Yeo, I.; Wong, T.Y.; Cheung, C.M.G. Incidence of fellow eye involvement in patients with unilateral exudative age-related macular degeneration. JAMA Ophthalmol. 2018, 136, 905–911. [Google Scholar] [CrossRef]
- Querques, G.; Sacconi, R.; Capuano, V.; Carnevali, A.; Colantuono, D.; Battista, M.; Borrelli, E.; Miere, A.; Parravano, M.; Costanzo, E.; et al. Treatment-naïve quiescent macular neovascularization secondary to AMD: The 2019 Young Investigator Lecture of Macula Society. Eur. J. Ophthalmol. 2021, 31, 3164–3176. [Google Scholar] [CrossRef] [PubMed]
- Nissen, A.H.K.; Kiilgaard, H.C.; van Dijk, E.H.C.; Hajari, J.N.; Huemer, J.; Iovino, C.; Schneider, M.; Sørensen, T.L.; Grauslund, J.; Subhi, Y. Exudative progression of treatment-naïve nonexudative macular neovascularization in age-related macular degeneration: A systematic review with meta-analyses. Am. J. Ophthalmol. 2024, 257, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Parrulli, S.; Monteduro, D.; Cereda, M.G.; Nguyen, V.; Staurenghi, G.; Cheung, C.M.G.; Gillies, M.; Teo, K.Y.C. Outer retinal layer thickening predicts the onset of exudative neovascular age-related macular degeneration. Am. J. Ophthalmol. 2021, 231, 19–27. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, M.; Kim, J.; Yoon, I.; Park, S.; Kim, C.G. Factors associated with the development of exudation in treatment-naive eyes with nonexudative macular neovascularization. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Solecki, L.; Loganadane, P.; Gauthier, A.S.; Simonin, M.; Puyraveau, M.; Delbosc, B.; Saleh, M. Predictive factors for exudation of quiescent choroidal neovessels detected by OCT angiography in the fellow eyes of eyes treated for a neovascular age-related macular degeneration. Eye 2021, 35, 644–650. [Google Scholar] [CrossRef]
- Bae, S.H.; Bae, K.; Yoon, C.K.; Park, U.C.; Park, K.H.; Lee, E.K. Morphological biomarkers predicting exudative conversion in type 1 nonexudative macular neovascularization using optical coherence tomography angiography. Retina 2024, 44, 1006–1014. [Google Scholar] [CrossRef]
- Wong, C.W.; Wong, T.Y.; Cheung, C.M.G. Polypoidal choroidal vasculopathy in Asians. J. Clin. Med. 2015, 4, 782–821. [Google Scholar] [CrossRef]
- Roisman, L.; Zhang, Q.; Wang, R.K.; Gregori, G.; Zhang, A.; Chen, C.L.; Durbin, M.K.; An, L.; Stetson, P.F.; Robbins, G.; et al. Optical coherence tomography angiography of asymptomatic neovascularization in intermediate age-related macular degeneration. Ophthalmology 2016, 123, 1309–1319. [Google Scholar] [CrossRef]
- Vidal-Oliver, L.; Herzig-de Almeida, E.; Spissinger, S.; Dolz-Marco, R.; Finger, R.P. Scan speed affects quantitative optical coherence tomography angiography vascular metrics. Sci. Rep. 2024, 14, 28997. [Google Scholar] [CrossRef]
- Gershoni, A.; Barayev, E.; Vainer, I.; Allon, R.; Yavnieli, R.; Shapira, Y.; Mimouni, M.; Geffen, N.; Nemet, A.Y.; Segal, O. Thickness measurements taken with the spectralis OCT increase with decreasing signal strength. BMC Ophthalmol. 2022, 22, 148. [Google Scholar] [CrossRef] [PubMed]
- Hanutsaha, P.; Guyer, D.R.; Yannuzzi, L.A.; Naing, A.; Slakter, J.S.; Sorenson, J.S.; Spaide, R.F.; Freund, K.B.; Feinsod, M.; Orlock, D.A. Indocyanine-green videoangiography of drusen as a possible predictive indicator of exudative maculopathy. Ophthalmology 1998, 105, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Laiginhas, R.; Yang, J.; Rosenfeld, P.J.; Falcão, M. Nonexudative macular neovascularization—A systematic review of prevalence, natural history, and recent insights from OCT angiography. Ophthalmol. Retina 2020, 4, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Maruko, I.; Chujo, K.; Hasegawa, T.; Arakawa, H.; Iida, T. Characteristics of treatment-naïve quiescent choroidal neovascularization detected by optical coherence tomography angiography in patients with age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2671–2677. [Google Scholar] [CrossRef]
- Teo, K.Y.C.; Yanagi, Y.; Wong, T.Y.; Charkaravarty, U.; Gemmy Cheung, C.M. Morphologic predictors and temporal characteristics of conversion from nonexudative to exudative age-related macular degeneration in the fellow eye. Ophthalmol. Retina 2021, 5, 126–140. [Google Scholar] [CrossRef]
- Serra, R.; Coscas, F.; Boulet, J.F.; Cabral, D.; Lupidi, M.; Coscas, G.J.; Souied, E.H. Predictive activation biomarkers of treatment-naive asymptomatic choroidal neovascularization in age-related macular degeneration. Retina 2020, 40, 1224–1233. [Google Scholar] [CrossRef]
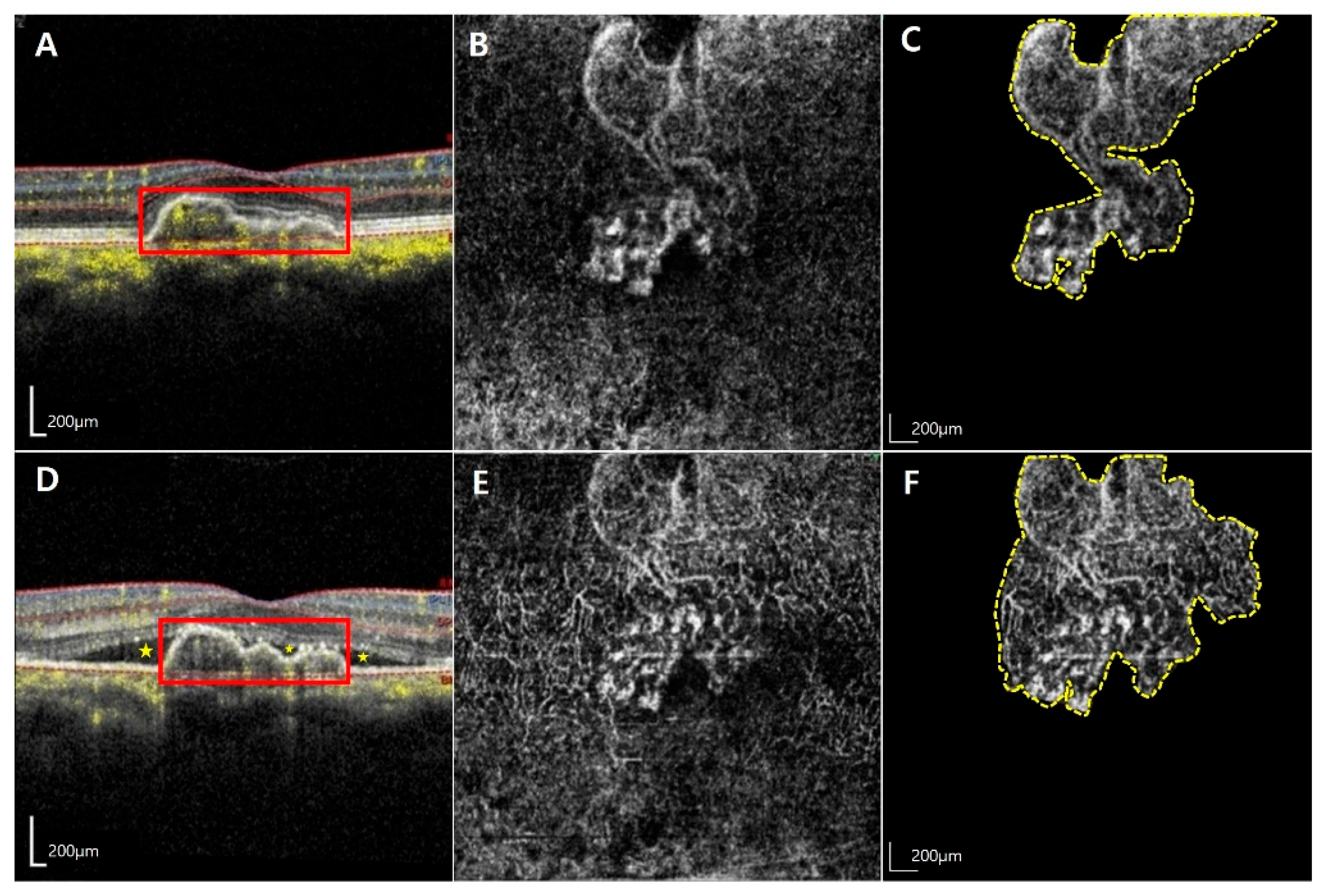
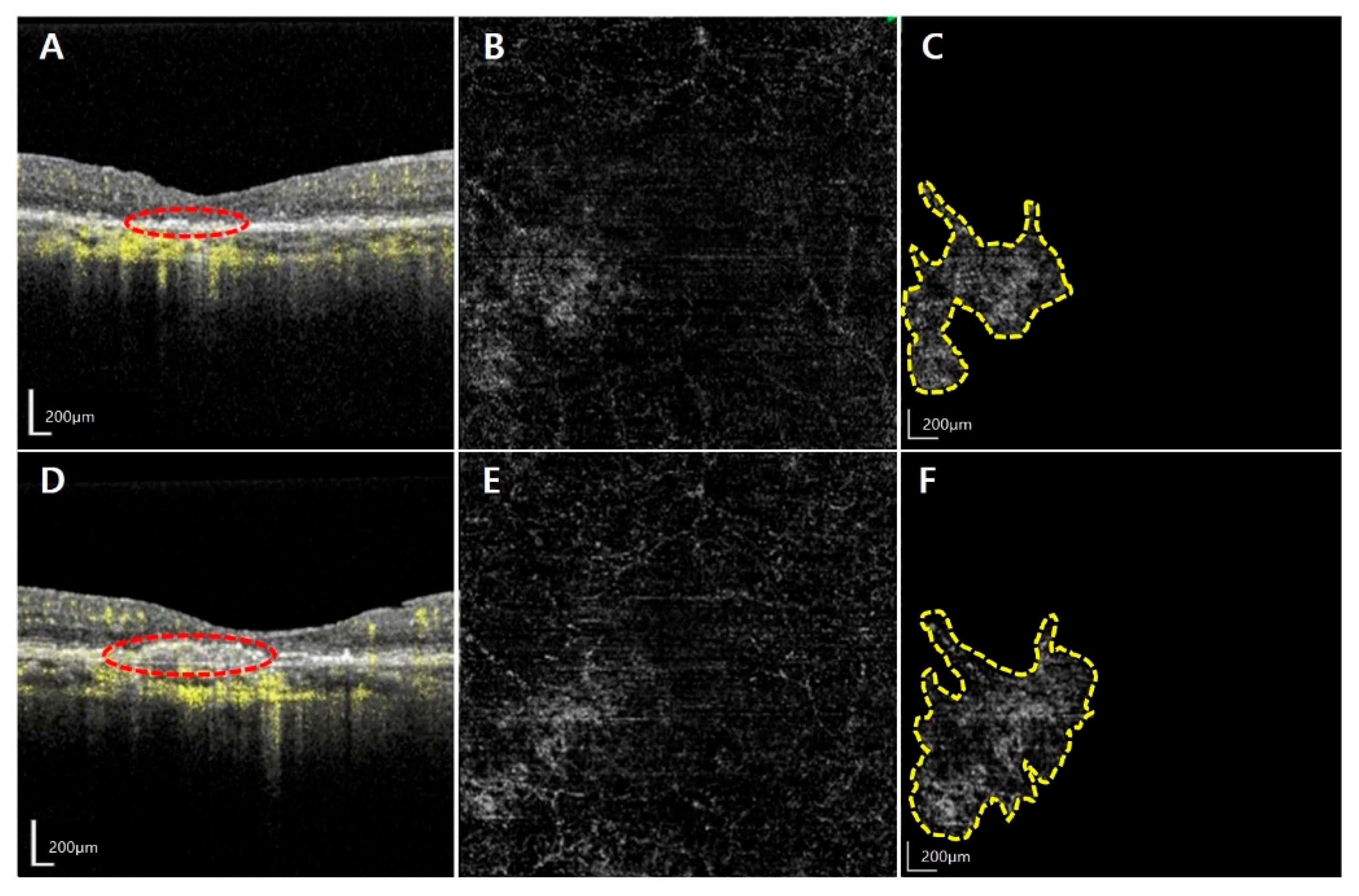
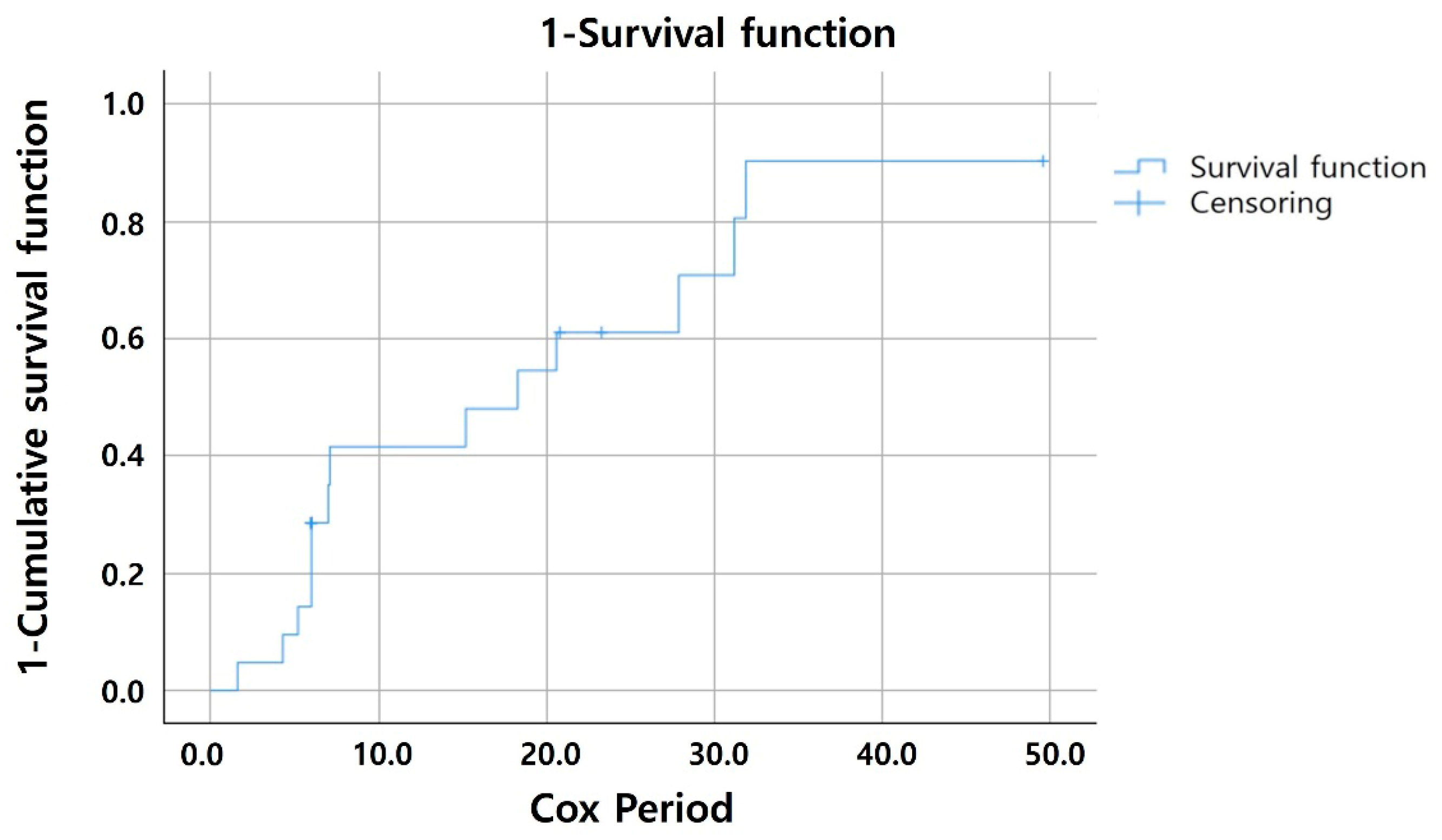

| Factors | Total (n = 21) | Activated MNV Group (n = 14) | Quiescent Group (n = 7) | p-Value |
|---|---|---|---|---|
| Age, years | 71.5 ± 9.1 | 72.1 ± 8.3 | 70.3 ± 9.9 | 0.799 |
| Sex, female (%)/male (%) | 8 (38.1)/13 (61.9) | 3 (21.4)/11 (78.6) | 5 (71.4)/2 (28.6) | 0.081 |
| Follow-up period, months | 15.1 ± 11.8 | 14.2 ± 9.7 | 16.8 ± 15.1 | 0.443 |
| Baseline BCVA, logMAR | 0.15 ± 0.18 | 0.18 ± 0.21 | 0.09 ± 0.06 | 0.689 |
| Baseline CMT, μm | 265.3 ± 37.1 | 278.9 ± 31.2 | 238.1 ± 32.9 | 0.020 * |
| Baseline SCT, μm | 245.2 ± 95.2 | 237.1 ± 98.9 | 261.5 ± 84.9 | 0.535 |
| Baseline ORLT, μm | 86.6 ± 5.3 | 87.6 ± 6.1 | 84.7 ± 1.8 | 0.360 |
| AMD status of the fellow eyes (%) | ||||
| Dry AMD | 6 (28.6) | 3 (21.4) | 3 (42.9) | |
| Neovascular AMD | 5 (23.8) | 4 (33.3) | 1 (14.3) | 0.529 |
| Geographic atrophy | 2 (9.5) | 2 (14.3) | 0 (0.0) | |
| MNV size at baseline, mm2 | 1.19 ± 1.27 | 1.48 ± 1.42 | 0.62 ± 0.58 | 0.149 |
| Factors | Activated MNV Group (n = 14) | p-Value | Quiescent Group (n = 7) | p-Value | ||
|---|---|---|---|---|---|---|
| Baseline | Progression | Baseline | Last Visit | |||
| BCVA, logMAR | 0.18 ± 0.21 | 0.22 ± 0.23 | 0.463 | 0.09 ± 0.06 | 0.08 ± 0.10 | 0.686 |
| CMT, μm | 278.9 ± 31.17 | 342.9 ± 111.3 | 0.087 | 238.1 ± 32.9 | 236.9 ± 37.0 | 0.606 |
| SCT, μm | 237.1 ± 98.9 | 264.7 ± 110.8 | 0.013 * | 261.5 ± 84.9 | 283.9 ± 93.3 | 0.128 |
| ORLT, μm | 87.6 ± 6.1 | 106.4 ± 22.2 | 0.012 * | 84.7 ± 1.8 | 85.4 ± 3.9 | 0.546 |
| MNV size, mm2 | 1.48 ± 1.42 | 2.00 ± 1.40 | 0.012 * | 0.62 ± 0.58 | 0.80 ± 0.80 | 0.017 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, G.Y.; Park, J.S.; Bae, K.W. Optical Coherence Tomography- and Optical Coherence Tomography Angiography-Based Evaluation in Treatment-Naïve Non-Exudative Macular Neovascularization. J. Clin. Med. 2025, 14, 6375. https://doi.org/10.3390/jcm14186375
Moon GY, Park JS, Bae KW. Optical Coherence Tomography- and Optical Coherence Tomography Angiography-Based Evaluation in Treatment-Naïve Non-Exudative Macular Neovascularization. Journal of Clinical Medicine. 2025; 14(18):6375. https://doi.org/10.3390/jcm14186375
Chicago/Turabian StyleMoon, Geun Young, Jong Seok Park, and Ki Woong Bae. 2025. "Optical Coherence Tomography- and Optical Coherence Tomography Angiography-Based Evaluation in Treatment-Naïve Non-Exudative Macular Neovascularization" Journal of Clinical Medicine 14, no. 18: 6375. https://doi.org/10.3390/jcm14186375
APA StyleMoon, G. Y., Park, J. S., & Bae, K. W. (2025). Optical Coherence Tomography- and Optical Coherence Tomography Angiography-Based Evaluation in Treatment-Naïve Non-Exudative Macular Neovascularization. Journal of Clinical Medicine, 14(18), 6375. https://doi.org/10.3390/jcm14186375






