Increase in Alveolar Septal Width Is a Histological Predictor of Chronic Lung Allograft Dysfunction and Survival in Lung Transplant Recipients—A Longitudinal Study
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Surveillance of Lung Transplant Recipients
2.3. Definition of CLAD
2.4. CLAD Phenotypes
2.5. Sampled Histological Material
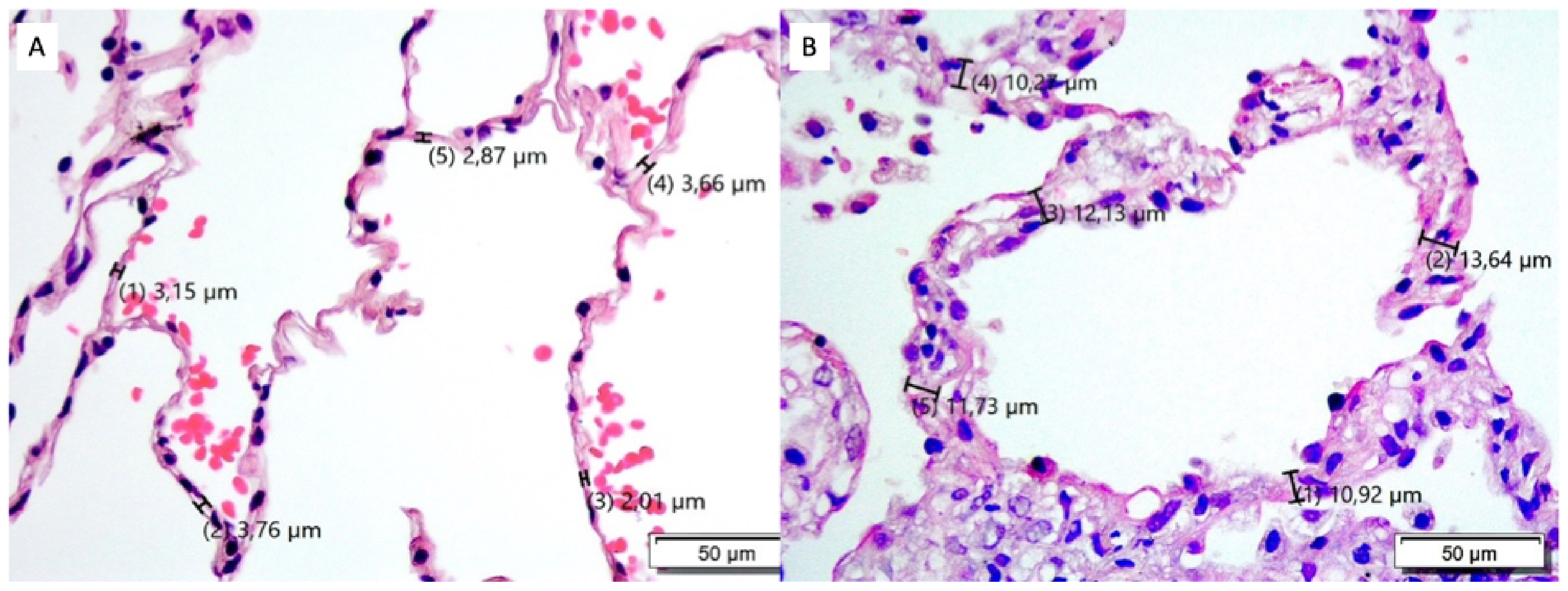
2.6. Morphometric Analysis
2.7. Molecular Analysis
2.8. Statistical Analysis
3. Results
3.1. Cohort
3.2. Sampled Histological Material
3.3. ASW Validation
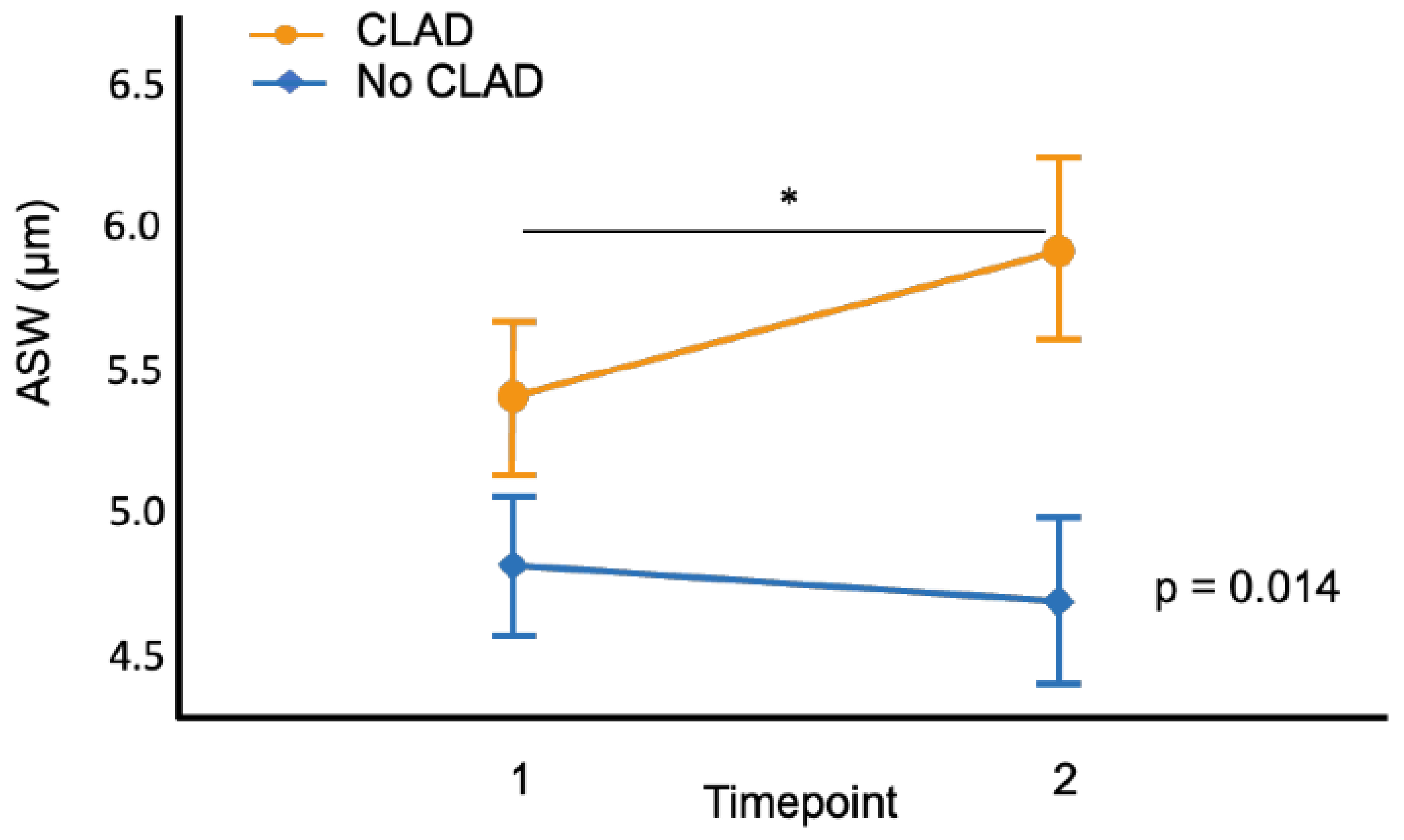
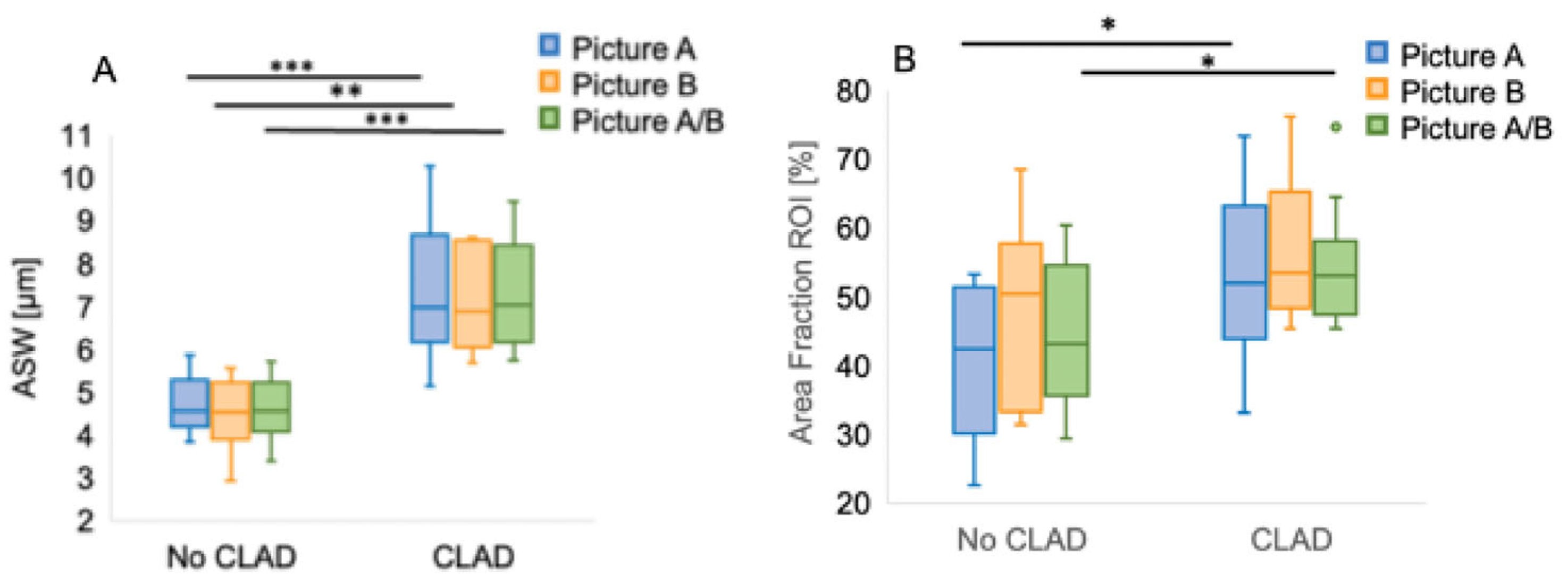
3.4. ASW and CLAD
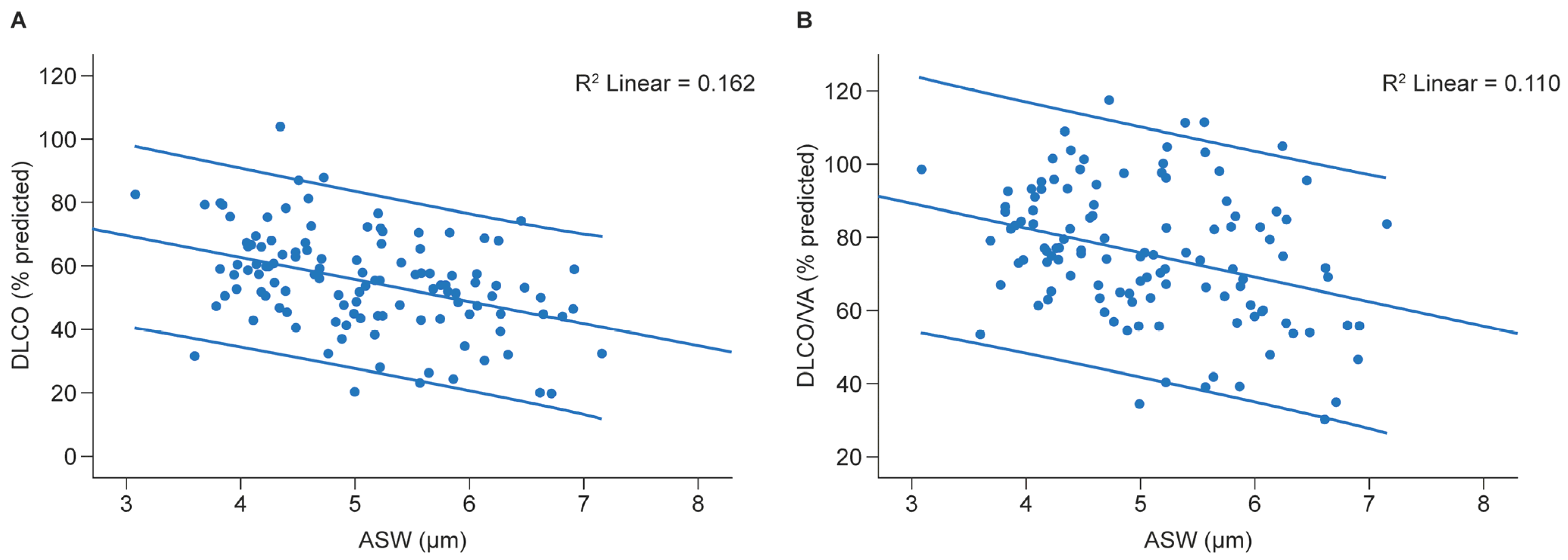
3.5. ASW and Diffusing Capacity of the Lung for Carbon Monoxide (DLCO)
3.6. Factors Associated with ASW
3.7. Extracellular Molecular Markers for ASW
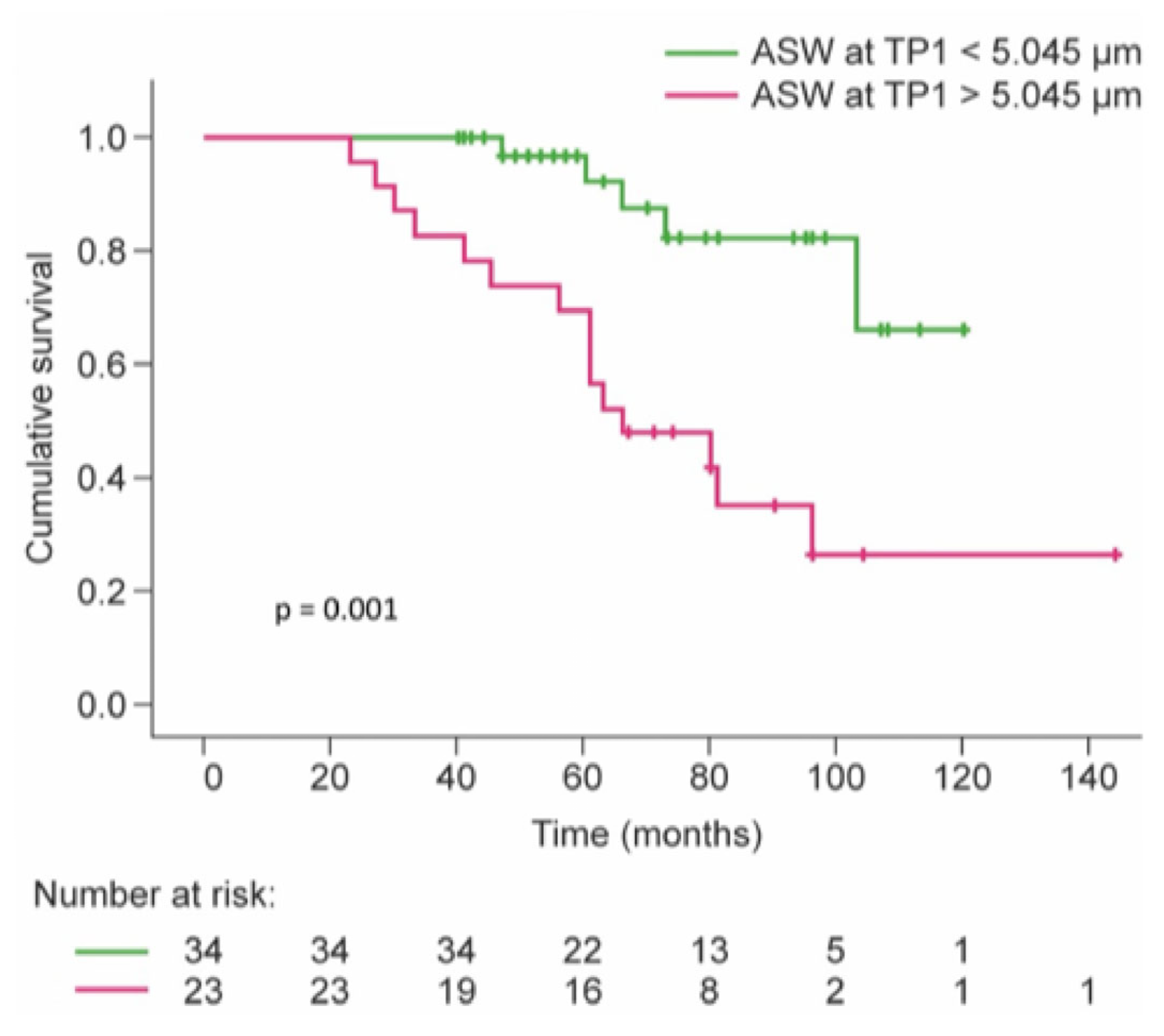
3.8. Survival
3.9. ASW Measurement in Clinical Routine
4. Discussion
4.1. Strengths and Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| ABB | air–blood barrier |
| ASW | alveolar septal width |
| ACR | acute cellular rejection |
| AMR | antibody-mediated rejection |
| AUC | area under the curve |
| BOS | bronchiolitis obliterans syndrome |
| CI | confidence interval |
| CLAD | chronic lung allograft dysfunction |
| CMV | cytomegalovirus |
| COL | collagen |
| CT | computed tomography |
| DLCO | diffusing capacity of the lung for carbon monoxide |
| ECMO | extracorporeal membrane oxygenation |
| FEV1 | forced expiratory volume in 1 s |
| FVC | forced vital capacity |
| ICU | intensive care unit |
| ISHLT | international society for heart and lung transplantation |
| LB | lymphocytic bronchiolitis |
| LOS | length of stay |
| LuTX | lung transplantation |
| PCR | polymerase chain reaction |
| PO2 | arterial oxygen pressure |
| RAS | restrictive allograft syndrome |
| ROI | region of interest |
| RPLP0 | ribosomal protein lateral stalk subunit P0 |
| TBB | transbronchial biopsy |
| TGFβ | transforming growth factor β |
| TLC | total lung capacity |
| TP | timepoint |
| UGLC | University Giessen Lung center |
| UIP | usual interstitial pneumonia |
| VA | alveolar volume |
References
- Chambers, D.C.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Meiser, B.; Potena, L.; Rossano, J.W.; Toll, A.E.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.S.; Cherikh, W.S.; Chambers, D.C.; Garcia, V.C.; Hachem, R.R.; Kreisel, D.; Puri, V.; Kozower, B.D.; Byers, D.E.; Witt, C.A.; et al. Bronchiolitis obliterans syndrome-free survival after lung transplantation: An International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. J. Heart Lung Transplant. 2019, 38, 5–16. [Google Scholar] [CrossRef]
- Verleden, G.M.; Glanville, A.R.; Lease, E.D.; Fisher, A.J.; Calabrese, F.; Corris, P.A.; Ensor, C.R.; Gottlieb, J.; Hachem, R.R.; Lama, V.; et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transplant. 2019, 38, 493–503. [Google Scholar] [CrossRef]
- Meyer, K.C.; Raghu, G.; Verleden, G.M.; Corris, P.A.; Aurora, P.; Wilson, K.C.; Brozek, J.; Glanville, A.R.; the ISHLT/ATS/ERS BOS Task Force Committee. An international ISHLT/ATS/ERS clinical practice guideline: Diagnosis and management of bronchiolitis obliterans syndrome. Eur. Respir. J. 2014, 44, 1479–1503. [Google Scholar] [CrossRef]
- Burton, C.M.; Iversen, M.; Carlsen, J.; Mortensen, J.; Andersen, C.B.; Steinbruchel, D.; Scheike, T. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. J. Heart Lung Transplant. 2009, 28, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.A.; Finlen Copeland, C.A.; Todd, J.L.; Snyder, L.D.; Martissa, J.A.; Palmer, S.M. Spirometrically significant acute rejection increases the risk for BOS and death after lung transplantation. Am. J. Transplant. 2012, 12, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Glanville, A.R.; Aboyoun, C.L.; Havryk, A.; Plit, M.; Rainer, S.; Malouf, M.A. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am. J. Respir. Crit. Care Med. 2008, 177, 1033–1040. [Google Scholar] [CrossRef]
- Husain, A.N.; Siddiqui, M.T.; Holmes, E.W.; Chandrasekhar, A.J.; McCabe, M.; Radvany, R.; Garrity, E.R. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am. J. Respir. Crit. Care Med. 1999, 159, 829–833. [Google Scholar] [CrossRef]
- Morrell, M.R.; Pilewski, J.M.; Gries, C.J.; Pipeling, M.R.; Crespo, M.M.; Ensor, C.R.; Yousem, S.A.; D’Cunha, J.; Shigemura, N.; Bermudez, C.A.; et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J. Heart Lung Transplant. 2014, 33, 1288–1294. [Google Scholar] [CrossRef]
- Stewart, S.; Fishbein, M.C.; Snell, G.I.; Berry, G.J.; Boehler, A.; Burke, M.M.; Glanville, A.; Gould, F.K.; Magro, C.; Marboe, C.C.; et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J. Heart Lung Transplant. 2007, 26, 1229–1242. [Google Scholar] [CrossRef]
- Yousem, S.A.; Berry, G.J.; Cagle, P.T.; Chamberlain, D.; Husain, A.N.; Hruban, R.H.; Marchevsky, A.; Ohori, N.P.; Ritter, J.; Stewart, S.; et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J. Heart Lung Transplant. 1996, 15, 1–15. [Google Scholar] [PubMed]
- Hachem, R.R. Acute Rejection and Antibody-Mediated Rejection in Lung Transplantation. Clin. Chest Med. 2017, 38, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.D.; Li, N.; Andersen, C.B.; Arrossi, A.V.; Askar, M.; Berry, G.J.; DeNicola, M.M.; Neil, D.A.; Pavlisko, E.N.; Reed, E.F.; et al. Banff study of pathologic changes in lung allograft biopsy specimens with donor-specific antibodies. J. Heart Lung Transplant. 2016, 35, 40–48. [Google Scholar] [CrossRef]
- Levine, D.J.; Glanville, A.R.; Aboyoun, C.; Belperio, J.; Benden, C.; Berry, G.J.; Hachem, R.; Hayes, D., Jr.; Neil, D.; Reinsmoen, N.L.; et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2016, 35, 397–406. [Google Scholar] [CrossRef]
- Calabrese, F.; Hirschi, S.; Neil, D.; Montero-Fernandez, A.; Timens, W.; Verbeken, E.; Chenard, M.P.; Ivanovic, M.; Le Pavec, J.; Pena, T.; et al. Alveolar Septal Widening as an “Alert” Signal to Look Into Lung Antibody-mediated Rejection: A Multicenter Pilot Study. Transplantation 2019, 103, 2440–2447. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 3rd ed.; The Guilford Press: New York, NY, USA, 2022. [Google Scholar]
- Hegewald, M.J. Diffusing capacity. Clin. Rev. Allergy Immunol. 2009, 37, 159–166. [Google Scholar] [CrossRef]
- Darley, D.R.; Ma, J.; Huszti, E.; Ghany, R.; Hutcheon, M.; Chow, C.W.; Tikkanen, J.; Keshavjee, S.; Singer, L.G.; Martinu, T. Diffusing capacity of the lung for carbon monoxide: Association with long-term outcomes after lung transplantation in a 20-year longitudinal study. Eur. Respir. J. 2022, 59, 2003639. [Google Scholar] [CrossRef]
- Verleden, S.E.; Von der Thusen, J.; Roux, A.; Brouwers, E.S.; Braubach, P.; Kuehnel, M.; Laenger, F.; Jonigk, D. When tissue is the issue: A histological review of chronic lung allograft dysfunction. Am. J. Transplant. 2020, 20, 2644–2651. [Google Scholar] [CrossRef]
- Hodge, S.; Holmes, M.; Banerjee, B.; Musk, M.; Kicic, A.; Waterer, G.; Reynolds, P.N.; Hodge, G.; Chambers, D.C. Posttransplant bronchiolitis obliterans syndrome is associated with bronchial epithelial to mesenchymal transition. Am. J. Transplant. 2009, 9, 727–733. [Google Scholar] [CrossRef]
- El-Gamel, A.; Sim, E.; Hasleton, P.; Hutchinson, J.; Yonan, N.; Egan, J.; Campbell, C.; Rahman, A.; Sheldon, S.; Deiraniya, A.; et al. Transforming growth factor beta (TGF-beta) and obliterative bronchiolitis following pulmonary transplantation. J. Heart Lung Transplant. 1999, 18, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Sun, K.H.; Wetter, J.B.; Wilson-Kanamori, J.R.; Hazelwood, L.A.; Henderson, N.C.; Adams, T.S.; Schupp, J.C.; Poli, S.D.; Rosas, I.O.; et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 2020, 11, 1920. [Google Scholar] [CrossRef] [PubMed]
- Tsitoura, E.; Trachalaki, A.; Vasarmidi, E.; Mastrodemou, S.; Margaritopoulos, G.A.; Kokosi, M.; Fanidis, D.; Galaris, A.; Aidinis, V.; Renzoni, E.; et al. Collagen 1a1 Expression by Airway Macrophages Increases In Fibrotic ILDs and Is Associated With FVC Decline and Increased Mortality. Front. Immunol. 2021, 12, 645548. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.J.; Hadley, R.; Cornett, A.M.; Dreffs, A.A.; Matthes, S.A.; Tsui, J.L.; Weiss, K.; Horowitz, J.C.; Fiore, V.F.; Barker, T.H.; et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 2012, 186, 866–876. [Google Scholar] [CrossRef]
- Morrone, C.; Smirnova, N.F.; Jeridi, A.; Kneidinger, N.; Hollauer, C.; Schupp, J.C.; Kaminski, N.; Jenne, D.; Eickelberg, O.; Yildirim, A.O. Cathepsin B promotes collagen biosynthesis, which drives bronchiolitis obliterans syndrome. Eur. Respir. J. 2021, 57, 2001416. [Google Scholar] [CrossRef]
- Goldin, J.; Cascella, M. Diffusing Capacity of the Lungs for Carbon Monoxide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Leiby, K.L.; Raredon, M.S.B.; Niklason, L.E. Bioengineering the Blood-gas Barrier. Compr. Physiol. 2020, 10, 415–452. [Google Scholar] [CrossRef]
- Bennett, D.; Bergantini, L.; Ferrara, P.; Cusi, M.G.; Scolletta, S.; Montagnani, F.; Paladini, P.; Sestini, P.; Refini, R.M.; Luzzi, L.; et al. Cytomegalovirus Infection Is Associated with Development of Chronic Lung Allograft Dysfunction. Lung 2022, 200, 513–522. [Google Scholar] [CrossRef]
- Kawashima, M.; Ma, J.; Huszti, E.; Levy, L.; Berra, G.; Renaud-Picard, B.; Takahagi, A.; Ghany, R.; Sato, M.; Keshavjee, S.; et al. Association between cytomegalovirus viremia and long-term outcomes in lung transplant recipients. Am. J. Transplant. 2024, 24, 1057–1069. [Google Scholar] [CrossRef]
- Haddad, T. Postoperative management and acute complications after lung transplantation. Curr. Chall. Thorac. Surg. 2023, 5. [Google Scholar] [CrossRef]
- Beeckmans, H.; Pagliazzi, A.; Kerckhof, P.; Hofkens, R.; Debackere, F.; Zajacova, A.; Bos, S.; Vanaudenaerde, B.M.; de Loor, H.; Naesens, M.; et al. Donor-derived cell-free DNA in chronic lung allograft dysfunction phenotypes: A pilot study. Front. Transplant. 2024, 3, 1513101. [Google Scholar] [CrossRef]
- Mohanty, R.P.; Moghbeli, K.; Singer, J.P.; Calabrese, D.R.; Hays, S.R.; Iasella, C.; Lieber, S.; Leard, L.E.; Shah, R.J.; Venado, A.; et al. Small airway brush gene expression predicts chronic lung allograft dysfunction and mortality. J. Heart Lung Transplant. 2024, 43, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Budding, K.; van de Graaf, E.A.; Kardol-Hoefnagel, T.; Kwakkel-van Erp, J.M.; Luijk, B.D.; Oudijk, E.D.; van Kessel, D.A.; Grutters, J.C.; Hack, C.E.; Otten, H.G. Soluble CD59 is a Novel Biomarker for the Prediction of Obstructive Chronic Lung Allograft Dysfunction After Lung Transplantation. Sci. Rep. 2016, 6, 26274. [Google Scholar] [CrossRef] [PubMed]
- Hudock, M.R.; Pinezich, M.R.; Mir, M.; Chen, J.; Bacchetta, M.; Vunjak-Novakovic, G.; Kim, J. Emerging Imaging Modalities for Functional Assessment of Donor Lungs Ex Vivo. Curr. Opin. Biomed. Eng. 2023, 25, 100432. [Google Scholar] [CrossRef] [PubMed]
- Bundesärztekammer. Richtlinie gemäß § 16 Abs. 1 S. 1 Nrn. 2 u. 5 TPG für die Wartelistenführung und Organvermittlung zur Lungentransplantation. Dtsch. Arztebl. Int. 2017, 114, A-1948/B–1648/C-1614. [Google Scholar]
- Weill, D.; Benden, C.; Corris, P.A.; Dark, J.H.; Davis, R.D.; Keshavjee, S.; Lederer, D.J.; Mulligan, M.J.; Patterson, G.A.; Singer, L.G.; et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2015, 34, 1–15. [Google Scholar] [CrossRef]
- Schneck, E.; Askevold, I.; Rath, R.; Hecker, A.; Reichert, M.; Guth, S.; Koch, C.; Sander, M.; Seeger, W.; Mayer, K.; et al. Chronic Lung Allograft Dysfunction Is Associated with Increased Levels of Cell-Free Mitochondrial DNA in Bronchoalveolar Lavage Fluid of Lung Transplant Recipients. J. Clin. Med. 2022, 11, 4142. [Google Scholar] [CrossRef]
| CLAD | |||||
|---|---|---|---|---|---|
| No (n = 31) | Yes (n = 26) | All (n = 57) | p | ||
| Donor characteristics | |||||
| Age, years | 52.9 ± 18.2 | 57.2 ± 16.4 | 55.0 ± 17.3 | 1.0 | |
| Sex, n (%) | 0.94 | ||||
| female | 14 (45) | 12 (48) | 26 (46) | ||
| male | 17 (55) | 14 (54) | 31 (54) | ||
| Smoking history, n (%) | |||||
| None | 22 (71) | 15 (58) | 37 (65) | 0.2 | |
| Slight | 3 (10) | 3 (12) | 6 (11) | 0.84 | |
| Medium | 3 (10) | 1 (4) | 4 (7) | 0.38 | |
| Strong | 0 (0) | 5 (19) | 5 (9) | 0.01 | |
| Missing | 3 (10) | 2 (8) | 5 (9) | ||
| Time on ventilation, days | 4 [2–6] | 3 [2–5] | 3 [2–6] | 0.58 | |
| PO2 100%, mm Hg | 452.0 ± 56.7 | 441.4 ± 69.4 | 447.4 ± 63.4 | 0.51 | |
| Blood type, n (%) | |||||
| 0 | 13 (42) | 6 (23) | 19 (33) | 0.13 | |
| A | 14 (45) | 16 (62) | 30 (53) | 0.22 | |
| B | 3 (10) | 3 (12) | 6 (11) | 0.82 | |
| AB | 1 (3) | 1 (4) | 2 (4) | 0.9 | |
| CMV serostatus, n (%) | 0.14 | ||||
| Negative | 13 (42) | 16 (62) | 29 (51) | ||
| Positive | 18 (58) | 10 (38) | 28 (49) | ||
| Recipient characteristics | |||||
| Age, years | 54.0 [38.0–63.0] | 56.5 [56.5–61.0] | 56.0 [43.5–61.0] | 1.0 | |
| Sex, n (%) | 0.6 | ||||
| Female | 15 (48) | 15 (60) | 30 (53) | ||
| Male | 16 (52) | 11 (40) | 27 (47) | ||
| Body mass index, kg/m2 | 23.30 ± 3.32 | 25.26 ± 4.26 | 23.22 ± 3.98 | 0.1 | |
| Lung disease, n (%) | 0.2 | ||||
| COPD/emphysema | 2 (6) | 6 (23) | 8 (14) | 0.07 | |
| Fibrosis—IPF | 8 (26) | 4 (15) | 12 (21) | 0.34 | |
| Fibrosis—other | 12 (39) | 12 (46) | 24 (42) | 0.57 | |
| Cystic fibrosis | 6 (19) | 2 (8) | 8 (14) | 0.21 | |
| Lymphangioleiomyomatosis | 2 (6) | 0 (0) | 2 (4) | 0.19 | |
| Pulmonary hypertension | 1 (3) | 2 (8) | 3 (5) | 0.45 | |
| CMV serostatus, n (%) | 0.06 | ||||
| Negative | 12 (39) | 4 (15) | 16 (28) | ||
| Positive | 18 (58) | 20 (77) | 38 (67) | ||
| Missing | 1 (3) | 2 (8) | 3 (5) | ||
| Last LAS a | 38.5 [36.0–56.6] | 36.9 [34.3–44.2] | 37.1 [35.6–51.9] | 0.94 | |
| Peri- and post-operative factors | |||||
| ECMO during surgery, n (%) | 0.97 | ||||
| No | 20 (65) | 16 (62) | 36 (63) | ||
| Yes | 11 (35) | 9 (35) | 20 (35) | ||
| Missing | 0 (0) | 1 (4) | 1 (2) | ||
| Incision–suture time, min | 357.7 ± 75.3 | 357.4 ± 85.2 | 357.6 ± 79.2 | 1.0 | |
| Ischemic time, min | 360 [240–420] | 300 [257–375] | 323 [241–390] | 0.64 | |
| Number of RBC units transfused | 3 [2–6] | 3 [0–4] | 3 [2–6] | 0.47 | |
| Post-LuTX time on ventilator, h | 48 [12–360] | 144 [12–772] | 72 [12–468] | 0.14 | |
| LOS in ICU, d | 16 [10–42] | 26 [14–54] | 20 [12–42] | 0.24 | |
| Number of acute rejections b | 2 [1–5] | 3 [1–4.5] | 3 [1–5] | 0.96 | |
| Cumulative CMV reactivation, n (%) b | 0.29 ± (0.74) | 0.38 ± (0.94) | 0.46 ± 1.0 | 0.36 | |
| LuTX center, n (%) | 0.82 | ||||
| Kerckhoff Clinic Bad Nauheim | 3 (10) | 3 (12) | 6 (11) | ||
| UGLC | 28 (90) | 23 (88) | 51 (89) | ||
| Immunsuppression 1, n (%) | 0.27 | ||||
| Tacrolimus | 31 (100) | 25 (96) | 56 (98) | ||
| Cyclophosporine | 0 (0) | 1 (4) | 1 (2) | ||
| Immunsuppression 2, n (%) | |||||
| Mycophenolate | 31 (100) | 26 (100) | 57 (100) | - | |
| CMV donor/recipient mismatch | 13 (48) | 14 (51) | 57(100) | 0.27 | |
| ASW at TP1 | ASW TP2 | |||
|---|---|---|---|---|
| B Coefficient (95% CI) | p | B Coefficient (95% CI) | p | |
| Donor characteristics | ||||
| Age | 0.004 (−0.008–0.016) | 0.482 | 0.004 (−0.014–0.018) | 0.78 |
| Sex | −0.233 (−0.638–0.172) | 0.254 | −0.156 (0.857–0.223) | 0.45 |
| Height | −0.015 (−0.035–0.006) | 0.166 | −0.019 (−0.03–0.00) | 0.09 |
| Slight smoking | −0.342 (−0.987–0.304) | 0.292 | −0.63 (−0.987–0.860) | 0.891 |
| Medium smoking | −0.145 (−0.917–0.627) | 0.707 | −0.95 (−1.199–1.009) | 0.86 |
| Strong smoking | 0.499 (−0.200–1.198) | 0.158 | 0.728 (−0.272–1.728) | 0.150 |
| TLC | −0.126 (−0.300–0.047) | 0.151 | −0.2 (−0.405–0.057) | 0.14 |
| Days on ventilation | 0.006 (−0.059–0.071) | 0.852 | −0.001 (−0.087–0.087) | 0.995 |
| CRP | 0.001 (−0.001–0.003) | 0.294 | −0.063 (−0.003–0.002) | 0.662 |
| PO2 100% | 0.000 (−0.003–0.004) | 0.774 | −0.003 (−0.004–0.004) | 0.982 |
| Blood type A a | 0.244 (−0.193–0.681) | 0.268 | 0.57 (−0.2–1.15) | 0.06 |
| Blood type B a | 0.500 (−0.199–1.198) | 0.157 | 0.59 (−0.345–1.527) | 0.21 |
| Blood type AB a | −0.809 (−1.917–0.300) | 0.149 | −0.404 (−1.89–1.083) | 0.59 |
| Rhesus factor | – | – | 0.162 (−0.25–1.031) | 0.227 |
| CMV serostatus | 0.318 (−0.082–0.717) | 0.117 | 0.024 (−0.496–0.593) | 0.860 |
| Recipient characteristics | ||||
| Last LAS b | 0.005 (−0.007–0.017) | 0.416 | −0.018 (0.897–−0.02) | 0.655 |
| EBV mismatch D/R | 0.046 (−0.338–0.430) | 0.811 | 0.061 (−0.695–1.057) | 0.67 |
| CMV mismatch D/R | 0.115 (−0.080–0.310) | 0.242 | −0.43 (−0.748–−0.112) | 0.008 c |
| TLC difference D/R | −0.022 (−0.273–0.230) | 0.864 | −0.069 (−0.393–0.256) | 0.67 |
| Peri- and post-operative factors | ||||
| ECMO before LuTX | 0.560 (−0.304–1.424) | 0.199 | 0.132 (−0.634–1.815) | 0.34 |
| ECMO during surgery | 0.256 (−0.169−0.680) | 0.233 | 0.004 (−0.571–0.578) | 0.98 |
| Incision–suture time | 0.002 (−0.001–0.005) | 0.239 | −0.093 (0.005–0.002) | 0.52 |
| Ischemic time | −0.002 (−0.004–0.001) | 0.184 | −0.124 (−0.005–0.002) | 0.36 |
| Duration of MAP <60 mm Hg | 0.001 (−0.004–0.007) | 0.602 | −0.124 (−0.009–0.004) | 0.40 |
| Cumulative vasopressor dose | 6.662 × 10−5 (0.000–0.000) | 0.103 | −0.048 (0.000–0.000) | 0.74 |
| Post-LuTX time on ventilator | 0.000 (0.000–0.001) | 0.293 | 0.21 (0.00–0.001) | 0.12 |
| LOS in ICU | 0.006 (−0.003–0.014) | 0.176 | 0.006 (0.00–0.013) | 0.051 |
| Cumulative no. of acute rejections | - | 0.063 (−0.266–0.428) | 0.64 | |
| Cumulative no. of EBV reactivations | - | 0.082 (−0.248–0.411) | 0.62 | |
| Cumulative no. of CMV reactivations | - | 0.323 (0.063–0.584) | 0.016 d | |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| PO2 100% | 1.007 (1.000–1.014) | 0.044 | 1.010 (1.003–1.017) | 0.003 |
| Age | 0.988 (0.958–1.020) | 0.462 | 0.994 (0.954–1.036) | 0.781 |
| Sex | 0.514 (0.196–1.347) | 0.176 | 0.613 (0.201–1.868) | 0.389 |
| ECMO during surgery | 2.804 (1.121–7.014) | 0.028 | 7.711 (2.339–25.421) | <0.001 |
| Post-LuTX time on ventilator | 1.002 (1.001–1.003) | 0.004 | 1.000 (0.999–1.002) | 0.55 |
| Cumulative no. of CMV reactivations | 1.616 (1.128–2.315) | 0.009 | 1.800 (1.133–2.858) | 0.013 |
| ASW at TP1 | 1.636 (1.081–2.476) | 0.020 | 0.143 (0.566–1.153) | 0.392 |
| ASW at TP2 | 1.823 (1.132–2.935) | 0.013 | 1.885 (1.086–3.269) | 0.024 |
| CLAD at TP2 | 1.265 (0.513–3.120) | 0.610 | 0.987 (0.00–26.18 × 103) | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhnert, S.; Rotert, A.M.; Sommerlad, J.; Yogeswaran, A.; Reichert, M.; Askevold, I.; Hecker, A.; Koch, C.; Bräuninger, A.; Gattenlöhner, S.; et al. Increase in Alveolar Septal Width Is a Histological Predictor of Chronic Lung Allograft Dysfunction and Survival in Lung Transplant Recipients—A Longitudinal Study. J. Clin. Med. 2025, 14, 6368. https://doi.org/10.3390/jcm14186368
Kuhnert S, Rotert AM, Sommerlad J, Yogeswaran A, Reichert M, Askevold I, Hecker A, Koch C, Bräuninger A, Gattenlöhner S, et al. Increase in Alveolar Septal Width Is a Histological Predictor of Chronic Lung Allograft Dysfunction and Survival in Lung Transplant Recipients—A Longitudinal Study. Journal of Clinical Medicine. 2025; 14(18):6368. https://doi.org/10.3390/jcm14186368
Chicago/Turabian StyleKuhnert, Stefan, Anna M. Rotert, Janine Sommerlad, Athiththan Yogeswaran, Martin Reichert, Ingolf Askevold, Andreas Hecker, Christian Koch, Andreas Bräuninger, Stefan Gattenlöhner, and et al. 2025. "Increase in Alveolar Septal Width Is a Histological Predictor of Chronic Lung Allograft Dysfunction and Survival in Lung Transplant Recipients—A Longitudinal Study" Journal of Clinical Medicine 14, no. 18: 6368. https://doi.org/10.3390/jcm14186368
APA StyleKuhnert, S., Rotert, A. M., Sommerlad, J., Yogeswaran, A., Reichert, M., Askevold, I., Hecker, A., Koch, C., Bräuninger, A., Gattenlöhner, S., Seeger, W., Hecker, M., & Dorfmüller, P. (2025). Increase in Alveolar Septal Width Is a Histological Predictor of Chronic Lung Allograft Dysfunction and Survival in Lung Transplant Recipients—A Longitudinal Study. Journal of Clinical Medicine, 14(18), 6368. https://doi.org/10.3390/jcm14186368






