Measuring and Correction Methods of H.-J. Haase Improve Binocular Vision in Patients with Severe Anisometropia †
Abstract
1. Introduction
2. Materials and Methods
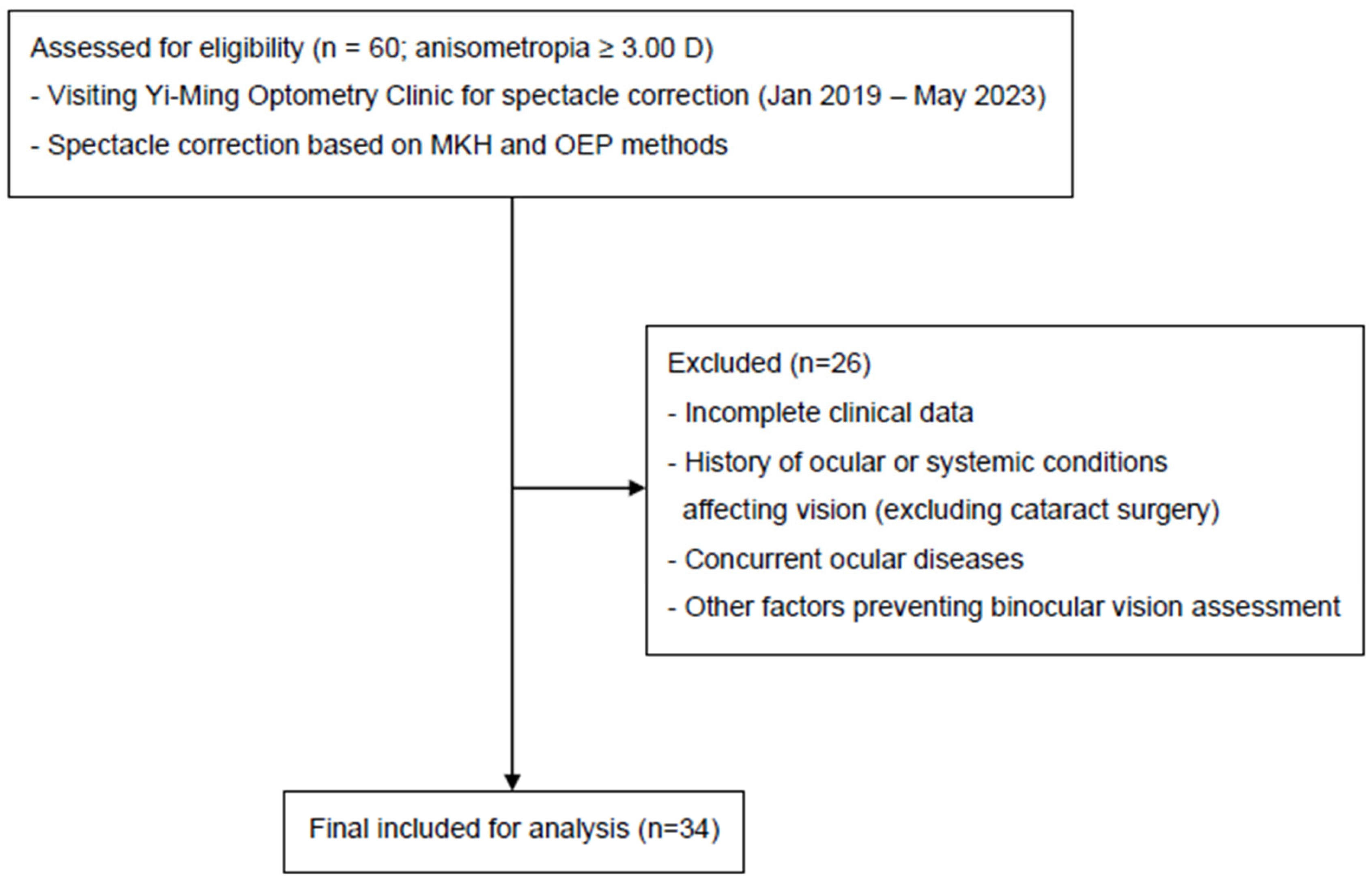
2.1. Inclusion and Exclusion Criteria
2.2. Primary Outcome
2.3. Equipment and Environment
2.4. Procedures
- Cross test: Alignment between a vertical line (right eye) and a horizontal line (left eye) was assessed. Prisms (base in/out) were adjusted until the cross aligned (Figure 1B(a)).
- Pointer test: A vertical pointer (right eye) was aligned with clock markings (left eye). Deviations were corrected with prism adjustments until alignment was achieved (Figure 1B(b)).
- Double pointer test: Alignment of vertical and horizontal pointers with clock markings was checked. Deviations (horizontal or vertical) were corrected with corresponding prisms until the crosshair aligned with the marking (Figure 1C(a)).
- Rectangle test: Right- and left-eye rectangles were aligned. Vertical misalignments were corrected using base-up or base-down prisms until the rectangles coincided (Figure 1C(b)).
- Stereo-balance test: Detected lateral displacement of triangles under stereo disparity. Deviations were corrected with prisms until triangles aligned with the central scale (Figure 1D(b)). After the heterophoria measurement, the next step was binocular balance testing.
2.5. The Measurement of Heterophoria by the OEP Method
- Setup: A prism dissociation of 6Δ base-up was placed in front of the right eye, while a 12Δ base-in measuring prism was placed in front of the left eye using a phoropter.
- Fixation target: Participants focused on a 20/30 visual acuity letter line at both near and distance while maintaining clarity.
- Alignment task: They were instructed to shift gaze to a lower target and indicate when the upper target aligned just above the lower one.
- Adjustment: Horizontal (12Δ) and vertical (6Δ) prisms were adjusted in one-diopter increments until alignment was reported.
- Repetition and averaging: The procedure was repeated three times, and average values were recorded.
- Criterion application: Sheard’s criterion was applied for exophoria cases, while Percival’s criterion was applied for esophoria cases.
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Bergaal, S.K.; Sharma, P.; Agarwal, T.; Saxena, R.; Phuljhele, S. Effect of induced anisometropia on stereopsis and surgical tasks in a simulated environment. Indian J. Ophthalmol. 2021, 69, 568–572. [Google Scholar] [CrossRef]
- Vincent, S.J.; Read, S.A. Progressive adult antimetropia. Clin. Exp. Optom. 2014, 97, 375–378. [Google Scholar] [CrossRef]
- Barrett, B.T.; Bradley, A.; Candy, T.R. The relationship between anisometropia and amblyopia. Prog. Retin. Eye Res. 2013, 36, 120–158. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.R.; Ponce, A.; Del Barco, L.J.; Díaz, J.A.; Pérez-Ocón, F. Impact of induced aniseikonia on stereopsis with random-dot stereogram. Optom. Vis. Sci. 2002, 79, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.R.; Ponce, A.; Anera, R.G. Induced aniseikonia diminishes binocular contrast sensitivity and binocular summation. Optom. Vis. Sci. 2004, 81, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.K. Errors of refraction and binocular optical defects. In Theory And Practice Of Optics And Refraction; Elsevier: New Delhi, India, 2008. [Google Scholar]
- Hashem, O.; Sheha, H. Ten-Year Outcomes of LASIK for Pediatric Myopic Anisometropia. Clin. Ophthalmol. 2022, 16, 4293–4301. [Google Scholar] [CrossRef]
- Winn, B.; Ackerley, R.G.; Brown, C.A.; Murray, F.K.; Paris, J.; John, M.S. Reduced aniseikonia in axial anisometropia with contact lens correction. Ophthalmic Physiol. Opt. 1988, 8, 341–344. [Google Scholar] [CrossRef]
- Yu, H.J.; Kuo, M.T.; Wu, P.C. Clinical Characteristics of Presenile Cataract: Change over 10 Years in Southern Taiwan. Biomed. Res. Int. 2021, 2021, 9385293. [Google Scholar] [CrossRef]
- Rutstein, R.P.; Fullard, R.J.; Wilson, J.A.; Gordon, A. Aniseikonia Induced by Cataract Surgery and Its Effect on Binocular Vision. Optom. Vis. Sci. 2015, 92, 201–207. [Google Scholar] [CrossRef]
- Vincent, S.J.; Collins, M.J.; Read, S.A.; Carney, L.G. Myopic anisometropia: Ocular characteristics and aetiological considerations. Clin. Exp. Optom. 2014, 97, 291–307. [Google Scholar] [CrossRef]
- Kundart, J. Diagnosis and Treatment of Aniseikonia: A Case Report and Review. Optom. Vis. Perform. 2018, 6. [Google Scholar]
- Haase, H.-J. Binokulare Korrektion: E. Sammlung von 10 Arbeiten aus d. Jahren 1957–1978 [d. Methodik u. Theorie von]; Schrickel: Düsseldorf, Germany, 1980. [Google Scholar]
- Haase, H.-J. Zur Fixationsdisparation: Eine Erweiterte Theorie und Praktische Folgerungen; Nachdruck der Gleichnamigen Zeitschriftenserie von 1980–1984; Mit Einem Nachtr. Stereo-Sehgleichgewicht [ua]; Verlag Optische Fachveröff: Heidelberg, Germany, 2000. [Google Scholar]
- Haase, H.-J. Winkelfehlsichtigkeiten Mit Fixationsdisparation; Bode: Pforzheim, Germany, 1999. [Google Scholar]
- Kundart, J. Prescribing Prism for Aniseikonia: A Case Report and Review. Optom. Vis. Perform. 2020, 8, 52. [Google Scholar]
- London, R.; Crelier, R.S. Fixation disparity analysis: Sensory and motor approaches. Optom.—J. Am. Optom. Assoc. 2006, 77, 590–608. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, T.P. Primary Care Optometry; Butterworth-Heinemann: Oxford, UK; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Politzer, T.; Schneider, R. Comparison of New Near Binocular Testing Sequence to the Standardized Optometric Extention Program 21 Point Examination. Ph.D. Thesis, Pacific University, Forest Grove, OR, USA, 2025. [Google Scholar]
- Bailey, R.N. The History of Ethics and Professionalism within Optometry in the United States of America 1898–2015, Part 1. Hindsight 2016, 47, 14–31. [Google Scholar]
- Lai, L.-J. Successful Treatment of Refractive Anisometropia with Prismatic Progressive Additional Lenses: A Technical Report. J. Clin. Exp. Ophthalmol. 2015, 6, 2. [Google Scholar]
- Kundart, J.; Citek, K.; Yudcovitch, L.; Hayes, J.R.; Ramaswamy, S. Diagnosis and treatment of aniseikonia from ocular asymmetry. Optom. Vis. Perform. 2025, 13, 94–116. [Google Scholar]
- Gerling, J.; de Paz, H.; Schroth, V.; Bach, M.; Kommerell, G. Ist die Feststellung einer Fixationsdisparation mit der Mess- und Korrektionsmethodik nach H.-J. Haase (MKH) verlässlich? Klin. Monatsblätter Augenheilkd. 2000, 216, 401–411. [Google Scholar] [CrossRef]
- Kromeier, M.; Schmitt, C.; Bach, M.; Kommerell, G. Bessern Prismen nach Hans-Joachim Haase die Stereosehschärfe? Klin. Monatsblätter Augenheilkd. 2002, 219, 422–428. [Google Scholar]
- Kromeier, M.; Schmitt, C.; Bach, M.; Kommerell, G. Do prisms according to Hans-Joachim Haase influence ocular prevalence? Klin. Monatsblätter Augenheilkd. 2002, 219, 851–857. [Google Scholar] [CrossRef]
- Tidbury, L.P.; Czanner, G.; Newsham, D. Fiat Lux: The effect of illuminance on acuity testing. Graefe's Arch. Clin. Exp. Ophthalmol. 2016, 254, 1091–1097. [Google Scholar] [CrossRef]
- Harris, W.F. Prentice's equation and generalizations. Optom. Vis. Sci. 2000, 77, 373–379. [Google Scholar] [CrossRef]
- Barrett, B.T. Assessment of Binocular Vision and Accommodation. In Clinical Procedures in Primary Eye Care, 4th ed.; Elliott, D.B., Ed.; W.B. Saunders: Oxford, UK, 2014; pp. 147–208. [Google Scholar]
- Fogt, N.; Toole, A.J.; Rogers, D.L. A review of proximal inputs to the near response. Clin. Exp. Optom. 2016, 99, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, M.; Wick, B. Clinical Management of Binocular Vision: Heterophoric, Accommodative, and Eye Movement Disorders; Lippincott Williams & Wilkins: Ambler, PA, USA, 2008. [Google Scholar]
- Goss, D.A. Ocular Accommodation, Convergence, and Fixation Disparity: A Manual of Clinical Analysis; Butterworth-Heinemann: Oxford, UK, 1995. [Google Scholar]
- Krarup, T.G.; Nisted, I.; Christensen, U.; Kiilgaard, J.F.; la Cour, M. The tolerance of anisometropia. Acta Ophthalmol. 2020, 98, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Strasburger, H. Seven Myths on Crowding and Peripheral Vision. Iperception 2020, 11, 2041669520913052. [Google Scholar] [CrossRef] [PubMed]
- Anstis, S.M. Letter: A chart demonstrating variations in acuity with retinal position. Vis. Res. 1974, 14, 589–592. [Google Scholar] [CrossRef]
- Lambertus, S.; Bax, N.M.; Fakin, A.; Groenewoud, J.M.M.; Klevering, B.J.; Moore, A.T.; Michaelides, M.; Webster, A.R.; van der Wilt, G.J.; Hoyng, C.B.; et al. Highly sensitive measurements of disease progression in rare disorders: Developing and validating a multimodal model of retinal degeneration in Stargardt disease. PLoS ONE 2017, 12, e0174020. [Google Scholar] [CrossRef]
- Goersch, H. Übertragung prismatischer Korrektionen aus der Meßbrille in die Korrektionsbrille. Dtsch. Opt. 1992, 12, 26–32. [Google Scholar]
- Mühlendyck, H.; Rüssmann, W.; Reinboth, J.J. The 4-prism diopter base-in test in diagnosis of exophoria with asthenopia and compensation by accommodative convergence. Ophthalmologe 1993, 90, 6–10. [Google Scholar]
- Simonsz, H.; van Els, J.; Ruijter, J.; Bakker, D.; Spekreijse, H. Preliminary report: Prescription of prism-glasses by the Measurement and Correction Method of H.-J. Haase or by conventional orthoptic examination: A multicenter, randomized, double-blind, cross-over study. Strabismus 2001, 9, 17–27. [Google Scholar] [CrossRef]
- Lie, I.; Opheim, A. Long-term acceptance of prisms by heterophorics. J. Am. Optom. Assoc. 1985, 56, 272–278. [Google Scholar]
- Lie, I.; Opheim, A. Long-term stability of prism correction of heterophorics and heterotropics; a 5 year follow-up. Part I: Heterophorics. J. Am. Optom. Assoc. 1990, 61, 491–498. [Google Scholar]
- Methling, D.; Jaschinski, W. Contrast sensitivity after wearing prisms to correct for heterophoria. Ophthalmic Physiol. Opt. 1996, 16, 211–215. [Google Scholar] [CrossRef]
- Kolb, H.; Fernandez, E.; Nelson, R. (Eds.) Webvision: The Organization of the Retina and Visual System; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Cline, D.; Hofstetter, H.W.; Griffin, J.R. Dictionary of Visual Science; Butterworth-Heinemann: Boston, MA, USA, 1997. [Google Scholar]
- Evans, B.J. Pickwell's Binocular Vision Anomalies; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Evans, B.J.W. (Ed.) Appendix 1—Confusing Aspects of Binocular Vision Tests. In Pickwell's Binocular Vision Anomalies, 5th ed.; Butterworth-Heinemann: Edinburgh, UK, 2007; pp. 340–341. [Google Scholar]
- Karania, R.; Evans, B.J. The Mallett Fixation Disparity Test: Influence of test instructions and relationship with symptoms. Ophthalmic. Physiol. Opt. 2006, 26, 507–522. [Google Scholar] [CrossRef]
- Wang, S.-H.; Ko, M.-L. MKH optometry benefits the binocular vision of patients with significant anisometropia. Investig. Ophthalmol. Vis. Sci. 2024, 65, 3067. [Google Scholar]

| Number = 34 | Mean ± SE (Min, Max) |
|---|---|
| Age (years) | 48.32 ± 2.05 (min 32, max 82) |
| Sex | |
| Male | 16 (47%) |
| Female | 18 (53%) |
| Sph/Cyl/SE | |
| OD | −4.62 ± 0.91 (min −14.00, max 5.12)/−0.89 ± 0.11 (min −3.25, max 0.00)/−5.06 ± 0.91 (min −14.19, max 4.69) |
| OS | −2.73 ± 0.69 (min −16.50, max 5.25)/−1.18 ± 0.16 (min −3.00, max 0.00)/−3.31 ± 0.71 (min −16.87, max 5.00) |
| Anisometropia (SE) | |5.51 ± 0.45| (min 3.00, max 14.46) |
| Phoria (▵) | 2.26 ± 0.32 (min 0.50, max 8.75) |
| VA (logMAR) | |
| OD | 0.02 ± 0.01 (min −0.10, max 0.20) |
| OS | 0.11 ± 0.06 (min 0.00, max 0.20) |
| MCH | OEP | p | |
|---|---|---|---|
| Mean ± SE (Min, Max) | Mean ± SE (Min, Max) | ||
| OUVA (logMAR) | −0.020 ± 0.010 (min −0.079, max 0.097) | 0.040 ± 0.010 (min 0.201, max 0.000) | <0.001 |
| ODH (▵) | 0.780 ± 0.128 (min 0.000, max 2.750) | 0.020 ± 0.020 (min 0.000, max 0.688) | <0.001 |
| OSH (▵) | 0.790 ± 0.119 (min 0.000, max 3.000) | 0.040 ± 0.025 (min 0.000, max 0.688) | <0.001 |
| ODV (▵) | 0.260 ± 0.093 (min 0.000, max 2.450) | 0.350 ± 0.131 (min 0.000, max 3.500) | 0.129 |
| OSV (▵) | 0.420 ± 0.177 (min 0.000, max 5.000) | 0.320 ± 0.132 (min 0.000, max 3.500) | 0.203 |
| SV (‘’) | 97.560 ± 7.888 (min 40.000, max 200.000) | 167.120 ± 17.295 (min 50.000, max 400.000) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-H.; Chen, Y.-Y.; Ko, M.-L. Measuring and Correction Methods of H.-J. Haase Improve Binocular Vision in Patients with Severe Anisometropia. J. Clin. Med. 2025, 14, 6367. https://doi.org/10.3390/jcm14186367
Wang S-H, Chen Y-Y, Ko M-L. Measuring and Correction Methods of H.-J. Haase Improve Binocular Vision in Patients with Severe Anisometropia. Journal of Clinical Medicine. 2025; 14(18):6367. https://doi.org/10.3390/jcm14186367
Chicago/Turabian StyleWang, Shun-Huan, Ya-Yu Chen, and Mei-Lan Ko. 2025. "Measuring and Correction Methods of H.-J. Haase Improve Binocular Vision in Patients with Severe Anisometropia" Journal of Clinical Medicine 14, no. 18: 6367. https://doi.org/10.3390/jcm14186367
APA StyleWang, S.-H., Chen, Y.-Y., & Ko, M.-L. (2025). Measuring and Correction Methods of H.-J. Haase Improve Binocular Vision in Patients with Severe Anisometropia. Journal of Clinical Medicine, 14(18), 6367. https://doi.org/10.3390/jcm14186367






