Association Between Periodontitis and Cancer: A Perspective Review of Mechanisms and Clinical Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.2.1. Research Question and PECOS Strategy
2.2.2. Inclusion Criteria
2.2.3. Exclusion Criteria
2.3. Selection of Studies
2.4. Data Extraction
3. Results
3.1. Results of the Literature Search
3.2. Study Characteristics
4. Discussion
4.1. Periodontitis and PC
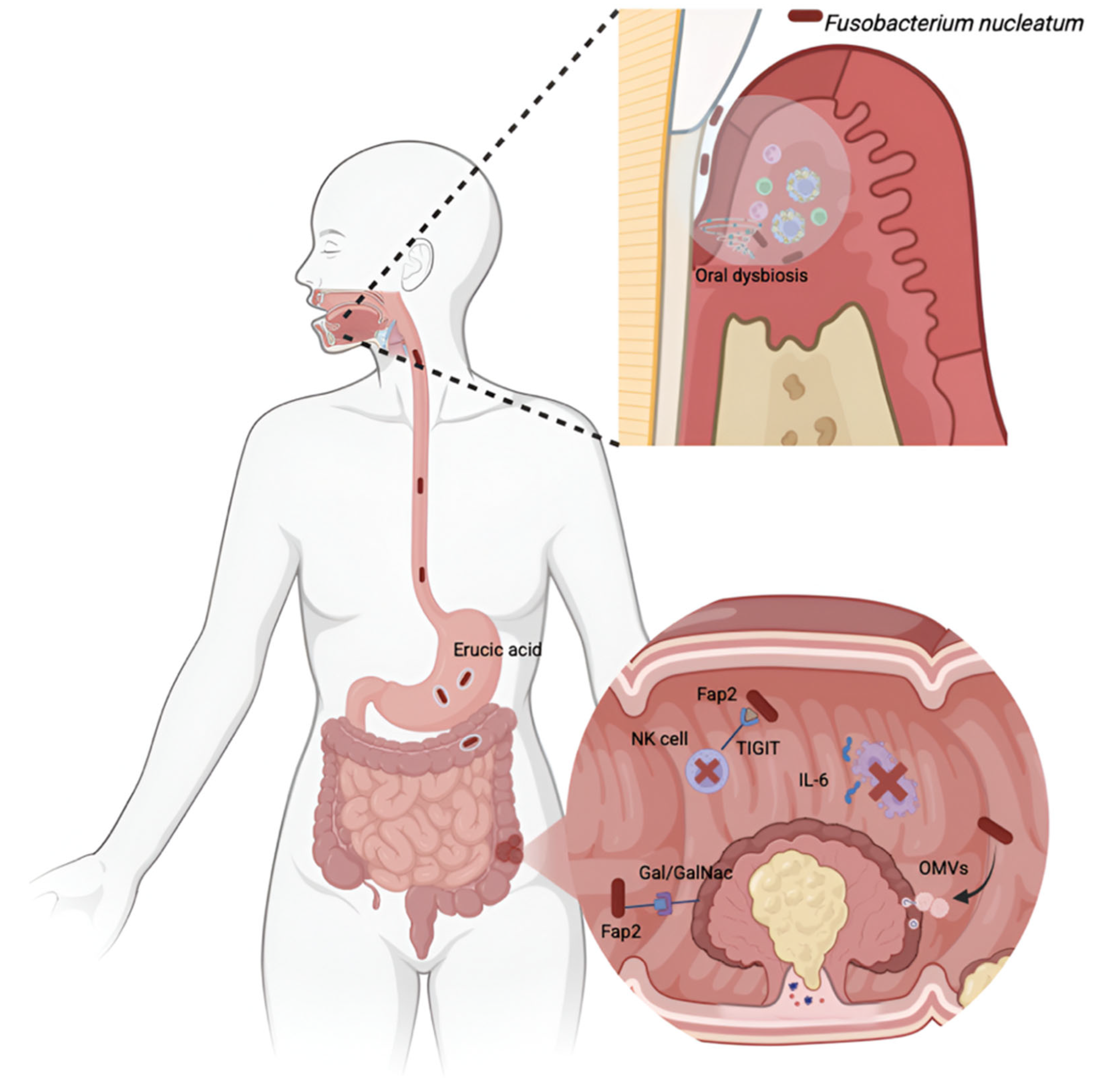
4.2. Periodontitis and CRC
4.3. Periodontitis and OSCC
4.4. Immune-Related Periodontitis
4.5. Periodontitis and OLP
4.6. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baelum, V.; López, R. Periodontal disease epidemiology—Learned and unlearned? Periodontol. 2000 2013, 62, 37–58. [Google Scholar] [CrossRef]
- Beck, J.D.; Papapanou, P.N.; Philips, K.H.; Offenbacher, S. Periodontal medicine: 100 years of progress. J. Dent. Res. 2019, 98, 1053–1062. [Google Scholar] [CrossRef]
- La Rosa, G.; Gattuso, G.; Pedullà, E.; Rapisarda, E.; Nicolosi, D.; Salmeri, M. Association of oral dysbiosis with oral cancer development (Review). Oncol. Lett. 2020, 19, 3045–3058. [Google Scholar] [CrossRef]
- Ye, C.; Liu, X.; Liu, Z.; Pan, C.; Zhang, X.; Zhao, Z.; Sun, H. Fusobacterium nucleatum in tumors: From tumorigenesis to tumor metastasis and tumor resistance. Cancer Biol. Ther. 2024, 25, 2306676. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Zhou, Y.; Ruf, S.; Meyle, J. Pathogenic mechanisms of Fusobacterium nucleatum on oral epithelial cells. Front. Oral Health 2022, 3, 831607. [Google Scholar] [CrossRef]
- Nie, F.; Zhang, J.; Tian, H.; Zhao, J.; Gong, P.; Wang, H.; Wang, S.; Yang, P.; Yang, C. The role of CXCL2-mediated crosstalk between tumor cells and macrophages in Fusobacterium nucleatum-promoted oral squamous cell carcinoma progression. Cell Death Dis. 2024, 15, 277. [Google Scholar] [CrossRef] [PubMed]
- Duizer, C.; Salomons, M.; van Gogh, M.; Gräve, S.; Schaafsma, F.A.; Stok, M.J.; Sijbranda, M.; Sivasamy, R.K.; Willems, R.J.L.; de Zoete, M.R. Fusobacterium nucleatum upregulates the immune inhibitory receptor PD-L1 in colorectal cancer cells via the activation of ALPK1. Gut Microbes 2025, 17, 2458203. [Google Scholar] [CrossRef]
- Mondal, T.; Chattopadhyay, D.; Saha Mondal, P.; Das, S.; Mondal, A.; Das, A.; Samanta, S.; Saha, T. Fusobacterium nucleatum modulates the Wnt/β-catenin pathway in colorectal cancer development. Int. J. Biol. Macromol. 2025, 299, 140196. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Sharma, Y.; Kumar, D. Unveiling the link between chronic inflammation and cancer. Metab. Open 2025, 25, 100347. [Google Scholar] [CrossRef]
- Mesa-López, M.J.; Bravo, M.; Egea, J.; El-Amrani, S.; Bonilla, M.; Alberca, F.; Mesa, F. Periodontitis as a field of cancerization: Association with carcinoembryonic antigen in colorectal cancer patients. Clin. Oral Investig. 2025, 29, 323. [Google Scholar] [CrossRef]
- Trumet, L.; Grötsch, B.; Agaimy, A.; Galler, K.; Geppert, C.; Winter, L.; Ries, J.; Kesting, M.; Weber, M. Multiplex immunofluorescence assessment of macrophages and IL-23R in inflammatory and malignant diseases of the oral mucosa: A pilot study. Front. Immunol. 2025, 16, 1569490. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, K.; Gao, L.; He, Q.; Ge, S. Microbial community composition in subgingival plaques and heterogeneity of tumor tissue TCRβ CDR3 repertoire in patients with moderate-to-severe periodontitis and oral squamous cell carcinoma. Technol. Health Care 2025, 33, 25–51. [Google Scholar] [CrossRef]
- Vitor, G.P.; Carvalho, A.P.; Esteves, R.P.; Miconi, W.G.; Costa, F.O.; Cota, M. Association between periodontitis and prostate cancer: A case–control study. J. Periodontol. 2025. early view. [Google Scholar] [CrossRef]
- Ma, K.S.-K.; Chen, S.T.; Dinh, Y.; Chiang, C.-H.; Van Dyke, T.E.; Sullivan, R.; Ananthakrishnan, A.N.; Hsia, Y.P.; Peng, C.-M. Periodontitis is an immune-related adverse event associated with immune checkpoint inhibitors: A multi-center cohort study. Cancer Lett. 2024, 598, 217100. [Google Scholar] [CrossRef]
- Reddy, S.S.; Rakesh, N.; Prashanth, R.; Choudhary, R.; Sruthy, S. Periodontitis and its role in oral cancer susceptibility: A case-control study. Indian J. Med. Paediatr. Oncol. 2024, 46, 03. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lin, P.Y.; Chen, C.C.; Chen, Y.K. Risk factors for the development of oral precancerous lesions in a cohort of 293 oral lichen planus patients with or without chronic periodontitis in southern Taiwan. J. Dent. Sci. 2023, 19, 594–600. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Wei, Y.; Zhong, Y.; Wang, Y.; Huang, R. Association between periodontal disease and prostate cancer: A systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e459–e465. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gu, C.; Li, S.; Gan, S.; Li, Y.; Xiang, S.; Gong, L.; Wang, S. Periodontal disease and the risk of prostate cancer: A meta-analysis of cohort studies. Int. Braz. J. Urol. 2021, 47, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Ohashi, Y.; Ushijima, T. How chronic inflammation fuels carcinogenesis as an environmental epimutagen. Discov. Oncol. 2025, 16, 1150. [Google Scholar] [CrossRef]
- Kanli, A.; Sunnetci-Akkouyunlu, D.; Kulcu-Sarikaya, N.; Urgutaş, C.; Akpinar, G.; Kasap, M. Potential common molecular mechanisms between periodontitis and prostate cancer: A network analysis of differentially expressed miRNAs. In Vivo 2025, 39, 795–809. [Google Scholar] [CrossRef]
- Martino, E.; Balestrieri, A.; Aragona, F.; Bifulco, G.; Mele, L.; Campanile, G.; Balestrieri, M.L.; D’onofrio, N. MiR-148a-3p promotes colorectal cancer cell ferroptosis by targeting SLC7A11. Cancers 2023, 15, 4342. [Google Scholar] [CrossRef]
- Ding, F.; Wu, H.; Han, X.; Jiang, X.; Xiao, Y.; Tu, Y.; Yu, M.; Lei, W.; Hu, S. The miR-148/152 family contributes to angiogenesis of human pluripotent stem cell-derived endothelial cells by inhibiting MEOX2. Mol. Ther. Nucleic Acids 2023, 15, 582–593. [Google Scholar] [CrossRef]
- Chickooree, D.; Zhu, K.; Ram, V.; Wu, H.J.; He, Z.J.; Zhang, S. A preliminary microarray assay of the miRNA expression signatures in buccal mucosa of oral submucous fibrosis patients. J. Oral Pathol. Med. 2016, 45, 691–697. [Google Scholar] [CrossRef]
- Aldilaijan, A.F.; Kim, Y.I.; Kim, C.W.; Yoon, Y.S.; Park, I.J.; Lim, S.B.; Kim, J.; Ro, J.-S.; Kim, J.C. Clinical implication of tissue carcinoembryonic antigen expression in association with serum carcinoembryonic antigen in colorectal cancer. Sci. Rep. 2023, 13, 7616. [Google Scholar] [CrossRef]
- Luo, T.; Li, J.; Pu, K.; Yang, G. Association between periodontitis and gastrointestinal cancer risk and prognosis: Evidence from a nested case–control study in Southwest China. Eur. J. Med. Res. 2025, 30, 225. [Google Scholar] [CrossRef]
- Nolde, M.; Alayash, Z.; Reckelkamm, S.L.; Kocher, T.; Ehmke, B.; Holtfreter, B.; Baurecht, H.; Georgakis, M.K.; Baumeister, S.-E. Downregulation of interleukin 6 signaling might reduce the risk of periodontitis: A drug target Mendelian randomization study. Front. Immunol. 2023, 14, 1160148. [Google Scholar] [CrossRef] [PubMed]
- Waldner, M.J.; Foersch, S.; Neurath, M.F. Interleukin-6—A key regulator of colorectal cancer development. Int. J. Biol. Sci. 2012, 8, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Dadgar-Zankbar, L.; Elahi, Z.; Shariati, A.; Khaledi, A.; Razavi, S.; Khoshbayan, A. Exploring the role of Fusobacterium nucleatum in colorectal cancer: Implications for tumor proliferation and chemoresistance. Cell Commun. Signal. 2024, 22, 547. [Google Scholar] [CrossRef]
- Rubinstein, M.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; Emgård, J.E.M.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Sheng, H.; Zhen, Y.; Wu, B.; Li, Z.; Chen, D.; Zhou, H. Oral Fusobacterium nucleatum resists the acidic pH of the stomach due to membrane erucic acid synthesized via enoyl-CoA hydratase-related protein FnFabM. J. Oral Microbiol. 2025, 17, 2453964. [Google Scholar]
- Udayasuryan, B.; Zhou, Z.; Ahmad, R.N.; Sobol, P.; Deng, C.; Nguyen, T.T.D.; Kodikalla, S.; Morrison, R.; Goswami, I.; Slade, D.J.; et al. Fusobacterium nucleatum infection modulates the transcriptome and epigenome of HCT116 colorectal cancer cells in an oxygen-dependent manner. Commun. Biol. 2024, 7, 551. [Google Scholar]
- Yamamoto, H.; Watanabe, Y.; Arai, H.; Umemoto, K.; Tateishi, K.; Sunakawa, Y. Microsatellite instability: A 2024 update. Cancer Sci. 2024, 115, 1738–1748. [Google Scholar] [CrossRef]
- Xu, C.C.; Fan, L.N.; Lin, Y.F.; Shen, W.Y.; Qi, Y.D.; Zhang, Y.; Chen, Z.; Wang, L.; Long, Y.; Hou, T.; et al. Fusobacterium nucleatum promotes colorectal cancer metastasis through miR-1322/CCL20 axis and M2 polarization. Gut Microbes 2021, 13, 1980347. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Wu, R.; Mao, M.; Ji, Y.; Wang, X.; Dou, S.; Yan, M.; Chen, W. Fusobacterium nucleatum-derived outer membrane vesicles promote immunotherapy resistance via changes in tryptophan metabolism in tumour-associated macrophages. J. Extracell. Vesicles 2025, 14, e70070. [Google Scholar] [CrossRef] [PubMed]
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontol. 2000 2020, 83, 213–233. [Google Scholar] [CrossRef]
- Shi, Y.T.; He, J.M.; Tong, Z.A.; Qian, Y.J.; Wang, Q.W.; Jia, D.J.C.; Zhu, W.; Zhao, Y.; Cai, B.; Chen, S.; et al. Ligature-induced periodontitis drives colorectal cancer: An experimental model in mice. J. Dent. Res. 2023, 102, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, Y.; Shi, X.; Zhao, Y.; Tang, Y.; Liu, S.; Zhu, X. Porphyromonas gingivalis outer membrane vesicles augments proliferation and metastasis of oral squamous cell carcinoma cells. BMC Oral Health 2025, 25, 701. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Li, J.; Liu, Z.; Chen, Q.; Chen, W.H.; Zhang, X.Z.; Luo, G.-F.; Shang, Z. Targeted elimination of the oral pathogen to overcome chemoresistance of oral squamous cell carcinoma by biologically derived nanotherapeutics. ACS Nano 2024, 18, 31794–31808. [Google Scholar] [CrossRef]
- Yáñez, L.; Soto, C.; Tapia, H.; Pacheco, M.; Tapia, J.; Osses, G.; Salinas, D.; Rojas-Celis, V.; Hoare, A.; Quest, A.F.G.; et al. Co-culture of P. gingivalis and F. nucleatum synergistically elevates IL-6 expression via TLR4 signaling in oral keratinocytes. Int. J. Mol. Sci. 2024, 25, 3611. [Google Scholar] [CrossRef]
- Mazurek-Mochol, M.; Bonsmann, T.; Mochol, M.; Poniewierska-Baran, A.; Pawlik, A. The role of interleukin 6 in periodontitis and its complications. Int. J. Mol. Sci. 2024, 25, 2146. [Google Scholar] [CrossRef]
- Kajihara, R.; Sakai, H.; Han, Y.; Amari, K.; Kawamoto, M.; Hakoyama, Y.; Nagashio, S.; Yamada, S.-I.; Sanjo, H.; Kurita, H. Presence of periodontitis may synergistically contribute to cancer progression via Treg and IL-6. Sci. Rep. 2022, 12, 11584. [Google Scholar] [CrossRef]
- Pai, S.I.; Matheus, H.R.; Guastaldi, F.P.S. Effects of periodontitis on cancer outcomes in the era of immunotherapy. Lancet Healthy Longev. 2023, 4, e166–e175. [Google Scholar]
- Sanadi, R.M.; Khandekar, P.; Chaudhari, S.; Javali, M.A.; Gurav, N.U. Association of periodontal disease with oral lichen planus: A systematic review and meta-analysis. J. Oral Maxillofac. Pathol. 2023, 27, 173. [Google Scholar] [PubMed]
- Nunes, G.P.; Pirovani, B.O.; Nunes, L.P.; Silva, A.N.A.; Morábito, M.J.S.D.; Nunes-Júnior, N.A.; Delbem, A.C.B.; Ferrisse, T.M. Does oral lichen planus aggravate the state of periodontal disease? A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 3357–3371. [Google Scholar] [CrossRef] [PubMed]
- Zaporowska-Stachowiak, I.; Springer, M.; Stachowiak, K.; Oduah, M.; Sopata, M.; Wieczorowska-Tobis, K.; Bryl, W. Interleukin 6 family of cytokines in cancers. J. Interferon Cytokine Res. 2024, 44, 45–49. [Google Scholar] [CrossRef] [PubMed]
| Author/Date | Study Design | N | Sample | Cancer Type | Main Outcomes |
|---|---|---|---|---|---|
| Mesa-López et al., 2025 [10] | Cross-sectional | 59 patients | Colonoscopy biopsy | CRC | In total, 69.5% of patients with CRC presented with periodontitis. A significant association was observed between CEA and periodontitis, likely due to the role of chronic inflammation. Periodontitis may create a favorable microenvironment for cancer development, acting like field cancerization, but does not appear to influence disease progression. |
| Trumet et al., 2025 [11] | Pilot | 29 patients with cancer and periodontitis | Gingiva biopsy | OSCC | Periodontitis showed a trend toward increased macrophage infiltration, similar to precancerous lesions and OSCC, suggesting a potential shared inflammatory axis in oral carcinogenesis. |
| Huang et al., 2025 [12] | Cross-sectional | 5 healthy, 5 moderate, 5 severe periodontitis with OSCC | Subgingival plaque and gingival/tumor tissue | OSCC | Microbial dysbiosis and reduced TCRβ repertoire diversity in patients with periodontitis and OSCC suggest a possible immuno-microbial pathway in oral carcinogenesis. |
| Vitor et al., 2025 [13] | Case–control | 152 cases, 220 controls | Periodontal examination | PC | Periodontitis was significantly associated with PC. Patients with PC exhibited worse periodontal status (higher PPD, CAL, BOP, and PISA). The association was stronger in patients with lower Gleason grades. Chronic inflammation may mediate this link. |
| Ma et al., 2024 [14] | Cohort | 868 treated with ICIs | Periodontal examination and irAEs | Various types of cancer | ICI treatment was associated with an increased risk of periodontitis. Interestingly, immune-related periodontitis was linked to improved overall and progression-free survival as well as concurrent immune-related cutaneous adverse events. |
| Reddy et al., 2024 [15] | Case–control | 63 cases, 63 controls | Questionnaire | OSCC | Periodontitis severity was significantly associated with oral cancer risk. Chronic inflammation, shifts in the oral microbiome, and cofactors such as smoking, alcohol, and poor oral hygiene contribute. Advanced periodontitis may act as an independent risk factor even in the absence of smoking or alcohol. Proinflammatory cytokines (IL-1β, TNF-α), pathogens like P. gingivalis, and oncogenic viruses contribute to carcinogenesis. |
| Huang et al., 2023 [16] | Retrospective | 293 patients | Biopsy | OLP | Periodontitis in patients with OLP is associated with a higher risk of precancerous lesions, possibly mediated by MMP-1/MMP-9, IL-17, and TNF-α. Additional risk is conferred by factors such as betel nut use, tobacco, and Candida infection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla, M.; Peñalver, I.; Mesa-López, M.J.; Mesa, F. Association Between Periodontitis and Cancer: A Perspective Review of Mechanisms and Clinical Evidence. J. Clin. Med. 2025, 14, 6334. https://doi.org/10.3390/jcm14176334
Bonilla M, Peñalver I, Mesa-López MJ, Mesa F. Association Between Periodontitis and Cancer: A Perspective Review of Mechanisms and Clinical Evidence. Journal of Clinical Medicine. 2025; 14(17):6334. https://doi.org/10.3390/jcm14176334
Chicago/Turabian StyleBonilla, Marco, Irene Peñalver, María José Mesa-López, and Francisco Mesa. 2025. "Association Between Periodontitis and Cancer: A Perspective Review of Mechanisms and Clinical Evidence" Journal of Clinical Medicine 14, no. 17: 6334. https://doi.org/10.3390/jcm14176334
APA StyleBonilla, M., Peñalver, I., Mesa-López, M. J., & Mesa, F. (2025). Association Between Periodontitis and Cancer: A Perspective Review of Mechanisms and Clinical Evidence. Journal of Clinical Medicine, 14(17), 6334. https://doi.org/10.3390/jcm14176334







