Risk of Abortion and Ectopic Pregnancy in Women with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Selection of Participants
2.3. Outcome
2.4. Variables
2.5. Statistics
2.6. Ethics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J. Clin. Endocrinol. Metab. 2020, 106, e1071–e1083. [Google Scholar] [CrossRef]
- Park, C.H.; Chun, S. Influence of combined oral contraceptives on polycystic ovarian morphology-related parameters in Korean women with polycystic ovary syndrome. Obstet. Gynecol. Sci. 2020, 63, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.; Laughlin, G.A.; Morales, A.J.; Yen, S.S. Inappropriate gonadotropin secretion in polycystic ovary syndrome: Influence of adiposity. J. Clin. Endocrinol. Metab. 1997, 82, 3728–3733. [Google Scholar] [CrossRef]
- Yang, S.W.; Yoon, S.H.; Kim, M.; Seo, Y.S.; Yuk, J.S. Risk of Gestational Diabetes and Pregnancy-Induced Hypertension with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study. J. Clin. Med. 2023, 12, 1738. [Google Scholar] [CrossRef]
- Choudhury, A.A.; Rajeswari, V.D. Polycystic ovary syndrome (PCOS) increases the risk of subsequent gestational diabetes mellitus (GDM): A novel therapeutic perspective. Life Sci. 2022, 310, 121069. [Google Scholar] [CrossRef]
- Mills, G.; Badeghiesh, A.; Suarthana, E.; Baghlaf, H.; Dahan, M.H. Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: A population-based study on 9.1 million pregnancies. Hum. Reprod. 2020, 35, 1666–1674. [Google Scholar] [CrossRef]
- Khomami, M.B.; Joham, A.E.; Boyle, J.A.; Piltonen, T.; Silagy, M.; Arora, C.; Misso, M.L.; Teede, H.J.; Moran, L.J. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity—A systematic review, meta-analysis, and meta-regression. Obes. Rev. 2019, 20, 659–674. [Google Scholar] [CrossRef]
- Homburg, R. Pregnancy complications in PCOS. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 281–292. [Google Scholar] [CrossRef]
- Khomami, M.B.; Shorakae, S.; Hashemi, S.; Harrison, C.L.; Piltonen, T.T.; Romualdi, D.; Tay, C.T.; Teede, H.J.; Vanky, E.; Mousa, A. Systematic review and meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Nat. Commun. 2024, 15, 5591. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Diao, F.; Cui, Y.; Mao, Y.; Wang, W.; Liu, J. The association between polycystic ovary syndrome and ectopic pregnancy after in vitro fertilization and embryo transfer. Am. J. Obstet. Gynecol. 2013, 209, 139.e1–139.e9. [Google Scholar] [CrossRef]
- Sha, T.; Wang, X.; Cheng, W.; Yan, Y. A meta-analysis of pregnancy-related outcomes and complications in women with polycystic ovary syndrome undergoing IVF. Reprod. Biomed. Online 2019, 39, 281–293. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, M.; Yang, S.W.; Lee, K.-M.; Han, S.W.; Seo, S.H.; Lee, A.; Sohn, I.S.; Kwon, H.S.; Cho, G.J.; et al. Evaluation of maternal rhesus blood type as a risk factor in adverse pregnancy outcomes in Korea: A nationwide health insurance database study. Obstet. Gynecol. Sci. 2020, 63, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Lim, N.-K.; Choi, Y.-M.; Hwang, K.-R.; Chae, S.-J.; Park, C.-W.; Choi, D.-S.; Kang, B.-M.; Lee, B.-S.; Kim, T.; et al. Prevalence of metabolic syndrome is higher among non-obese PCOS women with hyperandrogenism and menstrual irregularity in Korea. PLoS ONE 2014, 9, e99252. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam, E.A.-S.P.C.W.G. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; on behalf of theInternational PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- Jakubowicz, D.J.; Essah, P.A.; Seppälä, M.; Jakubowicz, S.; Baillargeon, J.-P.; Koistinen, R.; Nestler, J.E. Reduced serum glycodelin and insulin-like growth factor-binding protein-1 in women with polycystic ovary syndrome during first trimester of pregnancy. J. Clin. Endocrinol. Metab. 2004, 89, 833–839. [Google Scholar] [CrossRef]
- Okon, M.A.; Laird, S.M.; Tuckerman, E.M.; Li, T.-C. Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertil. Steril. 1998, 69, 682–690. [Google Scholar] [CrossRef]
- Falcetta, P.; Benelli, E.; Molinaro, A.; Di Cosmo, C.; Bagattini, B.; Del Ghianda, S.; Salvetti, G.; Fiore, E.; Pucci, E.; Fruzzetti, F.; et al. Effect of aging on clinical features and metabolic complications of women with polycystic ovary syndrome. J. Endocrinol. Investig. 2021, 44, 2725–2733. [Google Scholar] [CrossRef]
- Nybo Andersen, A.M.; Wohlfahrt, J.; Christens, P.; Olsen, J.; Melbye, M. Maternal age and fetal loss: Population based register linkage study. BMJ 2000, 320, 1708–1712. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive, M. Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2012, 98, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 193: Tubal Ectopic Pregnancy. Obstet. Gynecol. 2018, 131, e91–e103. [Google Scholar] [CrossRef] [PubMed]
- Boutzios, G.; Livadas, S.; Piperi, C.; Vitoratos, N.; Adamopoulos, C.; Hassiakos, D.; Iavazzo, C.; Diamanti-Kandarakis, E. Polycystic ovary syndrome offspring display increased oxidative stress markers comparable to gestational diabetes offspring. Fertil. Steril. 2013, 99, 943–950. [Google Scholar] [CrossRef]
- Jeffers, M.; O’Dwyer, P.; Curran, B.; Leader, M.; Gillan, J. Partial hydatidiform mole: A common but underdiagnosed condition: A 3-year retrospective clinicopathological and DNA flow cytometric analysis. Int. J. Gynecol. Pathol. 1993, 12, 315–323. [Google Scholar] [CrossRef]
- Gupta, S.; Surti, N.; Metterle, L.; Chandra, A.; Agarwal, A. Antioxidants and female reproductive pathologies. Arch. Med. Sci. Special Issues 2009, 2009, 173. [Google Scholar]
- Harma, M.; Erel, O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med. Wkly. 2003, 133, 563–566. [Google Scholar] [CrossRef]
- Ryu, K.-J.; Kim, M.S.; Kim, H.K.; Kim, Y.J.; Yi, K.W.; Shin, J.H.; Hur, J.Y.; Kim, T.; Park, H. Risk of type 2 diabetes is increased in nonobese women with polycystic ovary syndrome: The National Health Insurance Service-National Sample Cohort Study. Fertil. Steril. 2021, 115, 1569–1575. [Google Scholar] [CrossRef]
- McDonnell, R.; Hart, R.J. Pregnancy-related outcomes for women with polycystic ovary syndrome. Womens Health 2017, 13, 89–97. [Google Scholar] [CrossRef]
- Dou, Q.; Ma, L.-Y.; Li, P.-F.; Xu, X.-T.; Yu, G.; Zhang, D.; Xiang, Y.-G.; Tan, L. The influence of polycystic ovary syndrome on abortion rate after in vitro fertilization/intracytoplasmic sperm injection fresh cycle pregnancy. Sci. Rep. 2023, 13, 5978. [Google Scholar] [CrossRef]
- De Leo, V.; Musacchio, M.C.; Piomboni, P.; Di Sabatino, A.; Morgante, G. The administration of metformin during pregnancy reduces polycystic ovary syndrome related gestational complications. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 63–66. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 194: Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 131, e157–e171. [Google Scholar] [CrossRef]

| Control Group | PCOS Group | Total | p | |
|---|---|---|---|---|
| Number of participants | 169,998 | 44,714 | 214,712 | |
| Median age (years) | 33 [30–37] | 30 [27–32] | 32 [29–36] | <0.001 |
| Median follow-up period (days) | 1377 [561–2254] | 1534 [789–2430] | 1408 [614–2292] | <0.001 |
| Age at inclusion (years) | <0.001 | |||
| 20~24 | 9041 (5.3) | 5762 (12.9) | 14,803 (6.9) | |
| 25~29 | 32,547 (19.1) | 16,039 (35.9) | 48,586 (22.6) | |
| 30~34 | 62,052 (36.5) | 17,363 (38.8) | 79,415 (37) | |
| 35~39 | 44,900 (26.4) | 4924 (11) | 49,824 (23.2) | |
| 40~44 | 18,320 (10.8) | 575 (1.3) | 18,895 (8.8) | |
| 45~49 | 3138 (1.8) | 51 (0.1) | 3189 (1.5) | |
| SES | <0.001 | |||
| Mid~high SES | 168,253 (99) | 44,458 (99.4) | 212,711 (99.1) | |
| Low SES | 1745 (1) | 256 (0.6) | 2001 (0.9) | |
| Region | <0.001 | |||
| Urban area | 94,197 (55.4) | 23,033 (51.5) | 117,230 (54.6) | |
| Rural area | 75,801 (44.6) | 21,681 (48.5) | 97,482 (45.4) | |
| CCI | <0.001 | |||
| 0 | 135,612 (79.8) | 36,863 (82.4) | 172,475 (80.3) | |
| 1 | 23,934 (14.1) | 5778 (12.9) | 29,712 (13.8) | |
| ≥2 | 10,452 (6.1) | 2073 (4.6) | 12,525 (5.8) | |
| Delivery | <0.001 | |||
| Primi | 77,074 (45.3) | 37,561 (84) | 114,635 (53.4) | |
| Multi | 92,924 (54.7) | 7153 (16) | 100,077 (46.6) | |
| Multiple pregnancy | <0.001 | |||
| Singleton | 168,098 (98.9) | 44,541 (99.6) | 212,639 (99) | |
| Multiple | 1900 (1.1) | 173 (0.4) | 2073 (1) | |
| GDM | <0.001 | |||
| Absent | 161,328 (94.9) | 40,958 (91.6) | 202,286 (94.2) | |
| Present | 8670 (5.1) | 3756 (8.4) | 12,426 (5.8) | |
| PIH | <0.001 | |||
| Absent | 169,591 (99.8) | 44,681 (99.9) | 214,272 (99.8) | |
| Present | 407 (0.2) | 33 (0.1) | 440 (0.2) | |
| Adnexal surgery | <0.001 | |||
| Absent | 164,843 (97) | 43,297 (98.2) | 208,140 (97.2) | |
| Present | 5155 (3) | 787 (1.8) | 5942 (2.8) | |
| Uterine fibroids | <0.001 | |||
| Absent | 156,169 (91.9) | 42,921 (96) | 199,090 (92.7) | |
| Present | 13,829 (8.1) | 1793 (4) | 15,622 (7.3) | |
| Endometriosis | <0.001 | |||
| Absent | 162,748 (97.5) | 41,522 (92.9) | 207,270 (96.5) | |
| Present | 4250 (2.5) | 3192 (7.1) | 7442 (3.5) | |
| Obesity | 0.718 | |||
| Absent | 169,575 (99.8) | 44,607 (99.8) | 214,182 (99.8) | |
| Present | 423 (0.2) | 107 (0.2) | 530 (0.2) |
| Non-PCOS | PCOS | Total | p | |
|---|---|---|---|---|
| Number of participants | 169,998 | 44,714 | 214,712 | |
| Abortion | <0.001 | |||
| Absent | 157,522 (92.7) | 38,137 (85.3) | 195,659 (91.1) | |
| Present | 12,476 (7.3) | 6577 (14.7) | 19,053 (8.9) | |
| Ectopic pregnancy | <0.001 | |||
| Absent | 168,122 (98.9) | 43,249 (96.7) | 211,371 (98.4) | |
| Present | 1876 (1.1) | 1465 (3.3) | 3341 (1.6) | |
| Hydatidiform mole | <0.001 | |||
| Absent | 169,814 (99.9) | 44,634 (99.8) | 214,448 (99.9) | |
| Present | 184 (0.1) | 80 (0.2) | 264 (0.1) |
| Abortion | Ectopic Pregnancy | Hydatidiform Mole | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) a | p | RR (95% CI) a | p | RR (95% CI) a | p | |
| Crude | ||||||
| PCOS | 2.177 (2.109–2.248) | <0.001 | 3.036 (2.833–3.253) | <0.001 | 1.654 (1.272–2.151) | <0.001 |
| Adjusted a | ||||||
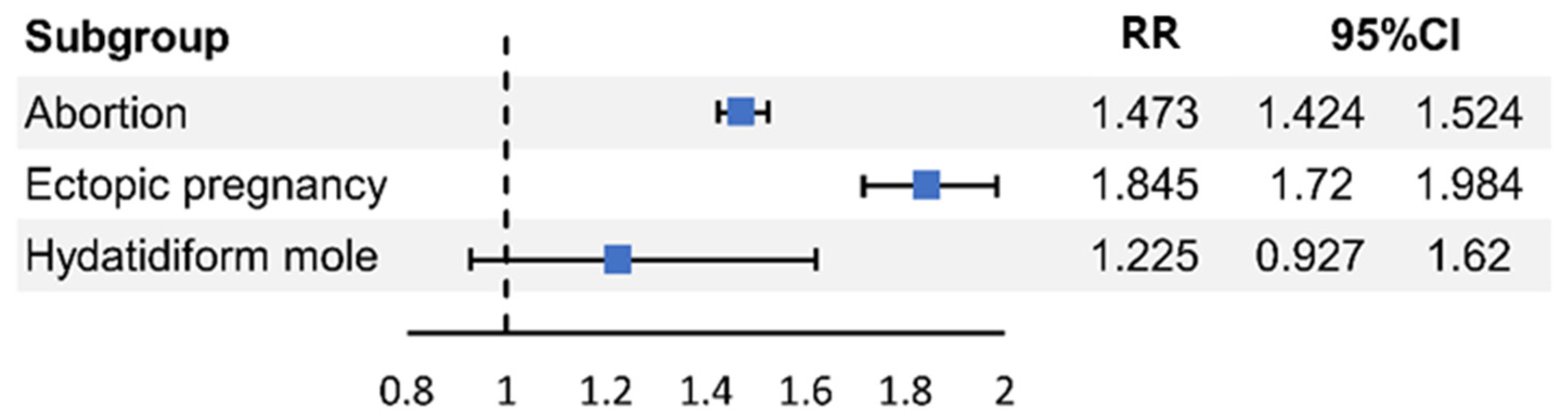
| PCOS | 1.473 (1.424–1.524) | <0.001 | 1.845 (1.716–1.984) | <0.001 | 1.225 (0.927–1.62) | 0.154 |
| Age at inclusion (years) | ||||||
| 20~24 | 1 | 1 | 1 | |||
| 25~29 | 1.249 (1.173–1.33) | <0.001 | 1.007 (0.894–1.134) | <0.001 | 0.566 (0.371–0.864) | 0.005 |
| 30~34 | 1.571 (1.478–1.67) | <0.001 | 0.968 (0.861–1.09) | <0.001 | 0.706 (0.475–1.049) | 0.143 |
| 35~39 | 1.677 (1.567–1.794) | <0.001 | 0.815 (0.706–0.941) | <0.001 | 0.594 (0.369–0.957) | 0.024 |
| 40~44 | 1.274 (1.164–1.394) | <0.001 | 0.447 (0.349–0.574) | 0.105 | 0.617 (0.326–1.167) | 0.185 |
| 44~49 | 0.435 (0.337–0.56) | <0.001 | 0.084 (0.027–0.262) | <0.001 | 2.351 (1.154–4.788) | <0.001 |
| SES | ||||||
| Mid~high SES | 1 | 1 | 1 | |||
| Low SES | 0.913 (0.765–1.09) | 0.314 | 1.129 (0.779–1.638) | 0.522 | 0 (0-Infinite) | 0.971 |
| Region | ||||||
| Urban area | 1 | 1 | 1 | |||
| Rural area | 0.952 (0.923–0.982) | 0.002 | 1.108 (1.033–1.188) | 0.004 | 1.011 (0.79–1.293) | 0.931 |
| CCI | ||||||
| 0 | 1 | 1 | 1 | |||
| 1 | 1.057 (1.01–1.105) | 0.957 | 1.083 (0.978–1.199) | 0.568 | 1.245 (0.89–1.743) | 0.63 |
| ≥2 | 1.12 (1.05–1.198) | 0.011 | 1.257 (1.091–1.448) | 0.011 | 1.28 (0.789–2.075) | 0.586 |
| Delivery | ||||||
| Primi | 1 | 1 | 1 | |||
| Multi | 0.227 (0.217–0.237) | <0.001 | 0.212 (0.189–0.237) | <0.001 | 0.376 (0.275–0.516) | <0.001 |
| Multiple pregnancy | ||||||
| Singleton | 1 | 1 | 1 | |||
| Multiple | 0.413 (0.288–0.594) | <0.001 | 0.829 (0.392–1.754) | 0.624 | 0 (0-Infinite) | 0.975 |
| GDM | 0.904 (0.786–1.041) | 0.16 | 0.992 (0.687–1.432) | 0.964 | 0.245 (0.034–1.764) | 0.163 |
| PIH | 1.202 (0.756–1.912) | 0.437 | 0.997 (0.247–4.015) | 0.996 | 0 (0-Infinite) | 0.988 |
| Adnexal surgery before inclusion | 0.907 (0.817–1.007) | 0.067 | 1.063 (0.846–1.336) | 0.601 | 0.816 (0.348–1.916) | 0.641 |
| Uterine fibroids | 1.093 (1.028–1.162) | 0.004 | 0.984 (0.793–1.22) | 0.88 | 1.644 (1.085–2.489) | 0.019 |
| Endometriosis | 1 (0.912–1.096) | 0.994 | 1.324 (1.152–1.522) | <0.001 | 0.912 (0.447–1.861) | 0.8 |
| Obesity | 0.814 (0.576–1.149) | 0.241 | 1.249 (0.643–2.428) | 0.512 | 0 (0-Infinite) | 0.986 |
| 20~29 Years a | 30~39 Years a | 40~49 Years a | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) a | p | RR (95% CI) a | p | RR (95% CI) a | p | |
| Abortion | ||||||
| No PCOS | 1 | 1 | 1 | |||
| PCOS | 1.379 (1.307–1.454) | <0.001 | 1.535 (1.468–1.605) | <0.001 | 3.152 (2.526–3.934) | <0.001 |
| Ectopic pregnancy | ||||||
| No PCOS | 1 | 1 | 1 | |||
| PCOS | 2.009 (1.808–2.233) | <0.001 | 1.767 (1.597–1.956) | <0.001 | 1.475 (0.673–3.232) | 0.332 |
| Hydatidiform mole | ||||||
| No PCOS | 1 | 1 | 1 | |||
| PCOS | 1.228 (0.807–1.868) | 0.338 | 1.358 (0.924–1.997) | 0.12 | 1.287 (0.302–5.493) | 0.733 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuk, J.-S.; Yoon, S.-H.; Yang, S.-W. Risk of Abortion and Ectopic Pregnancy in Women with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study. J. Clin. Med. 2025, 14, 6325. https://doi.org/10.3390/jcm14176325
Yuk J-S, Yoon S-H, Yang S-W. Risk of Abortion and Ectopic Pregnancy in Women with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study. Journal of Clinical Medicine. 2025; 14(17):6325. https://doi.org/10.3390/jcm14176325
Chicago/Turabian StyleYuk, Jin-Sung, Sang-Hee Yoon, and Seung-Woo Yang. 2025. "Risk of Abortion and Ectopic Pregnancy in Women with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study" Journal of Clinical Medicine 14, no. 17: 6325. https://doi.org/10.3390/jcm14176325
APA StyleYuk, J.-S., Yoon, S.-H., & Yang, S.-W. (2025). Risk of Abortion and Ectopic Pregnancy in Women with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study. Journal of Clinical Medicine, 14(17), 6325. https://doi.org/10.3390/jcm14176325






