Association of the Lactate/Albumin Ratio with Mortality and Hypovolemia in Critically Ill Patients: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Study Endpoint
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
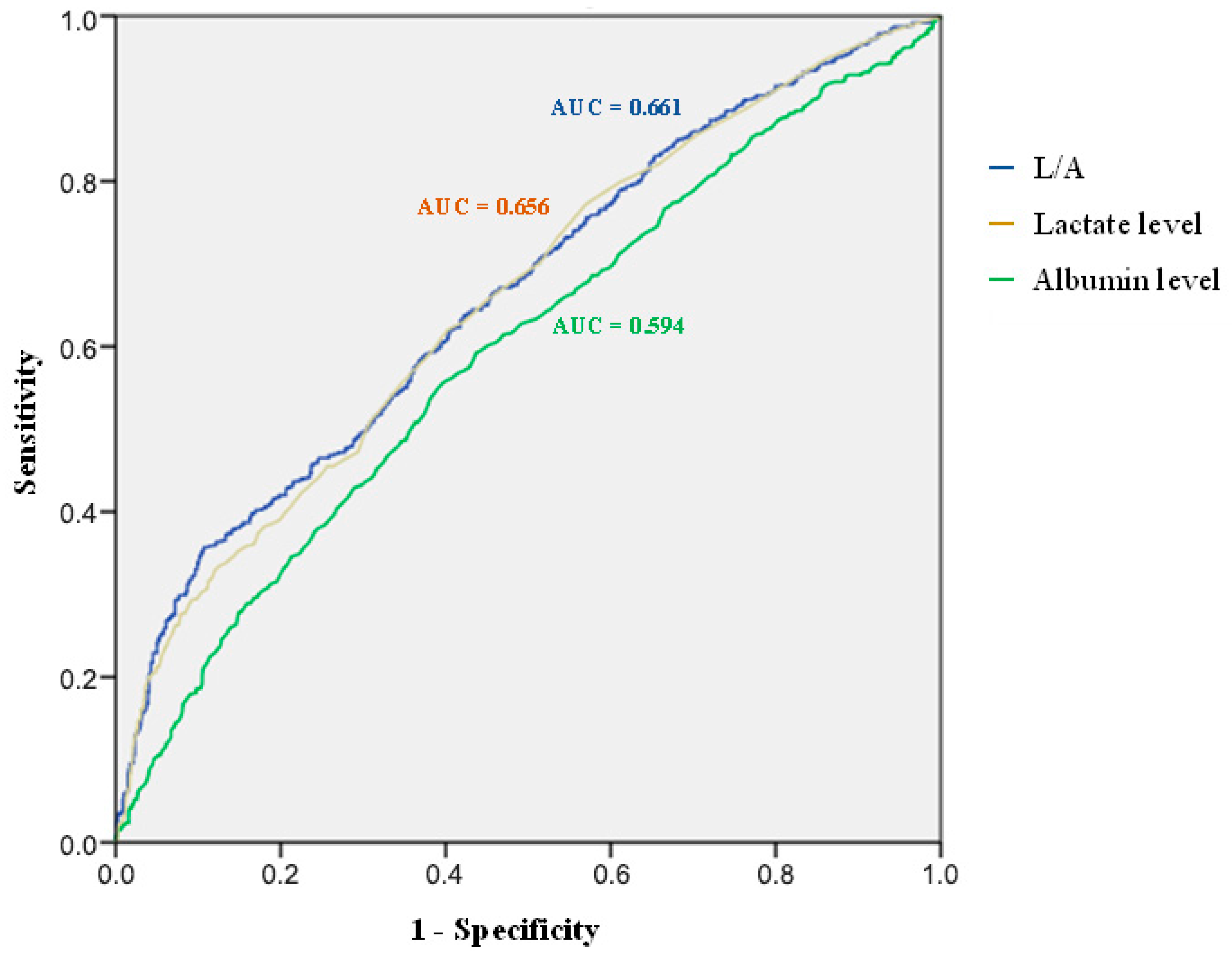
3.2. Association Between L/A and 30-Day Mortality
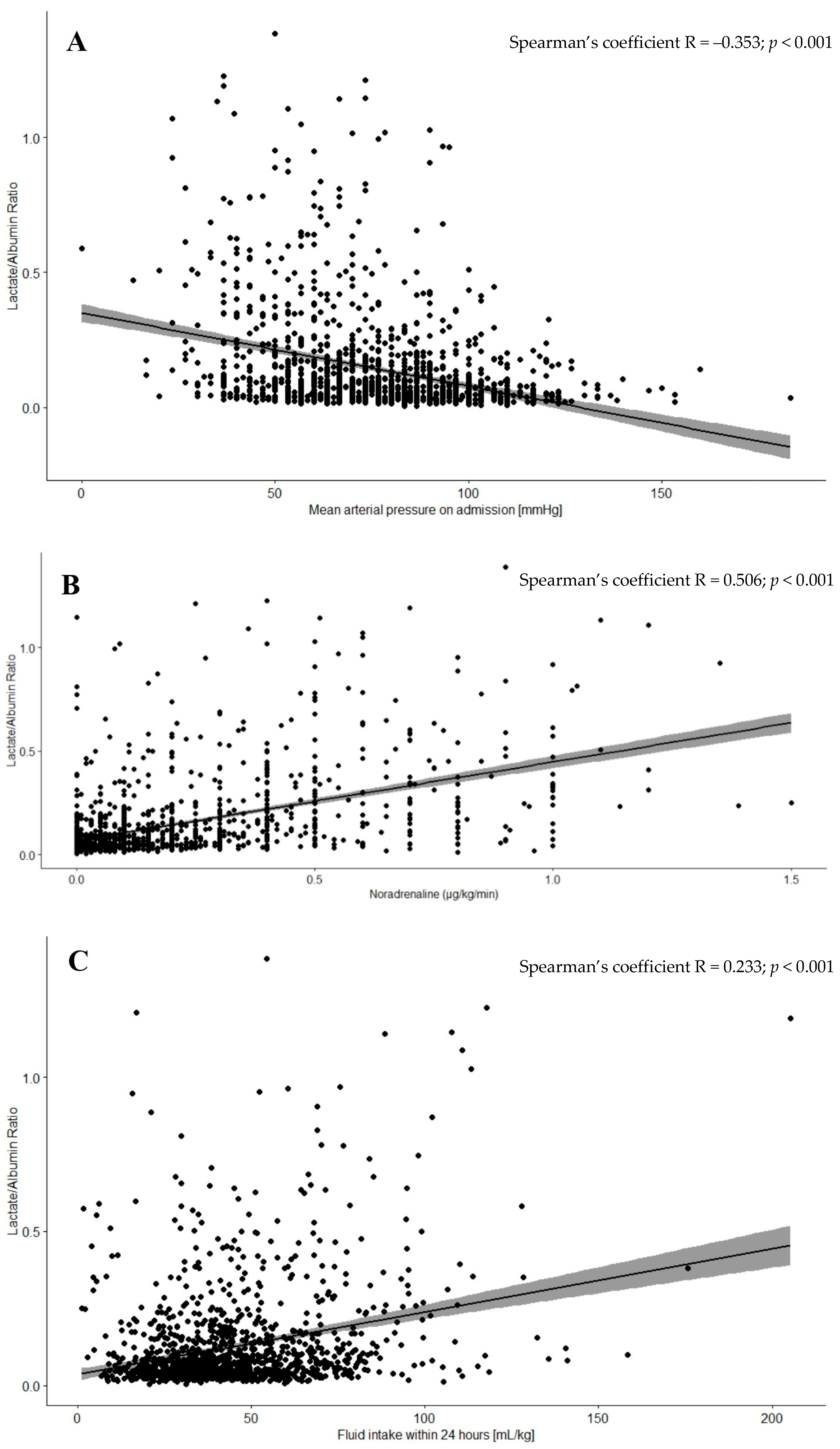
3.3. Correlation Between Hypovolemia Markers and L/A
3.4. Association Between L/A and CRRT Requirement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| CI | Confidence Interval |
| CRRT | Continuous Renal Replacement Therapy |
| HR | Hazard Ratio |
| ICU | Intensive Care Unit |
| IQR | Interquartile Range |
| L/A | Lactate/Albumin Ratio |
| OR | Odds Ratio |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
| SOFA | Sequential Organ Failure Assessment |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- Fuller, B.M.; Dellinger, R.P. Lactate as a Hemodynamic Marker in the Critically Ill. Curr. Opin. Crit. Care 2012, 18, 267. [Google Scholar] [CrossRef]
- Alshiakh, S.M. Role of serum lactate as prognostic marker of mortality among emergency department patients with multiple conditions: A systematic review. SAGE Open Med. 2023, 11, 20503121221136401. [Google Scholar] [CrossRef]
- Khodashahi, R.; Sarjamee, S. Early lactate area scores and serial blood lactate levels as prognostic markers for patients with septic shock: A systematic review. Infect. Dis. 2020, 52, 451–463. [Google Scholar] [CrossRef]
- Odom, S.R.; Howell, M.D.; Silva, G.S.; Nielsen, V.M.; Gupta, A.; Shapiro, N.I.; Talmor, D. Lactate clearance as a predictor of mortality in trauma patients. J. Trauma Acute Care Surg. 2013, 74, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Klemm, G.; Markart, S.; Hermann, A.; Staudinger, T.; Hengstenberg, C.; Heinz, G.; Zilberszac, R. Lactate as a Predictor of 30-Day Mortality in Cardiogenic Shock. J. Clin. Med. 2024, 13, 1932. [Google Scholar] [CrossRef] [PubMed]
- Sakal, C.; Ak, R.; Taşçı, A.; Kırkpantur, E.D.; Akoğlu, E.Ü.; Ozturk, T.C. Admission blood lactate levels of patients diagnosed with cerebrovascular disease effects on short- and long-term mortality risk. Int. J. Clin. Pract. 2021, 75, e14161. [Google Scholar] [CrossRef] [PubMed]
- Akirov, A.; Masri-Iraqi, H.; Atamna, A.; Shimon, I. Low Albumin Levels Are Associated with Mortality Risk in Hospitalized Patients. Am. J. Med. 2017, 130, 1465.e11–1465.e19. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.; Wu, Q.; Cao, J.; Qiu, T. The association between albumin and C-reactive protein in older adults. Medicine 2023, 102, E34726. [Google Scholar] [CrossRef]
- Phillips, A.; Gerald Shaper, A.; Whincup, P.H. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet 1989, 2, 1434–1436. [Google Scholar] [CrossRef]
- Gharipour, A.; Razavi, R.; Gharipour, M.; Mukasa, D. Lactate/albumin ratio: An early prognostic marker in critically ill patients. Am. J. Emerg. Med. 2020, 38, 2088–2095. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, K.; Wu, B.; Lin, W.; Chen, S.; Xu, X.; Peng, C.; Xie, D. Association between lactate/albumin ratio and mortality in patients with heart failure after myocardial infarction. ESC Heart. Fail. 2023, 10, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guo, H.; Chen, X.; Zhang, Q. Association between lactate/albumin ratio and all-cause mortality in patients with acute respiratory failure: A retrospective analysis. PLoS ONE 2021, 16, e0255744. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Choi, B.; Eun, S.; Bae, G.E.; Koo, C.M.; Kim, M.K. Using the lactate-to-albumin ratio to predict mortality in patients with sepsis or septic shock: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1743–1752. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, Q.; Li, W.; Hu, D.; Guan, Y.; Wang, J. Elevated lactate/albumin ratio is associated with poor prognosis in sepsis patients: A systematic review and meta-analysis. J, Med. Biochem. 2024, 43, 334–349. [Google Scholar] [CrossRef]
- Yi, X.; Jin, D.; Huang, S.; Xie, Z.; Zheng, M.; Zhou, F.; Jin, Y. Association between lactate-to-albumin ratio and 28-days all-cause mortality in patients with sepsis-associated liver injury: A retrospective cohort study. BMC Infect. Dis. 2024, 24, 65. [Google Scholar] [CrossRef]
- Zhu, X.; Xue, J.; Liu, Z.; Dai, W.; Xu, H.; Zhou, Q.; Zhao, S.; Zhou, Q.; Chen, W. The Lactate/Albumin Ratio Predicts Mortality in Critically Ill Patients with Acute Kidney Injury: An Observational Multicenter Study on the eICU Database. Int. J. Gen. Med. 2021, 14, 10511–10525. [Google Scholar] [CrossRef]
- Macdonald, S. Fluid Resuscitation in Patients Presenting with Sepsis: Current Insights. Open Access Emerg. Med. 2022, 14, 633–638. [Google Scholar] [CrossRef]
- Wrzosek, A.; Drygalski, T.; Garlicki, J.; Woroń, J.; Szpunar, W.; Polak, M.; Droś, J.; Wordliczek, J.; Zajączkowska, R. The volume of infusion fluids correlates with treatment outcomes in critically ill trauma patients. Front. Med. 2023, 9, 1040098. [Google Scholar] [CrossRef]
- Wilms, H.; Mittal, A.; Haydock, M.D.; van den Heever, M.; Devaud, M.; Windsor, J.A. A systematic review of goal directed fluid therapy: Rating of evidence for goals and monitoring methods. J. Crit. Care 2014, 29, 204–209. [Google Scholar] [CrossRef]
- Wang, B.; Chen, G.; Cao, Y.; Xue, J.; Li, J.; Wu, Y. Correlation of lactate/albumin ratio level to organ failure and mortality in severe sepsis and septic shock. J. Crit. Care 2015, 30, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Wang, H.X.; Huang, X.H.; Ma, L.Q.; Yang, Z.J.; Wang, H.L.; Xu, B.; Luo, M.Q. Association between lactate-to-albumin ratio and short-time mortality in patients with acute respiratory distress syndrome. J. Clin. Anesth. 2024, 99, 111632. [Google Scholar] [CrossRef]
- Jin, P.; Bian, Y.; Cui, Q.; Liang, X.; Sun, Y.; Zheng, Q. Association between lactate/albumin ratio and 28-day all-cause mortality in critically ill patients with acute myocardial infarction. Sci. Rep. 2024, 14, 23677. [Google Scholar] [CrossRef]
- Gök, A.; Kasapoğlu, U.S.; Delen, L.A.; Özmen, F.; Banazılı, S. Lactate/Albumin Ratio as a Prognostic Factor for Short-time Mortality in Critically Ill Patients with Coronavirus Disease-2019. Turk. J. Intensive. Care 2021, 19, 62–72. [Google Scholar] [CrossRef]
- Shadvar, K.; Nader-Djalal, N.; Vahed, N.; Sanaie, S.; Iranpour, A.; Mahmoodpoor, A.; Vahedian-Azimi, A.; Samim, A.; Rahimi-Bashar, F. Comparison of lactate/albumin ratio to lactate and lactate clearance for predicting outcomes in patients with septic shock admitted to intensive care unit: An observational study. Sci. Rep. 2022, 12, 13047. [Google Scholar] [CrossRef]
- Kearney, D.; Reisinger, N.; Lohani, S. Integrative Volume Status Assessment. POCUS J. 2022, 7, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, L.; Karadjian, O.; Braam, B.; Mavrakanas, T.; Weber, C. Bioimpedance-Guided Monitoring of Volume Status in Patients With Kidney Disease: A Systematic Review and Meta-Analysis. Can. J. Kidney Health Dis. 2023, 10, 20543581231185433. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Phases of fluid management and the roles of human albumin solution in perioperative and critically ill patients. Curr. Med. Res. Opin. 2020, 36, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.W.; Marx, G. Evidence-based fluid management in the ICU. Curr. Opin. Anaesthesiol. 2016, 29, 158–165. [Google Scholar] [CrossRef]
- Kashani, K.; Omer, T.; Shaw, A.D. The Intensivist’s Perspective of Shock, Volume Management, and Hemodynamic Monitoring. Clin. J. Am. Soc. Nephrol. 2022, 17, 706–716. [Google Scholar] [CrossRef]
- Lee, J.W.; You, J.; Chung, S.; Choa, M.; Kong, T.; Ko, D.R.; Hwang, Y.J.; Lee, Y.H.; Park, I.; Kim, S. Lactate/albumin ratio for the prediction of the development of sepsis-induced acute kidney injury in the emergency department. J. Korean Soc. Emerg. Med. 2019, 30, 22–32. [Google Scholar]
- Shi, X.; Zhong, L.; Lu, J.; Hu, B.; Shen, Q.; Gao, P. Clinical significance of the lactate-to-albumin ratio on prognosis in critically ill patients with acute kidney injury. Ren. Fail. 2024, 46, 2350238. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Min, J.; Lu, J.; Zhong, L.; Luo, H. Association between lactate/albumin ratio and prognosis in critically ill patients with acute kidney injury undergoing continuous renal replacement therapy. Ren. Fail. 2024, 46, 2374451. [Google Scholar] [CrossRef] [PubMed]
- Barbar, S.D.; Clere-Jehl, R.; Bourredjem, A.; Hernu, R.; Montini, F.; Bruyère, R.; Lebert, C.; Bohé, J.; Badie, J.; Eraldi, J.-P.; et al. Timing of Renal-Replacement Therapy in Patients with Acute Kidney Injury and Sepsis. N. Engl. J. Med. 2018, 379, 1431–1442. [Google Scholar] [CrossRef]
- Katulka, R.J.; Al Saadon, A.; Sebastianski, M.; Featherstone, R.; Vandermeer, B.; Silver, S.A.; Gibney, R.T.N.; Bagshaw, S.M.; Rewa, O.G. Determining the optimal time for liberation from renal replacement therapy in critically ill patients: A systematic review and meta-analysis (DOnE RRT). Crit. Care 2020, 24, 50. [Google Scholar] [CrossRef]
- Van Der Mullen, J.; Wise, R.; Vermeulen, G.; Moonen, P.J.; Malbrain, M.L.N.G. Assessment of hypovolaemia in the critically ill. Anaesthesiol. Intensive. Ther. 2018, 50, 150–159. [Google Scholar] [CrossRef]
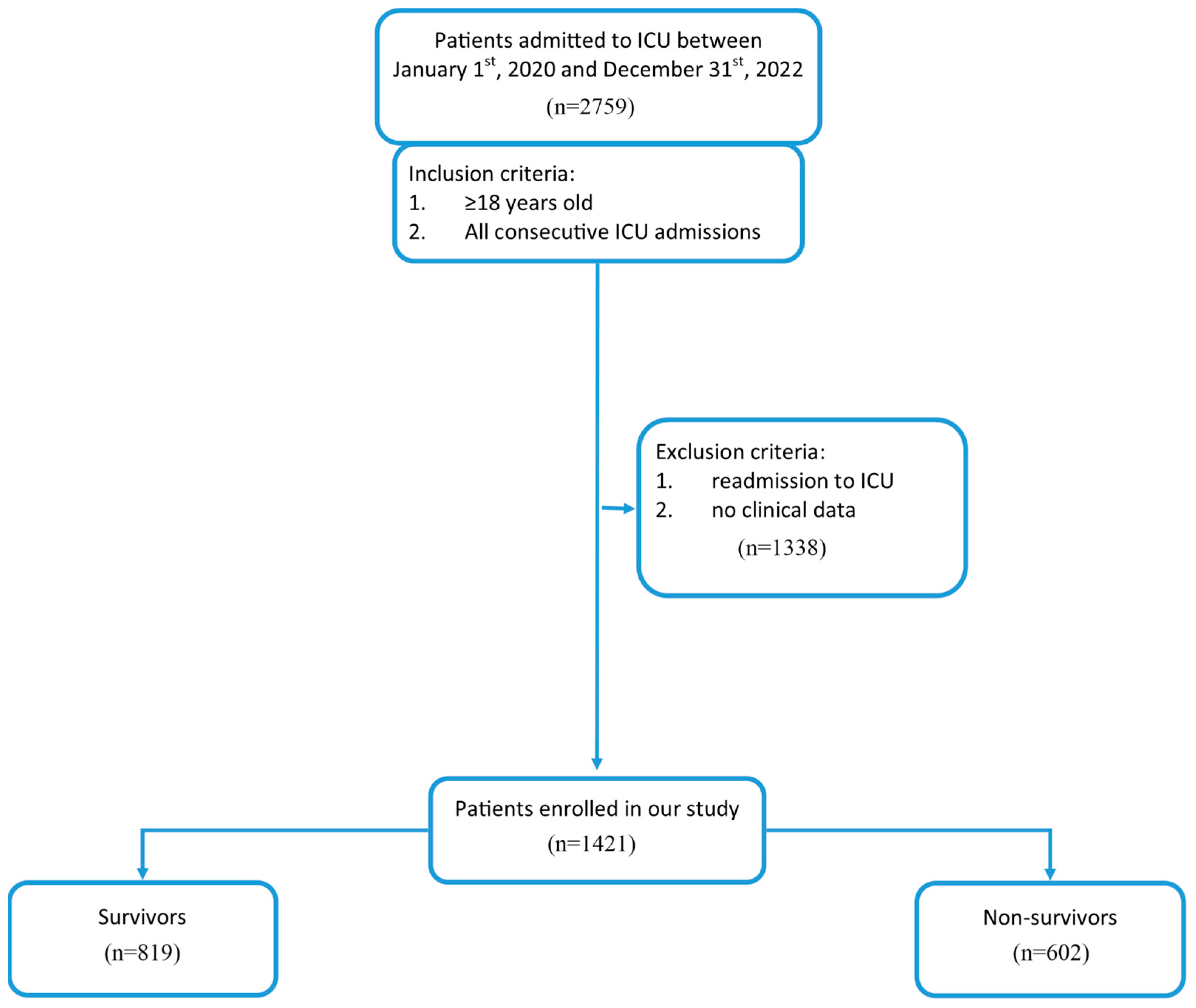
| Parameter | Survivors (n = 819, 57.6%) | Non-Survivors (n = 602, 42.4%) | p-Value |
|---|---|---|---|
| Age [years], median (IQR) | 62 (47; 70) | 67 (55; 74) | <0.001 |
| Male gender, n (%) | 502 (61.3) | 379 (63.0) | 0.280 |
| Body mass index, mean (SD) a | 27.79 (±6.33) | 28.73 (±6.91) | 0.090 |
| Comorbidities | |||
| Diabetes mellitus, n (%) | 203 (24.8) | 175 (29.1) | 0.041 |
| Arterial hypertension, n (%) | 388 (47.4) | 337 (56.0) | 0.001 |
| Obstructive pulmonary disease, n (%) | 55 (6.7) | 59 (9.8) | 0.022 |
| Ischemic heart disease, n (%) | 108 (13.2) | 132 (21.9) | <0.001 |
| Chronic kidney disease, n (%) | 94 (11.5) | 88 (14.6) | 0.048 |
| Chronic liver disease, n (%) | 21 (2.6) | 37 (6.1) | 0.001 |
| Heart failure, n (%) | 121 (14.8) | 130 (21.6) | 0.001 |
| Active malignancy, n (%) | 96 (11.7) | 85 (14.1) | 0.104 |
| Previous stroke, n (%) | 51 (6.2) | 32 (5.3) | 0.272 |
| Admission category | |||
| Medical, n (%) | 454 (55.4) | 386 (64.1) | 0.001 |
| Surgical, n (%) | 365 (44.6) | 216 (35.9) | 0.001 |
| Admission diagnosis category | |||
| Respiratory, n (%) | 238 (29.1) | 194 (32.2) | 0.281 |
| Cardiovascular b, n (%) | 156 (19.0) | 164 (27.2) | <0.001 |
| Other medical, n (%) | 14 (1.7) | 6 (1.0) | 0.260 |
| Non-operative trauma, n (%) | 46 (5.6) | 22 (3.7) | 0.087 |
| Postoperative, n (%) | 365 (44.6) | 216 (35.9) | 0.001 |
| Parameter | AUC | 95%CI of AUC | p-Value |
|---|---|---|---|
| L/A | 0.661 | 0.633–0.690 | <0.001 |
| Lactate level | 0.656 | 0.628–0.685 | <0.001 |
| Albumin level | 0.594 | 0.564–0.624 | <0.001 |
| Parameter | L/A < 0.06 (n = 632, 44.48%) | L/A ≥ 0.06 (n = 789, 55.52%) | p-Value |
|---|---|---|---|
| SOFA score, mean (SD) | 8.71 (±3.33) | 11.3 (±3.70) | <0.001 |
| ICU length of stay [days], mean (SD) | 16.80 (±16.31) | 14.14 (±15.92) | 0.002 |
| Hospital length of stay [days], mean (SD) | 25.36 (±18.16) | 21.17 (±18.66) | <0.001 |
| Lactate level [mmol/L], median (IQR) | 1.10 (0.90; 1.50) | 3.80 (2.40; 7.00) | <0.001 |
| Albumin level [mg/mL], median (IQR) | 32.40 (28.20; 36.48) | 27.30 (22.30; 32.30) | <0.001 |
| L/A, median (IQR) | 0.04 (0.28; 0.47) | 0.13 (0.085; 0.258) | <0.001 |
| Anion gap [mEq/L], median (IQR) 1 | 10.00 (7.80; 12.40) | 12.90 (9.70; 16.55) | <0.001 |
| Base excess [mmol/L], median (IQR) 2 | −2.00 (−4.90; 1.20) | −6.50 (−11.00; −2.00) | <0.001 |
| pH, median (IQR) | 7.344 (7.28; 7.40) | 7.270 (7.16; 7.35) | <0.001 |
| Mean arterial pressure on admission [mmHg], median (IQR) | 83.33 (71.67; 96.67) | 70.00 (56.67; 86.67) | <0.001 |
| Heart rate [bpm], median (IQR) | 85 (75; 100) | 95 (75; 115) | <0.001 |
| Dose of norepinephrine within 24 h [µg/kg/min], median (IQR) | 0.05 (0.00; 0.12) | 0.20 (0.05; 0.40) | <0.001 |
| Fluid intake within 24 h [mL/kg], median (IQR) 3 | 36.94 (27.70; 48.05) | 43.91 (33.14; 62.21) | <0.001 |
| CRRT during ICU stay, n (%) | 142 (22.5) | 337 (42.7) | <0.001 |
| 30-day mortality, n (%) | 198 (31.3) | 404 (51.2) | <0.001 |
| Variable | HR (95%CI) | p-Value |
|---|---|---|
| Age (1 year) | 1.010 (1.005–1.016) | <0.001 |
| SOFA score (1 point) | 1.148 (1.119–1.178) | <0.001 |
| L/A ≥ 0.06 (0/1) | 1.423 (1.183–1.712) | <0.001 |
| Variable | OR (95%CI) | p-Value |
|---|---|---|
| Age (1 year) | 0.993 (0.985–1.002) | 0.115 |
| Diabetes mellitus (0/1) | 1.083 (0.807–1.454) | 0.596 |
| Arterial hypertension (0/1) | 1.361 (1.027–1.805) | 0.032 |
| Obstructive pulmonary disease (0/1) | 0.756 (0.481–1.189) | 0.226 |
| Ischemic heart disease (0/1) | 1.089 (0.773–1.535) | 0.625 |
| Chronic kidney disease (0/1) | 2.814 (1.966–4.029) | <0.001 |
| Chronic liver disease (0/1) | 1.974 (1.104–3.530) | 0.022 |
| Active malignancy (0/1) | 1.191 (0.836–1.696) | 0.333 |
| Previous stroke (0/1) | 0.807 (0.571–1.140) | 0.224 |
| Sepsis on admission (0/1) | 2.940 (2.304–3.750) | <0.001 |
| Surgical procedure before admission (0/1) | 1.002 (0.779–1.289) | 0.985 |
| L/A ≥ 0.06 (0/1) | 2.134 (1.652–2.757) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Droś, J.; Świstek, R.; Kasongo, P.; Konieczyński, J.; Bielański, P.; Sajdyk, A.; Wrzosek, A.; Składzień, T.; Depukat, R.; Marusińska, M.; et al. Association of the Lactate/Albumin Ratio with Mortality and Hypovolemia in Critically Ill Patients: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 6321. https://doi.org/10.3390/jcm14176321
Droś J, Świstek R, Kasongo P, Konieczyński J, Bielański P, Sajdyk A, Wrzosek A, Składzień T, Depukat R, Marusińska M, et al. Association of the Lactate/Albumin Ratio with Mortality and Hypovolemia in Critically Ill Patients: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(17):6321. https://doi.org/10.3390/jcm14176321
Chicago/Turabian StyleDroś, Jakub, Rafał Świstek, Patryk Kasongo, Jakub Konieczyński, Piotr Bielański, Agnieszka Sajdyk, Anna Wrzosek, Tomasz Składzień, Rafał Depukat, Maria Marusińska, and et al. 2025. "Association of the Lactate/Albumin Ratio with Mortality and Hypovolemia in Critically Ill Patients: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 17: 6321. https://doi.org/10.3390/jcm14176321
APA StyleDroś, J., Świstek, R., Kasongo, P., Konieczyński, J., Bielański, P., Sajdyk, A., Wrzosek, A., Składzień, T., Depukat, R., Marusińska, M., Czech, K., Frączek, K., Paciorek, K., Skoczeń, W., Stachera, B., Chaba, W., Peszek, A., Pabian, G., Pawlik, M., ... Terlecki, M. (2025). Association of the Lactate/Albumin Ratio with Mortality and Hypovolemia in Critically Ill Patients: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(17), 6321. https://doi.org/10.3390/jcm14176321






