Oral Contraceptive Use and Reproductive History in Relation to Metabolic Syndrome Among Women from KNHANES 2010–2023
Abstract
1. Introduction
2. Materials and Methods
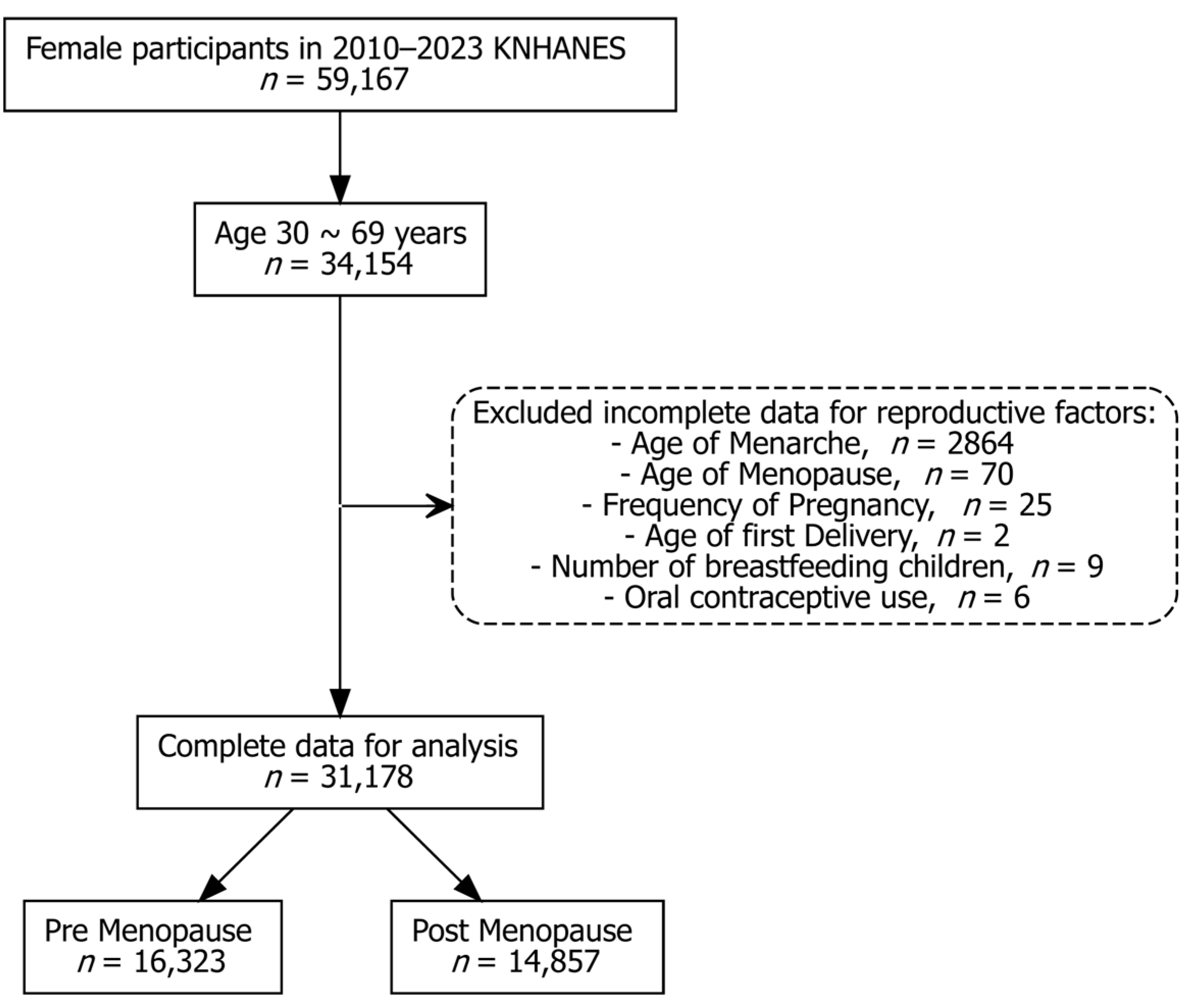
2.1. Data Source and Study Population
2.2. Covariates for Adjustment
2.3. Metabolic Syndrome as the Study Outcome
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics by Metabolic Syndrome
3.2. Associations Between Covariates and Metabolic Syndrome by Menopausal Status
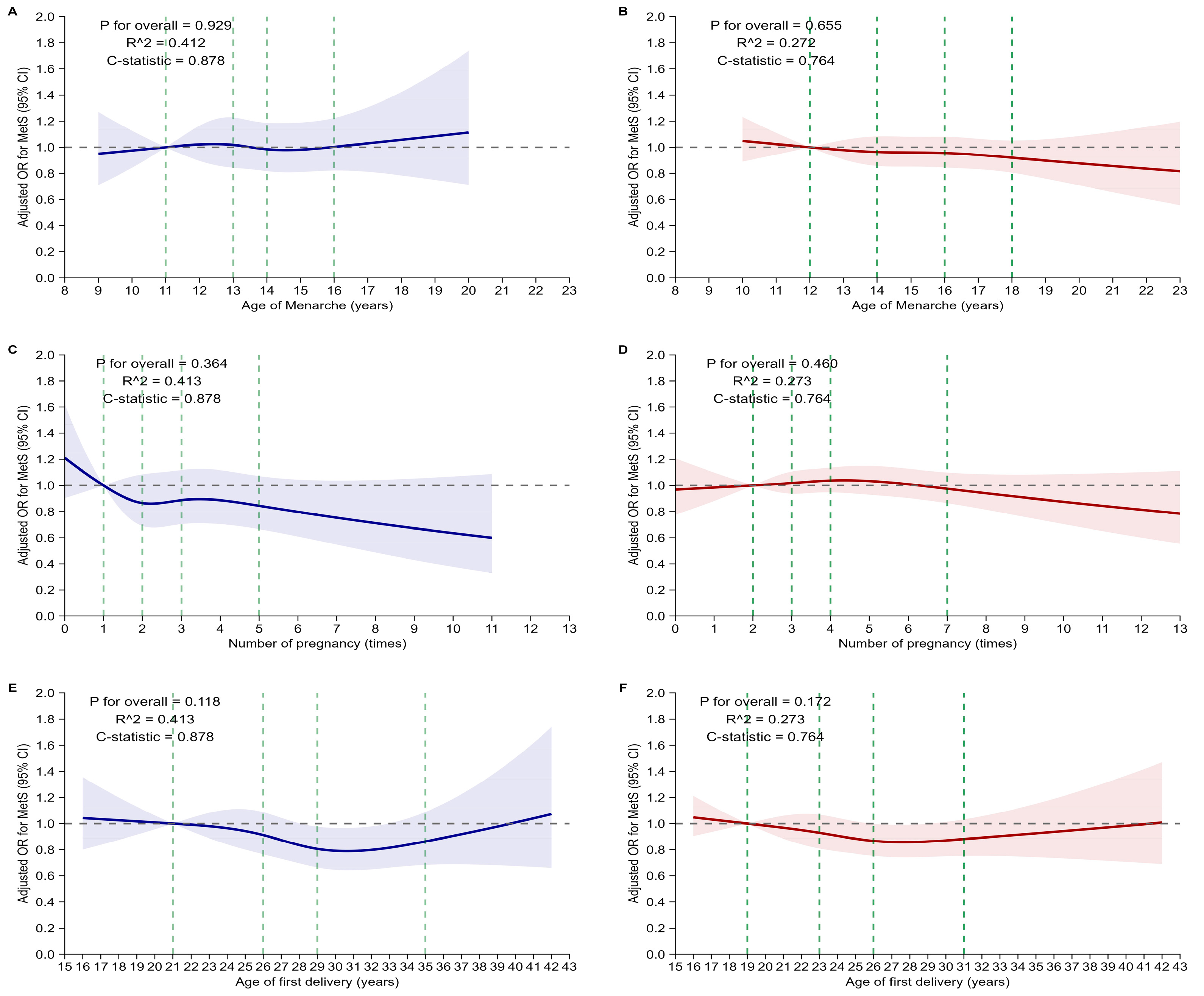
3.3. Associations Between Reproductive Factors and Metabolic Syndrome
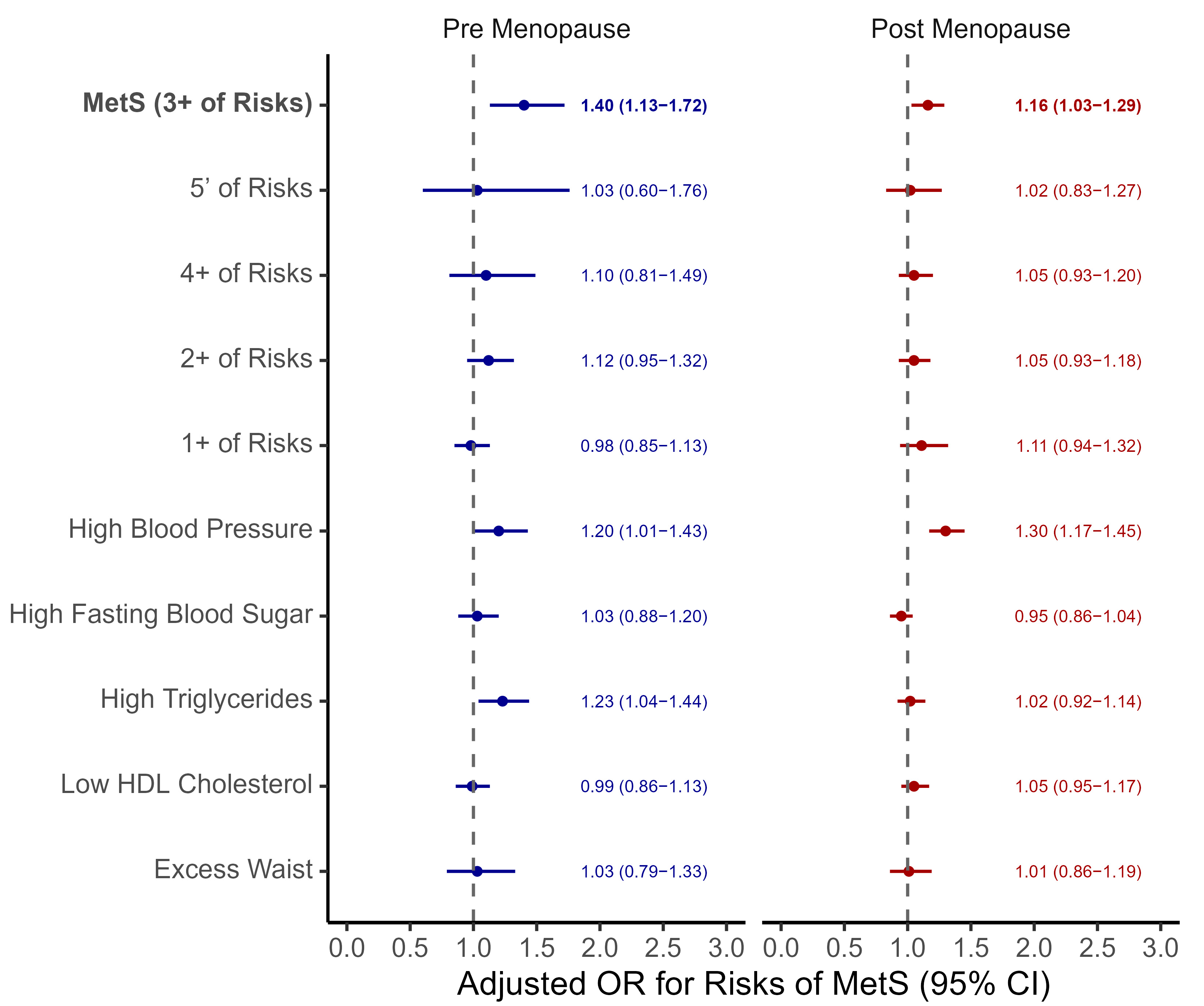
3.4. Association Between Oral Contraceptive Use and Metabolic Syndrome
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MetS | Metabolic syndrome |
| OC | Oral contraceptive |
| KNHANES | Korea National Health and Nutrition Examination Survey |
| HDL | High-density lipoprotein |
| SMD | Standardized mean difference |
| RCS | Restricted cubic spline |
References
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk a Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef] [PubMed]
- Rich-Edwards, J.W.; Fraser, A.; Lawlor, D.A.; Catov, J.M. Pregnancy Characteristics and Women’s Future Cardiovascular Health: An Underused Opportunity to Improve Women’s Health? Epidemiol. Rev. 2014, 36, 57–70. [Google Scholar] [CrossRef]
- Nichols, A.R.; Chavarro, J.E.; Oken, E. Reproductive Risk Factors across the Female Lifecourse and Later Metabolic Health. Cell Metab. 2024, 36, 240–262. [Google Scholar] [CrossRef]
- Grandi, S.M.; Filion, K.B.; Yoon, S.; Ayele, H.T.; Doyle, C.M.; Hutcheon, J.A.; Smith, G.N.; Gore, G.C.; Ray, J.G.; Nerenberg, K.; et al. Cardiovascular Disease-Related Morbidity and Mortality in Women With a History of Pregnancy Complications. Circulation 2019, 139, 1069–1079. [Google Scholar] [CrossRef]
- Kwan, B.-S.; Yang, J.; Jo, H.C.; Baek, J.C.; Kim, R.B.; Park, J.E. Age at Menarche and Its Association With Adult-Onset Metabolic Syndrome and Related Disorders in Women: A Cross-Sectional Study of a Nationally Representative Sample Over 10 Years. Asia Pac. J. Public. Health 2024, 36, 558–564. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Early Menarche and Risk of Metabolic Syndrome: A Systematic Review and Meta-Analysis. J. Womens Health 2019, 28, 77–86. [Google Scholar] [CrossRef]
- Glueck, C.J.; Morrison, J.A.; Wang, P.; Woo, J.G. Early and Late Menarche Are Associated with Oligomenorrhea and Predict Metabolic Syndrome 26 Years Later. Metab. Clin. Exp. 2013, 62, 1597–1606. [Google Scholar] [CrossRef]
- Stöckl, D.; Meisinger, C.; Peters, A.; Thorand, B.; Huth, C.; Heier, M.; Rathmann, W.; Kowall, B.; Stöckl, H.; Döring, A. Age at Menarche and Its Association with the Metabolic Syndrome and Its Components: Results from the KORA F4 Study. PLoS ONE 2011, 6, e26076. [Google Scholar] [CrossRef]
- Cho, G.J.; Park, H.T.; Shin, J.H.; Kim, T.; Hur, J.Y.; Kim, Y.T.; Lee, K.W.; Kim, S.H. The Relationship between Reproductive Factors and Metabolic Syndrome in Korean Postmenopausal Women: Korea National Health and Nutrition Survey 2005. Menopause 2009, 16, 998–1003. [Google Scholar] [CrossRef]
- Bai, L.; Yang, X.; Sun, Z.; Luo, Z.; Li, L.; Liang, X.; Zhou, J.; Meng, L.; Peng, Y.; Qin, Y. Reproductive Factors and Metabolic Syndrome among Chinese Women Aged 40 Years and Older. J. Diabetes 2023, 15, 36–46. [Google Scholar] [CrossRef]
- Zuo, R.; Ge, Y.; Xu, J.; He, L.; Liu, T.; Wang, B.; Sun, L.; Wang, S.; Zhu, Z.; Wang, Y. The Association of Female Reproductive Factors with Risk of Metabolic Syndrome in Women from NHANES 1999–2018. BMC Public Health 2023, 23, 2306. [Google Scholar] [CrossRef] [PubMed]
- Halperin, I.J.; Kumar, S.S.; Stroup, D.F.; Laredo, S.E. The Association between the Combined Oral Contraceptive Pill and Insulin Resistance, Dysglycemia and Dyslipidemia in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. Hum. Reprod. 2011, 26, 191–201. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, P.X.; Moreira, P.; de Almeida, D.C.; de Souza, A.A.; do Carmo Franco, M. Oral Contraceptives in Adolescents: A Retrospective Population-Based Study on Blood Pressure and Metabolic Dysregulation. Eur. J. Clin. Pharmacol. 2024, 80, 1097–1103. [Google Scholar] [CrossRef]
- Silva-Bermudez, L.S.; Toloza, F.J.K.; Perez-Matos, M.C.; de Souza, R.J.; Banfield, L.; Vargas-Villanueva, A.; Mendivil, C.O. Effects of Oral Contraceptives on Metabolic Parameters in Adult Premenopausal Women: A Meta-Analysis. Endocr. Connect. 2020, 9, 978–998. [Google Scholar] [CrossRef]
- Sitruk-Ware, R.; Nath, A. Characteristics and Metabolic Effects of Estrogen and Progestins Contained in Oral Contraceptive Pills. Best. Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 13–24. [Google Scholar] [CrossRef]
- Surendran, A.; Zhang, H.; Stamenkovic, A.; Ravandi, A. Lipidomics and Cardiovascular Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167806. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.-H.; Oh, K. Data Resource Profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Erdoğan, K.; Sanlier, N. Metabolic Syndrome and Menopause: The Impact of Menopause Duration on Risk Factors and Components. Int. J. Womens Health 2024, 16, 1249–1256. [Google Scholar] [CrossRef]
- Christakis, M.K.; Hasan, H.; De Souza, L.R.; Shirreff, L. The Effect of Menopause on Metabolic Syndrome: Cross-Sectional Results from the Canadian Longitudinal Study on Aging. Menopause 2020, 27, 999–1009. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Garcia-Contreras, C.; Vazquez-Gomez, M. Nature and Nurture in the Early-Life Origins of Metabolic Syndrome. Curr. Pharm. Biotechnol. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The Importance of Nutrition in Pregnancy and Lactation: Lifelong Consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef]
- Godsland, I.F.; Crook, D.; Devenport, M.; Wynn, V. Relationships between Blood Pressure, Oral Contraceptive Use and Metabolic Risk Markers for Cardiovascular Disease. Contraception 1995, 52, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.A.; Blyler, C.A.; Bello, N.A. Oral Contraceptive Pills and Hypertension: A Review of Current Evidence and Recommendations. Hypertension 2023, 80, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Würtz, P.; Auro, K.; Morin-Papunen, L.; Kangas, A.J.; Soininen, P.; Tiainen, M.; Tynkkynen, T.; Joensuu, A.; Havulinna, A.S.; et al. Effects of Hormonal Contraception on Systemic Metabolism: Cross-Sectional and Longitudinal Evidence. Int. J. Epidemiol. 2016, 45, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Godsland, I.F.; Crook, D.; Simpson, R.; Proudler, T.; Felton, C.; Lees, B.; Anyaoku, V.; Devenport, M.; Wynn, V. The Effects of Different Formulations of Oral Contraceptive Agents on Lipid and Carbohydrate Metabolism. N. Engl. J. Med. 1990, 323, 1375–1381. [Google Scholar] [CrossRef]
- Khoo, C.; Campos, H.; Judge, H.; Sacks, F.M. Effects of Estrogenic Oral Contraceptives on the Lipoprotein B Particle System Defined by Apolipoproteins E and C-III Content. J. Lipid Res. 1999, 40, 202–212. [Google Scholar] [CrossRef]
- Pechère-Bertschi, A.; Maillard, M.; Stalder, H.; Bischof, P.; Fathi, M.; Brunner, H.R.; Burnier, M. Renal Hemodynamic and Tubular Responses to Salt in Women Using Oral Contraceptives. Kidney Int. 2003, 64, 1374–1380. [Google Scholar] [CrossRef]
- Kim, S.-W.; Jeon, J.-H.; Lee, W.-K.; Lee, S.; Kim, J.-G.; Lee, I.-K.; Park, K.-G. Long-Term Effects of Oral Contraceptives on the Prevalence of Diabetes in Post-Menopausal Women: 2007-2012 KNHANES. Endocrine 2016, 53, 816–822. [Google Scholar] [CrossRef]
- Dou, W.; Huang, Y.; Liu, X.; Huang, C.; Huang, J.; Xu, B.; Yang, L.; Liu, Y.; Lei, X.; Li, X.; et al. Associations of Oral Contraceptive Use With Cardiovascular Disease and All-Cause Death: Evidence From the UK Biobank Cohort Study. J. Am. Heart Assoc. 2023, 12, e030105. [Google Scholar] [CrossRef]
- Nguyen, A.T. U.S. Medical Eligibility Criteria for Contraceptive Use, 2024. MMWR Recomm. Rep. 2024, 73, 1–126. [Google Scholar] [CrossRef]
- Khalafi, M.; Habibi Maleki, A.; Sakhaei, M.H.; Rosenkranz, S.K.; Pourvaghar, M.J.; Ehsanifar, M.; Bayat, H.; Korivi, M.; Liu, Y. The Effects of Exercise Training on Body Composition in Postmenopausal Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2023, 14, 1183765. [Google Scholar] [CrossRef]
| Overall (n = 31,178) | No MetS (n = 21,660) | MetS (n = 8324) | SMD * | |
|---|---|---|---|---|
| Age (years), mean (±SD) | 50.1 (±11.1) | 47.4 (±10.5) | 57.4 (±9.1) | 0.993 |
| Age groups, n (%) | 0.973 | |||
| 30–39 years | 6893 (22.1) | 6067 (28.0) | 468 (5.6) | |
| 40–49 years | 7846 (25.2) | 6488 (30.0) | 1077 (12.9) | |
| 50–59 years | 8591 (27.6) | 5659 (26.1) | 2650 (31.8) | |
| 60–69 years | 7848 (25.2) | 3446 (15.9) | 4129 (49.6) | |
| BMI (kg/m2), mean (±SD) | 23.6 (±3.6) | 22.7 (±3.0) | 26.1 (±3.7) | 1.046 |
| BMI groups, n (%) | 0.967 | |||
| <18.5 | 1304 (4.2) | 1184 (5.5) | 44 (0.5) | |
| 18.5–24.9 | 20,447 (65.8) | 16,322 (75.4) | 3384 (40.7) | |
| 25.0–29.9 | 7690 (24.7) | 3744 (17.3) | 3703 (44.6) | |
| 30.0–34.9 | 1403 (4.5) | 343 (1.6) | 997 (12.0) | |
| ≧35.0 | 246 (0.8) | 43 (0.2) | 183 (2.2) | |
| Education level, n (%) | 0.757 | |||
| ≦Elementary | 5484 (17.6) | 2422 (11.2) | 2851 (34.3) | |
| Middle school | 3574 (11.5) | 1972 (9.1) | 1468 (17.7) | |
| High school | 10,848 (34.8) | 7853 (36.3) | 2596 (31.2) | |
| ≧College | 11,240 (36.1) | 9398 (43.4) | 1395 (16.8) | |
| Income level, n (%) | 0.162 | |||
| Q1: Low | 6046 (19.5) | 3846 (17.8) | 1929 (23.2) | |
| Q2: Low–middle | 6229 (20.1) | 4253 (19.7) | 1736 (20.9) | |
| Q3: Middle | 6240 (20.1) | 4333 (20.1) | 1690 (20.4) | |
| Q4: Middle–high | 6280 (20.2) | 4504 (20.9) | 1566 (18.9) | |
| Q5: High | 6246 (20.1) | 4626 (21.5) | 1376 (16.6) | |
| No Spouse, n (%) | 4118 (14.0) | 2326 (11.5) | 1633 (20.2) | 0.243 |
| Smoking status, n (%) | 0.075 | |||
| Never smoker | 27,693 (89.0) | 19,209 (88.8) | 7461 (89.9) | |
| Ex-smoker | 1868 (6.0) | 1368 (6.3) | 396 (4.8) | |
| Current smoker | 1560 (5.0) | 1054 (4.9) | 446 (5.4) | |
| Drinking habit, n (%) | 0.290 | |||
| Never drinking | 3507 (11.3) | 2745 (12.7) | 653 (7.9) | |
| Normal drinking | 23,350 (75.1) | 16,516 (76.4) | 5941 (71.6) | |
| High drinking | 4235 (13.6) | 2344 (10.8) | 1706 (20.6) | |
| Walking days per week, n (%) | 0.085 | |||
| 0 day | 16,481 (52.9) | 11,521 (53.2) | 4347 (52.2) | |
| 1–3 days | 9770 (31.3) | 6953 (32.1) | 2442 (29.4) | |
| 4–7 days | 4917 (15.8) | 3180 (14.7) | 1531 (18.4) | |
| Exercise days per week, n (%) | 0.165 | |||
| 0 day | 25,437 (81.6) | 17,303 (79.9) | 7121 (85.6) | |
| 1–3 days | 3826 (12.3) | 2976 (13.7) | 743 (8.9) | |
| 4–7 days | 1912 (6.1) | 1379 (6.4) | 459 (5.5) | |
| Age of Menarche (years), mean (±SD) | 14.1 (±2.0) | 13.9 (±1.9) | 14.7 (±2.1) | 0.376 |
| Pregnancies (times), mean (±SD) | 3.2 (±1.9) | 2.94 (±1.8) | 3.74 (±1.9) | 0.432 |
| Age at First Delivery (years), mean (±SD) | 26.1 (±4.1) | 26.64 (±4.0) | 24.73 (±3.9) | 0.459 |
| Number of Breastfed Children, mean (±SD) | 1.3 (±1.8) | 1.05 (±1.6) | 1.90 (±2.2) | 0.430 |
| Post Menopause, n (%) | 14,857 (47.7) | 8159 (37.7) | 6187 (74.3) | 0.775 |
| Used Oral Contraceptives, n (%) | 4858 (15.6) | 2928 (13.5) | 1756 (21.1) | 0.180 |
| Pre Menopause | Post Menopause | |
|---|---|---|
| Variables | Adjusted OR (95% CI)* | Adjusted OR (95% CI) * |
| Age per 1 year | 1.11 (1.10–1.12) | 1.09 (1.08–1.10) |
| BMI per 1 kg/m2 | 1.45 (1.42–1.48) | 1.31 (1.29–1.33) |
| Education level, College | 1.00 (ref.) | 1.00 (ref.) |
| High school | 1.06 (0.90–1.25) | 1.06 (0.91–1.23) |
| Middle school | 1.12 (0.84–1.51) | 1.15 (0.97–1.36) |
| Elementary | 1.33 (0.96–1.85) | 1.19 (1.00–1.42) |
| Income level, Q5: High | 1.00 (ref.) | 1.00 (ref.) |
| Q4: Middle–high | 1.16 (0.93–1.46) | 1.09 (0.95–1.26) |
| Q3: Middle | 1.26 (1.00–1.59) | 1.17 (1.01–1.35) |
| Q2: Low–middle | 1.31 (1.04–1.66) | 1.20 (1.04–1.38) |
| Q1: Low | 1.45 (1.14–1.84) | 1.27 (1.10–1.48) |
| Spouse, Yes | 1.00 (ref.) | 1.00 (ref.) |
| No | 1.04 (0.81–1.33) | 0.98 (0.87–1.10) |
| Smoking status, Never smoker | 1.00 (ref.) | 1.00 (ref.) |
| Ex-smoker | 1.03 (0.77–1.38) | 1.22 (0.95–1.55) |
| Current smoker | 1.42 (1.03–1.96) | 1.60 (1.26–2.04) |
| Drinking habit, Never drinking | 1.00 (ref.) | 1.00 (ref.) |
| Normal drinking | 1.28 (1.04–1.57) | 1.29 (1.07–1.54) |
| High drinking | 2.13 (1.58–2.88) | 1.41 (1.16–1.73) |
| Walking days per week, 0 days | 1.00 (ref.) | 1.00 (ref.) |
| 1–3 days | 1.15 (0.99–1.34) | 1.05 (0.95–1.17) |
| 4–7 days | 0.98 (0.81–1.20) | 1.08 (0.96–1.22) |
| Exercise days per week, 0 days | 1.00 (ref.) | 1.00 (ref.) |
| 1–3 days | 0.95 (0.76–1.19) | 0.77 (0.67–0.90) |
| 4–7 days | 0.82 (0.61–1.10) | 0.62 (0.52–0.75) |
| Age at Menarche per 1 year | 1.00 (0.96–1.04) | 0.99 (0.96–1.01) |
| Pregnancies per 1 time | 0.98 (0.94–1.03) | 1.00 (0.97–1.03) |
| Age at First Delivery per 1 year | 0.99 (0.97–1.01) | 0.99 (0.98–1.01) |
| Breast Feeding per 1 child | 0.99 (0.93–1.04) | 0.99 (0.96–1.02) |
| Oral Contraceptives, Not used | 1.00 (ref.) | 1.00 (ref.) |
| Used | 1.40 (1.13–1.72) | 1.16 (1.03–1.29) |
| Oral Contraceptive Duration | Pre Menopause | Post Menopause | ||
|---|---|---|---|---|
| MetS Cases/Sub Total Cases | Adjusted OR (95% CI) * | MetS Cases/Sub Total Cases | Adjusted OR (95% CI) * | |
| Never used | 350/3392 | 1.00 | 995/2590 | 1.00 |
| <1 year | 16/180 | 0.97 (0.44–2.16) | 119/263 | 1.13 (0.79–1.63) |
| 1–3 years | 19/96 | 1.48 (0.72–2.84) | 151/321 | 1.12 (0.80–1.58) |
| 3–5 years | 2/19 | 1.50 (0.06–40.62) | 65/106 | 1.26 (0.73–2.16) |
| ≧5 years | 3/26 | 0.44 (0.13–1.49) | 48/76 | 2.13 (1.27–3.57) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, I.A.; Jo, J.; Lee, J.; Lim, H.; Cheon, Y.-H.; Kim, R.B. Oral Contraceptive Use and Reproductive History in Relation to Metabolic Syndrome Among Women from KNHANES 2010–2023. J. Clin. Med. 2025, 14, 6319. https://doi.org/10.3390/jcm14176319
Cho IA, Jo J, Lee J, Lim H, Cheon Y-H, Kim RB. Oral Contraceptive Use and Reproductive History in Relation to Metabolic Syndrome Among Women from KNHANES 2010–2023. Journal of Clinical Medicine. 2025; 14(17):6319. https://doi.org/10.3390/jcm14176319
Chicago/Turabian StyleCho, In Ae, Jaeyoon Jo, Jeesun Lee, Hyunjin Lim, Yun-Hong Cheon, and Rock Bum Kim. 2025. "Oral Contraceptive Use and Reproductive History in Relation to Metabolic Syndrome Among Women from KNHANES 2010–2023" Journal of Clinical Medicine 14, no. 17: 6319. https://doi.org/10.3390/jcm14176319
APA StyleCho, I. A., Jo, J., Lee, J., Lim, H., Cheon, Y.-H., & Kim, R. B. (2025). Oral Contraceptive Use and Reproductive History in Relation to Metabolic Syndrome Among Women from KNHANES 2010–2023. Journal of Clinical Medicine, 14(17), 6319. https://doi.org/10.3390/jcm14176319






