Phase II Cardiac Rehabilitation Under Compulsory Insurance in Kazakhstan: A Five-Year Cohort Analysis of Clinical and Economic Outcomes
Abstract
1. Introduction
Aim of Study
2. Materials and Methods
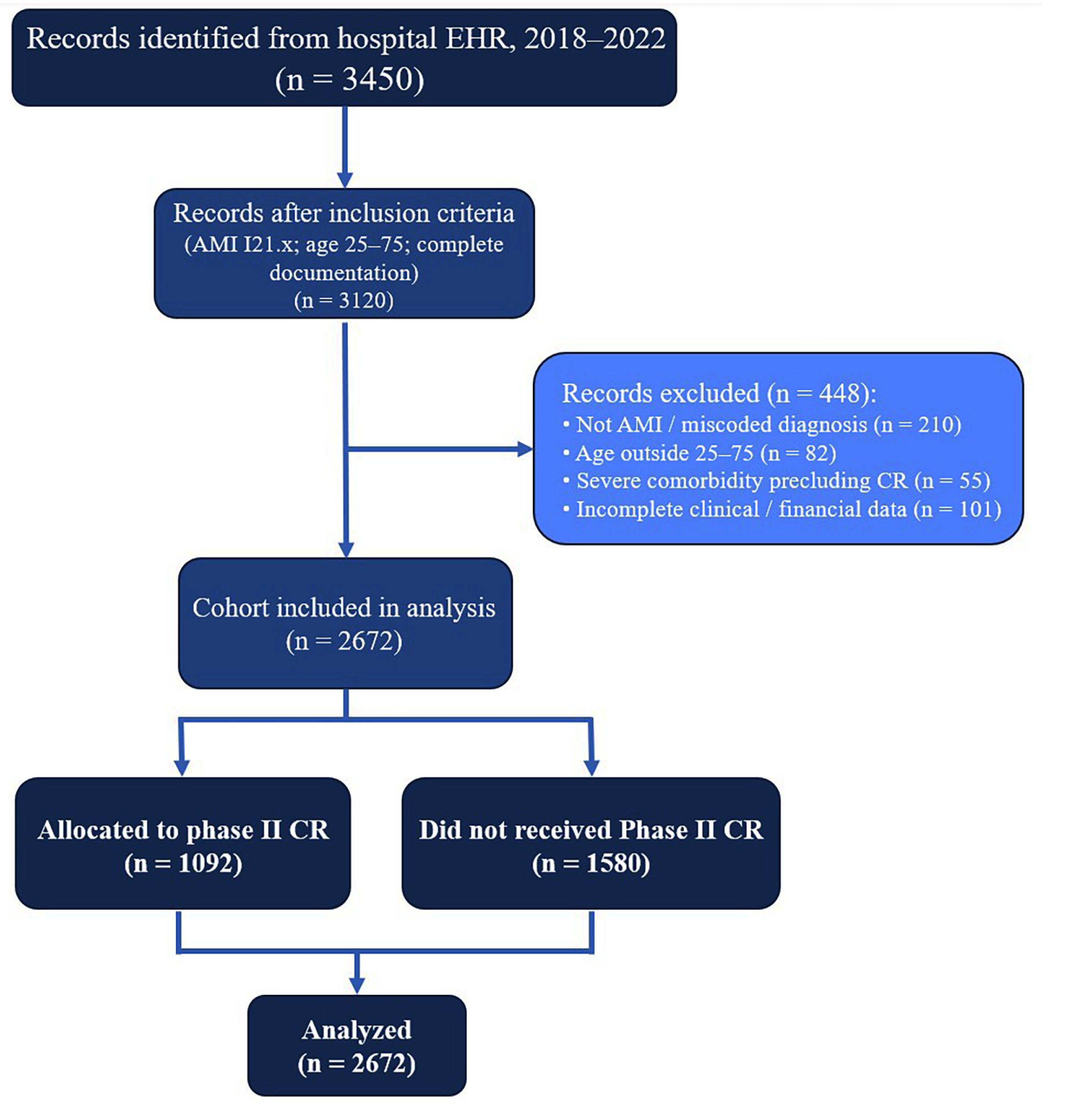
2.1. Study Design and Setting
2.2. Study Population and Group Formation
- CR group: Patients who completed second-stage (phase II) cardiac rehabilitation, which, in Kazakhstan, is delivered as an inpatient program in specialized hospital departments. In accordance with local health policy and similar to other post-Soviet countries, completion is determined by fulfilling an individualized number of prescribed bed days according to a standardized national protocol rather than a fixed number of exercise sessions [10,14,15]. This approach reflects both the regulatory framework and clinical practice in the region.
- Non-CR group: Patients who received the same standard therapy, but without a course of rehabilitation treatment (without the 2nd stage of rehabilitation).
2.3. Sample Size
2.4. Data Collection
2.5. Primary Outcomes
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Socio-Demographic and Clinical Characteristics
3.2. Initial Hospitalization Costs and Rehabilitation Timing
3.3. Change in Left Ventricular Ejection Fraction
- Dependent Variable: second LVEF
- Fixed Factor: rehabilitation (group: yes/no)
- Covariate: initial LVEF
3.4. Subsequent Hospitalizations, Length of Stay, and Costs
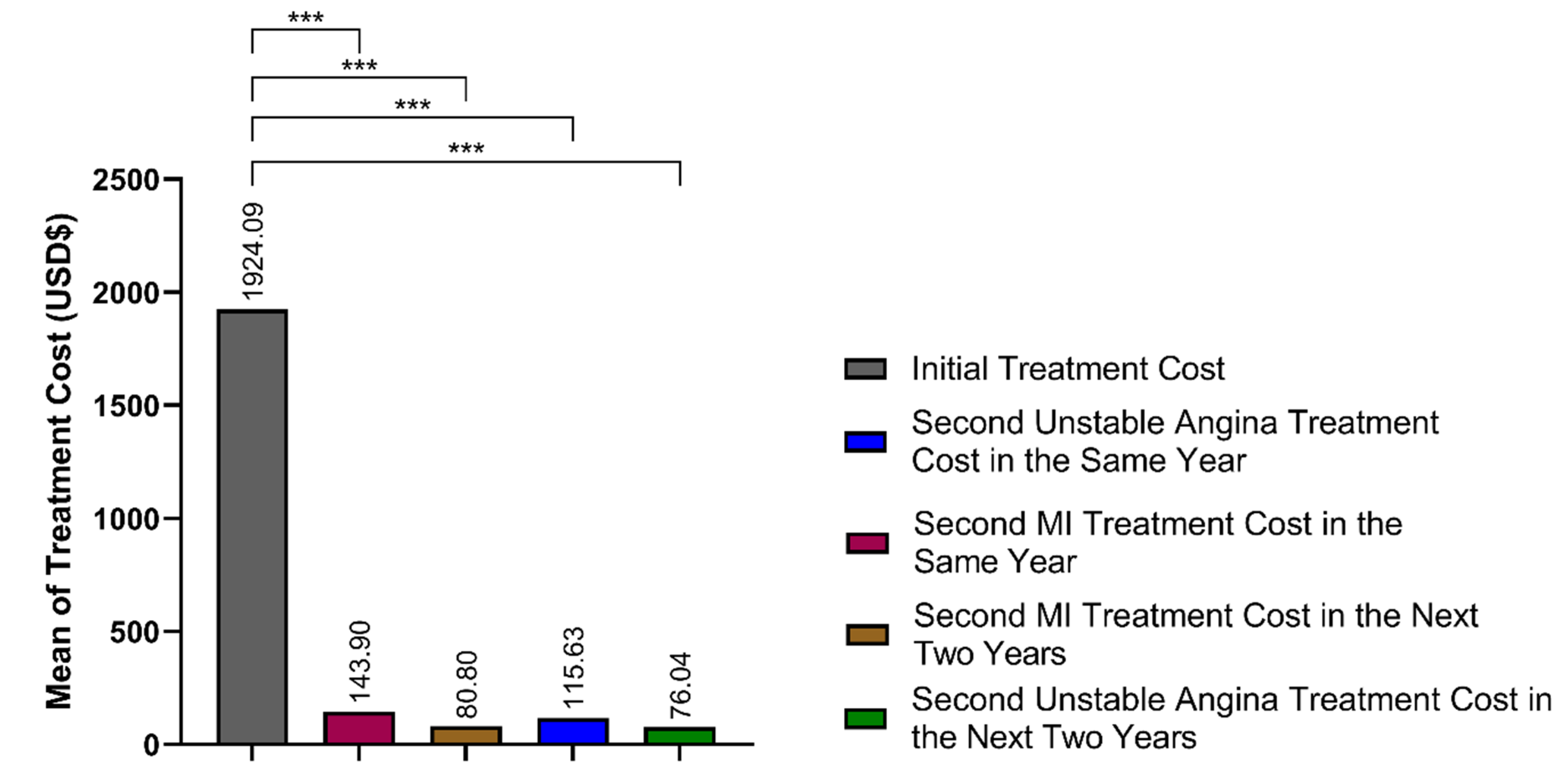
3.5. Cumulative Cost Comparison
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CVDs | Cardiovascular diseases |
| CR | Cardiac rehabilitation |
| AMI | Acute myocardial infarction |
| LVEF | Left ventricular ejection fraction |
| ICD-10 | International Classification of Diseases, Tenth Revision |
| PCI | Percutaneous coronary intervention |
| CABG | Coronary artery bypass grafting |
References
- World Health Organization. Cardiovascular Diseases (CVDs); WHO: Geneava, Switzerland, 2025; Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 31 July 2025).
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics—2016 Update. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Oldridge, N.; Thompson David, R.; Zwisler, A.-D.; Rees, K.; Martin, N.; Taylor Rod, S. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease. JACC 2016, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dibben, G.; Faulkner, J.; Oldridge, N.; Rees, K.; Thompson, D.R.; Zwisler, A.D.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2021, 11, CD001800. [Google Scholar] [CrossRef]
- Thomas, R.J.; Beatty, A.L.; Beckie, T.M.; Brewer, L.C.; Brown, T.M.; Forman, D.E.; Franklin, B.A.; Keteyian, S.J.; Kitzman, D.W.; Regensteiner, J.G.; et al. Home-Based Cardiac Rehabilitation: A Scientific Statement From the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation 2019, 140, e69–e89. [Google Scholar] [CrossRef]
- Oldridge, N.B. Economic Evaluation of Cardiac Rehabilitation. In Cardiovascular Prevention and Rehabilitation; Perk, J., Gohlke, H., Hellemans, I., Sellier, P., Mathes, P., Monpère, C., McGee, H., Saner, H., Eds.; Springer: London, UK, 2007; pp. 494–501. [Google Scholar] [CrossRef]
- Edwards, K.; Jones, N.; Newton, J.; Foster, C.; Judge, A.; Jackson, K.; Arden, N.K.; Pinedo-Villanueva, R. The cost-effectiveness of exercise-based cardiac rehabilitation: A systematic review of the characteristics and methodological quality of published literature. Health Econ. Rev. 2017, 7, 37. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Spaulding, E.M.; Marvel, F.A.; LaFave, S.; Yu, J.; Mota, D.; Lorigiano, T.-J.; Huynh, P.P.; Shan, R.; Yesantharao, P.S.; et al. Cost-effectiveness of a Digital Health Intervention for Acute Myocardial Infarction Recovery. Med. Care 2021, 59, 1023–1030. [Google Scholar] [CrossRef]
- Zhakhina, G.; Gaipov, A.; Salustri, A.; Gusmanov, A.; Sakko, Y.; Yerdessov, S.; Bekbossynova, M.; Abbay, A.; Sarria-Santamera, A.; Akbilgic, O. Incidence, mortality and disability-adjusted life years of acute myocardial infarction in Kazakhstan: Data from unified national electronic healthcare system 2014–2019. Front. Cardiovasc. Med. 2023, 10, 1127320. [Google Scholar] [CrossRef]
- Babayeva, A.; Ushurova, K.; Serikova, G.; Kanapina, A.; Assemov, A. Current Status and Problems of Cardiac Rehabilitation in Kazakhstan. Master’s Thesis, Kazakh National Medical University, Almaty, Kazakhstan, 2021; pp. 293–297. [Google Scholar]
- Oldridge, N.B.; Pakosh, M.T.; Thomas, R.J. Cardiac rehabilitation in low- and middle-income countries: A review on cost and cost-effectiveness. Int. Health 2016, 8, 77–82. [Google Scholar] [CrossRef]
- Pesah, E.; Turk-Adawi, K.; Supervia, M.; Lopez-Jimenez, F.; Britto, R.; Ding, R.; Babu, A.; Sadeghi, M.; Sarrafzadegan, N.; Cuenza, L.; et al. Cardiac rehabilitation delivery in low/middle-income countries. Heart 2019, 105, 1806. [Google Scholar] [CrossRef]
- Tessler, J.; Ahmed, I.; Bordoni, B. Cardiac Rehabilitation. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Euroaspire, I. A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur. J. Prev. Cardiol. 2016, 23, 636–648. [Google Scholar]
- World Health Organization. Rehabilitation Guideline After Myocardial Infarction, 2013; WHO: Geneava, Switzerland, 2021. [Google Scholar]
- Taylor, R.S.; Sagar, V.A.; Davies, E.J.; Briscoe, S.; Coats, A.J.S.; Dalal, H.; Lough, F.; Rees, K.; Singh, S.J.; Mordi, I.R. Exercise-based rehabilitation for heart failure. Cochrane Database Syst. Rev. 2014, 2014, CD003331. [Google Scholar] [CrossRef] [PubMed]
- Dibben, G.O.; Faulkner, J.; Oldridge, N.; Rees, K.; Thompson, D.R.; Zwisler, A.-D.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease: A meta-analysis. Eur. Heart J. 2023, 44, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, J.A.; Isiozor, N.M.; Kunutsor, S.K. Objectively Assessed Cardiorespiratory Fitness and All-Cause Mortality Risk: An Updated Meta-analysis of 37 Cohort Studies Involving 2,258,029 Participants. Mayo Clin. Proc. 2022, 97, 1054–1073. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.; Larsen, K.S.R.; Petersen, A.K. Validity of the Talk Test as a Method to Estimate Ventilatory Threshold and Guide Exercise Intensity in Cardiac Patients. J. Cardiopulm. Rehabil. Prev. 2020, 40, 330–334. [Google Scholar] [CrossRef]
- Lawler, P.R.; Filion, K.B.; Eisenberg, M.J. Efficacy of exercise-based cardiac rehabilitation post–myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Am. Heart J. 2011, 162, 571–584.e572. [Google Scholar] [CrossRef]
- Graham, H.L.; Lac, A.; Lee, H.; Benton, M.J. Predicting Long-Term Mortality, Morbidity, and Survival Outcomes Following a Cardiac Event: A Cardiac Rehabilitation Study. Rehabil. Process Outcome 2019, 8, 1179572719827610. [Google Scholar] [CrossRef]
- Frederix, I.; Hansen, D.; Coninx, K.; Vandervoort, P.; Vandijck, D.; Hens, N.; Van Craenenbroeck, E.; Van Driessche, N.; Dendale, P. Medium-Term Effectiveness of a Comprehensive Internet-Based and Patient-Specific Telerehabilitation Program With Text Messaging Support for Cardiac Patients: Randomized Controlled Trial. J. Med. Internet Res. 2015, 17, e185. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.; O’Carroll, V. The effectiveness of cardiac telerehabilitation in comparison to centre-based cardiac rehabilitation programmes: A literature review. J. Telemed. Telecare 2022, 30, 631–646. [Google Scholar] [CrossRef]
- Hautala, A.J.; Kiviniemi, A.M.; Mäkikallio, T.; Koistinen, P.; Ryynänen, O.P.; Martikainen, J.A.; Seppänen, T.; Huikuri, H.V.; Tulppo, M.P. Economic evaluation of exercise-based cardiac rehabilitation in patients with a recent acute coronary syndrome. Scand. J. Med. Sci. Sports 2017, 27, 1395–1403. [Google Scholar] [CrossRef]
- Wittboldt, S.; Margret, L.; Annica, R.F.; Björn, E.; Bäck, M. Exercise-based cardiac rehabilitation after acute myocardial infarction in Sweden—Standards, costs, and adherence to European guidelines (The Perfect-CR study). Physiother. Theory Pract. 2024, 40, 366–376. [Google Scholar] [CrossRef]
- Simões, V.A.L.; Mendes, F.d.S.N.S.; Avellar, A.M.; da Silva, G.M.S.; Carneiro, F.M.; Silva, P.S.; Mazzoli-Rocha, F.; Silva, R.S.; Vieira, M.C.; Costa, C.J.d.N.; et al. Cost-effectiveness of an exercise-based cardiovascular rehabilitation program in patients with chronic Chagas cardiomyopathy in Brazil: An analysis from the PEACH study. Trop. Med. Int. Health 2022, 27, 630–638. [Google Scholar] [CrossRef]
- Serón, P.; Gaete, M.; Oliveros, M.-J.; Román, C.; Lanas, F.; Velásquez, M.; Reveco, R.; Bustos, L.; Rojas, R. Cost-Effectiveness of Exercise-Based Cardiac Rehabilitation in Chilean Patients Surviving Acute Coronary Syndrome. J. Cardiopulm. Rehabil. Prev. 2019, 39, 168–174. [Google Scholar] [CrossRef]
- Clark, A.M.; King-Shier, K.M.; Duncan, A.; Spaling, M.; Stone, J.A.; Jaglal, S.; Angus, J. Factors influencing referral to cardiac rehabilitation and secondary prevention programs: A systematic review. J. Cardiovasc. Risk 2013, 20, 692–700. [Google Scholar] [CrossRef]
- Turk-Adawi, K.; Supervia, M.; Lopez-Jimenez, F.; Pesah, E.; Ding, R.; Britto, R.R.; Bjarnason-Wehrens, B.; Derman, W.; Abreu, A.; Babu, A.S.; et al. Cardiac Rehabilitation Availability and Density around the Globe. eClinicalMedicine 2019, 13, 31–45. [Google Scholar] [CrossRef]
- Oerkild, B.; Frederiksen, M.; Hansen, J.F.; Prescott, E. Home-based cardiac rehabilitation is an attractive alternative to no cardiac rehabilitation for elderly patients with coronary heart disease: Results from a randomised clinical trial. BMJ Open 2012, 2, e001820. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, J.; Kidawa, T.M.; Rynkowska-Kidawa, M.; IrzmaŃski, T.R.; Kowalski, T.J. Evaluation of frequency of occurrence of cognitive impairment in the course of arterial hypertension in an elderly population. Psychogeriatrics 2020, 20, 406–411. [Google Scholar] [CrossRef]
- Taylor, R.S.; Fredericks, S.; Jones, I.; Neubeck, L.; Sanders, J.; De Stoutz, N.; Thompson, D.R.; Wadhwa, D.N.; Grace, S.L. Global perspectives on heart disease rehabilitation and secondary prevention: A scientific statement from the Association of Cardiovascular Nursing and Allied Professions, European Association of Preventive Cardiology, and International Council of Cardiovascular Prevention and Rehabilitation. Eur. Heart J. 2023, 44, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Rawstorn, J.C.; Gant, N.; Direito, A.; Beckmann, C.; Maddison, R. Telehealth exercise-based cardiac rehabilitation: A systematic review and meta-analysis. Heart 2016, 102, 1183–1192. [Google Scholar] [CrossRef]
| Total | CR Users | Non-CR Users | p Value | ||||
|---|---|---|---|---|---|---|---|
| Socio-demographic | Age (mean ± SD) | 53.8 ± 6.6 | 53.87 ± 6.6 | 53.79 ± 6.5 | 0.766 | ||
| 25–34 | 1.19% | ||||||
| 35–44 | 8.57% | ||||||
| 45–54 | 35.18% | ||||||
| ≥55 | 55.06% | ||||||
| Chi-Square Test | |||||||
| χ2 | df | p Value | |||||
| Male | 90.2% | 89% | 91.1% | 3.124 | 1 | 0.077 | |
| Female | 9.8% | 11% | 8.9% | ||||
| Education | Incomplete secondary education | 3% | 2.8% | 3.0% | 1.298 | 4 | 0.862 |
| Incomplete university education | 2% | 1.7% | 2.2% | ||||
| School education | 37% | 36.4% | 37.4% | ||||
| Secondary specialized education | 35.1% | 35.5% | 34.8% | ||||
| University education | 22.9% | 23.4% | 22.5% | ||||
| Residency status | Urban | 81.1% | 82.7% | 80% | 3.054 | 1 | 0.081 |
| Rural | 18.9% | 17.3% | 20% | ||||
| Living status | Currently alive | 87.9% | 86.6% | 88.8% | 2.854 | 1 | 0.091 |
| Currently deceased | 12.1% | 13.4% | 11.2% | ||||
| Current disability | Yes | 12.3% | 14.4% | 10.9% | 7.290 | 1 | 0.007 ** |
| No | 87.7% | 85.6% | 89.1% | ||||
| Remaining on disability for 2 years of observation after MI | Yes | 39.7% | 43.1% | 37.3 | 9.244 | 1 | 0.002 ** |
| No | 60.3% | 56.9% | 62.7% | ||||
| Mean of disability (mean month ± SD) | 8.8 ± 11.2 | 9.68 ± 11.4 | 8.21 ± 11 | 0.001 ** | |||
| MI occurrence at first hospitalization | Primary MI | 78.6% | 78.9% | 78.4% | 0.104 | 1 | 0.747 |
| Recurrent MI | 21.4% | 21.1% | 21.6% | ||||
| Type of MI | ST elevation MI (STEMI) | 56.1% | 58.5% | 54.5% | 4.244 | 1 | 0.039 * |
| Non-STEMI | 43.9% | 41.5% | 45.5% | ||||
| Treatment received at first hospitalization | Drug treatment | 23.1% | 21.3% | 24.2% | 7.194 | 2 | 0.027 * |
| PCI | 60.7% | 60.3% | 61.0% | ||||
| CABG | 16.2% | 18.3% | 14.8% | ||||
| Yes | No | p Value | |
|---|---|---|---|
| Cardiac rehabilitation | 40.9% | 59.1% | <0.001 *** |
| Initial treatment cost (USD, mean ± SD) | 1988.80 ± 1290.1 | 1879.36 ± 1104.8 | 0.019 ** 0.019 ** |
| Initial treatment cost (KZT, mean ± SD) | 1,046,736.86 ± 679,003.5 | 989,139.52 ± 581,503.7 | |
| Time taken to complete rehabilitation (months ± SD) | 1.89 (±0.49) | ||
| CR Users | Non-CR Users | p Value (Independent-Samples t-Test) | ||||
|---|---|---|---|---|---|---|
| Initial LVEF (mean ± SD) | 51.75 ± 10.8 | 47.77 ± 13 | <0.001 *** | |||
| Second LVEF (mean ± SD) | 53.71 ± 9.2 | 48.98 ± 10.4 | <0.001 *** | |||
| Univariate General Linear Model | ||||||
| Type III SS | df | MS | F | Sig. | Partial Eta Squared | |
| Rehabilitation | 4173.987 | 1 | 4173.987 | 74.019 | <0.001 | 0.027 * |
| Initial LVEF (covariate) | 117,133.398 | 1 | 117,133.398 | 2077.163 | <0.001 | 0.438 |
| Error | 150,507.685 | 2669 | 56.391 | |||
| CR Users | Non-CR Users | χ2 | df | p Value | |||
|---|---|---|---|---|---|---|---|
| Repeat MI in same year | Frequency | Yes | 43.7% | 56.3% | 0.424 | 1 | 0.515 |
| No | 40.7% | 59.3% | |||||
| Treatment received for repeat MI in the same year | Drug treatment | 50.0% | 50.0% | 8.496 | 7 | 0.291 | |
| PCI | 28.6% | 71.4% | |||||
| CABG | 49.3% | 50.7% | |||||
| Bed days (mean ± SD) | 9.44 ± 3.5 | 9.33 ± 3.6 | 0.865 | ||||
| Treatment payment (USD, mean ± SD) | 3175.54 ± 1455.5 | 2955.51 ± 1365.6 | 0.385 | ||||
| Treatment payment (KZT, mean ± SD) | 1,671,337.78 ± 766,081.1 | 1,555,535.06 ± 718,787.5 | 0.385 | ||||
| Repeat MI in the next 2 years after primary hospitalization | Frequency | Yes | 39.7% | 60.3% | 0.074 | 1 | 0.786 |
| No | 40.9% | 59.1% | |||||
| Treatment received for repeat MI in the same year | Drug treatment | 25.0% | 75.0% | 9.372 | 6 | 0.154 | |
| PCI | 43.9% | 56.1% | |||||
| CABG | 100.0% | 0.0% | |||||
| Bed days (mean ± SD) | 10.14 ± 5 | 9.01 ± 3.1 | 0.129 | ||||
| Treatment payment (USD, mean ± SD) | 2169.45 ± 956.3 | 1745.67 ± 869.3 | 0.017 * | ||||
| Treatment payment (KZT, mean ± SD) | 1,141,817.16 ± 503.360.7 | 918.775.78 ± 457.578.4 | 0.017 * | ||||
| Emergency hospitalization for unstable angina during the same year | Frequency | Yes | 42.2% | 57.8% | 0.138 | 1 | 0.711 |
| No | 40.8% | 59.2% | |||||
| Treatment received for repeat MI in the same year | Drug treatment | 38.4% | 61.6% | 5.062 | 7 | 0.652 | |
| PCI | 42.0% | 58.0% | |||||
| CABG | 53.3% | 46.7% | |||||
| Bed days (mean ± SD) | 8.85 ± 4.1 | 8.89 ± 4.3 | 0.943 | ||||
| Treatment payment (USD, mean ± SD) | 1806.79 ± 1329.3 | 1617.61 ± 1249.8 | 0.327 | ||||
| Treatment payment (KZT, mean ± SD) | 950.943.34 ± 699.668.8 | 851.374.24 ± 657.824.2 | 0.327 | ||||
| Emergency hospitalization for unstable angina in the next 2 years after primary MI | Frequency | Yes | 37.6% | 62.4% | 1.228 | 1 | 0.268 |
| No | 41.2% | 58.8% | |||||
| Treatment received for repeat MI in the same year | Drug treatment | 38.8% | 61.2% | 6.495 | 7 | 0.483 | |
| PCI | 34.4% | 65.6% | |||||
| CABG | 50.0% | 50.0% | |||||
| Bed days (mean ± SD) | 9.75 ± 5.5 | 9.14 ± 5.1 | 0.337 | ||||
| Treatment payment (USD, mean ± SD) | 1443.40 ± 1090.2 | 1556.78 ± 1053.2 | 0.556 | ||||
| Treatment payment (KZT, mean ± SD) | 759.686.65 ± 573.816.6 | 819.359.33 ± 554.334.9 | 0.560 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeyeva, Y.; Yermukhanova, L.S.; Nurbakyt, A.N.; Kurmanalina, G.L.; Walkowiak, D.; Nogayeva, M.G.; Afshar, A. Phase II Cardiac Rehabilitation Under Compulsory Insurance in Kazakhstan: A Five-Year Cohort Analysis of Clinical and Economic Outcomes. J. Clin. Med. 2025, 14, 6317. https://doi.org/10.3390/jcm14176317
Sergeyeva Y, Yermukhanova LS, Nurbakyt AN, Kurmanalina GL, Walkowiak D, Nogayeva MG, Afshar A. Phase II Cardiac Rehabilitation Under Compulsory Insurance in Kazakhstan: A Five-Year Cohort Analysis of Clinical and Economic Outcomes. Journal of Clinical Medicine. 2025; 14(17):6317. https://doi.org/10.3390/jcm14176317
Chicago/Turabian StyleSergeyeva, Yelena, Lyudmila S. Yermukhanova, Ardak N. Nurbakyt, Gulnara L. Kurmanalina, Dariush Walkowiak, Maral G. Nogayeva, and Alireza Afshar. 2025. "Phase II Cardiac Rehabilitation Under Compulsory Insurance in Kazakhstan: A Five-Year Cohort Analysis of Clinical and Economic Outcomes" Journal of Clinical Medicine 14, no. 17: 6317. https://doi.org/10.3390/jcm14176317
APA StyleSergeyeva, Y., Yermukhanova, L. S., Nurbakyt, A. N., Kurmanalina, G. L., Walkowiak, D., Nogayeva, M. G., & Afshar, A. (2025). Phase II Cardiac Rehabilitation Under Compulsory Insurance in Kazakhstan: A Five-Year Cohort Analysis of Clinical and Economic Outcomes. Journal of Clinical Medicine, 14(17), 6317. https://doi.org/10.3390/jcm14176317





