Reconstructive Arthroplasty for Malignant Bone Tumors of the Knee—A Single-Center Experience of Functionality and Quality of Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Patient Characteristics and Follow-Up Examination
2.3. Subgroup Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients and Tumor Characteristics
3.2. Primary Outcome Analysis—Functional Outcome and Quality of Life
3.3. Secondary Outcome Analysis
3.3.1. Implant-Related Complication
3.3.2. Survival Analysis
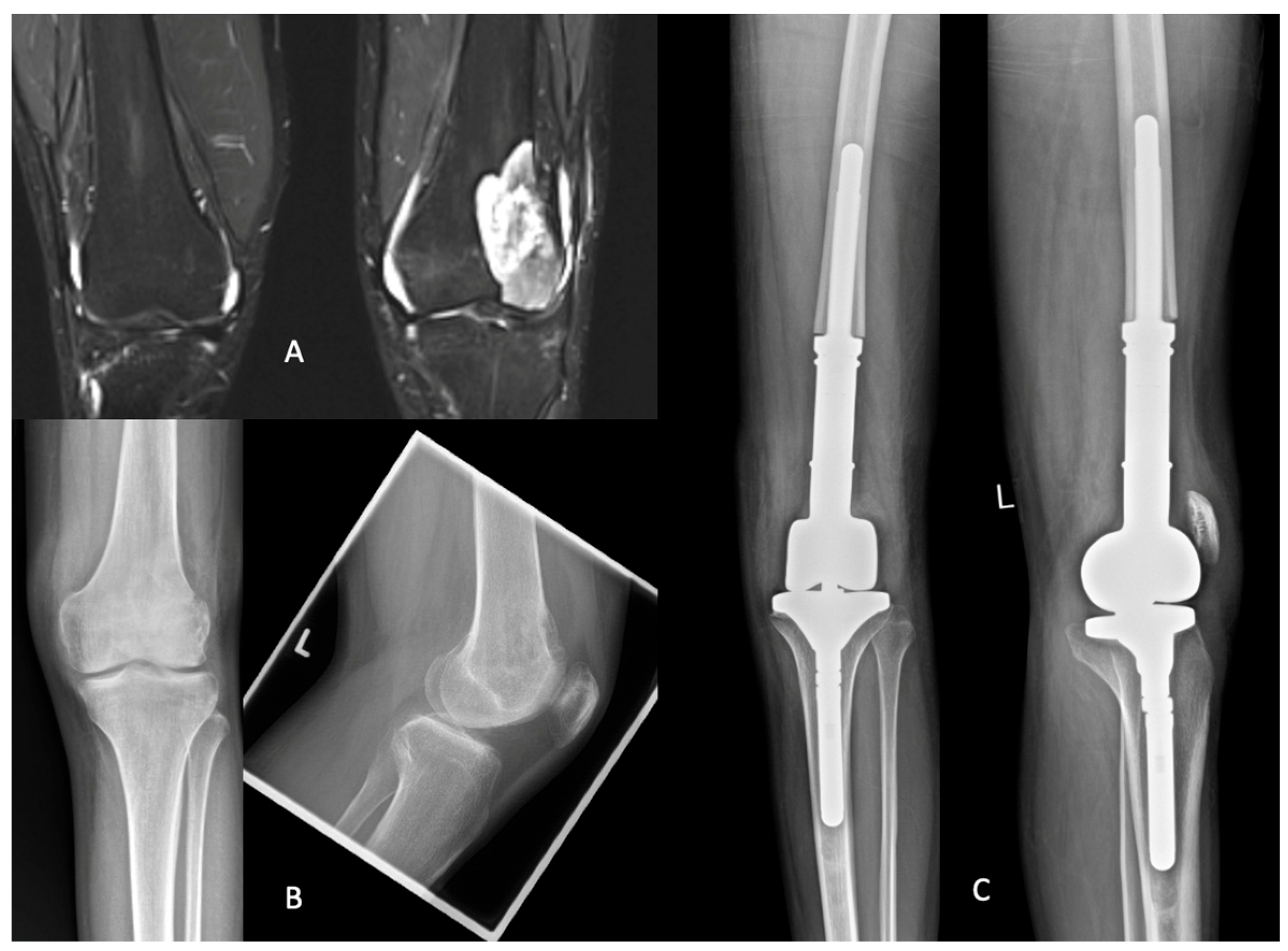
3.3.3. Example Case
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bădilă, A.E.; Rădulescu, D.M.; Niculescu, A.-G.; Grumezescu, A.M.; Rădulescu, M.; Rădulescu, A.R. Recent Advances in the Treatment of Bone Metastases and Primary Bone Tumors: An Up-to-Date Review. Cancers 2021, 13, 4229. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Downie, S.; Stillie, A.; Moran, M.; Sudlow, C.; Simpson, H. Patient-reported assessment of outcome after surgery for bone metastases. Orthop. Rev. 2021, 13, 9062. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Sakai, Y.; Kawamoto, T.; Fukase, N.; Kawakami, Y.; Takemori, T.; Fujiwara, S.; Kitayama, K.; Yahiro, S.; Miyamoto, T.; et al. Surgical outcomes of metastatic bone tumors in the extremities (Surgical outcomes of bone metastases). J. Bone Oncol. 2021, 27, 100352. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; Henrichs, M.-P.; Gosheger, G.; Guder, W.; Nottrott, M.; Andreou, D.; Bormann, E.; Eveslage, M.; Hauschild, G.; Streitbürger, A. Tumour endoprosthesis replacement in the proximal tibia after intra-articular knee resection in patients with sarcoma and recurrent giant cell tumour. Int. Orthop. 2018, 42, 2475–2481. [Google Scholar] [CrossRef]
- He, X.; Gao, Z.; Xu, H.; Zhang, Z.; Fu, P. A meta-analysis of randomized control trials of surgical methods with osteosarcoma outcomes. J. Orthop. Surg. 2017, 12, 5. [Google Scholar] [CrossRef]
- Holm, C.E.; Bardram, C.; Riecke, A.F.; Horstmann, P.; Petersen, M.M. Implant and limb survival after resection of primary bone tumors of the lower extremities and reconstruction with mega-prostheses fifty patients followed for a mean of forteen years. Int. Orthop. 2018, 42, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Pala, E.; Trovarelli, G.; Calabrò, T.; Angelini, A.; Abati, C.N.; Ruggieri, P. Survival of Modern Knee Tumor Megaprostheses: Failures, Functional Results, and a Comparative Statistical Analysis. Clin. Orthop. 2015, 473, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Andreou, D.; Tunn, P.-U.; Szkandera, J.; Liegl-Atzwanger, B.; Leithner, A. Diagnosis and treatment of soft-tissue sarcomas of the extremities and trunk. EFORT Open Rev. 2017, 2, 421–431. [Google Scholar] [CrossRef]
- Smolle, M.A.; Andreou, D.; Tunn, P.-U.; Leithner, A. Advances in tumour endoprostheses: A systematic review. EFORT Open Rev. 2019, 4, 445–459. [Google Scholar] [CrossRef]
- Henderson, E.R.; O’Connor, M.I.; Ruggieri, P.; Windhager, R.; Funovics, P.T.; Gibbons, C.L.; Guo, W.; Hornicek, F.J.; Temple, H.T.; Letson, G.D. Classification of failure of limb salvage after reconstructive surgery for bone tumours: A modified system Including biological and expandable reconstructions. Bone Jt. J. 2014, 96-B, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.; Wu, P.-K.; Chen, C.-F.; Chen, W.-M.; Liu, C.-L.; Chen, T.-H. Bone-prosthesis composite with rotating hinged-knee prosthesis in limb salvage surgery for high-grade sarcoma around the knee. J. Arthroplast. 2015, 30, 90–94. [Google Scholar] [CrossRef]
- Albergo, J.I.; Gaston, C.L.; Aponte-Tinao, L.A.; Ayerza, M.A.; Muscolo, D.L.; Farfalli, G.L.; Jeys, L.M.; Carter, S.R.; Tillman, R.M.; Abudu, A.T.; et al. Proximal Tibia Reconstruction After Bone Tumor Resection: Are Survivorship and Outcomes of Endoprosthetic Replacement and Osteoarticular Allograft Similar? Clin. Orthop. 2017, 475, 676–682. [Google Scholar] [CrossRef]
- Pala, E.; Trovarelli, G.; Angelini, A.; Maraldi, M.; Berizzi, A.; Ruggieri, P. Megaprosthesis of the knee in tumor and revision surgery. Acta Biomed. Atenei Parm. 2017, 88, 129–138. [Google Scholar] [CrossRef]
- Zimel, M.N.; Farfalli, G.L.; Zindman, A.M.; Riedel, E.R.; Morris, C.D.; Boland, P.J.; Healey, J.H. Revision Distal Femoral Arthroplasty With the Compress(®) Prosthesis Has a Low Rate of Mechanical Failure at 10 Years. Clin. Orthop. 2016, 474, 528–536. [Google Scholar] [CrossRef]
- Simard, S.; Thewes, B.; Humphris, G.; Dixon, M.; Hayden, C.; Mireskandari, S.; Ozakinci, G. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J. Cancer Surviv. Res. Pract. 2013, 7, 300–322. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Wen, Y.; Wang, H.; Sun, H.; Liang, W.; Zhang, B.; Humphris, G. Fear of cancer recurrence in adolescent and young adult cancer survivors: A systematic review of the literature. Psychooncology 2019, 28, 675–686. [Google Scholar] [CrossRef]
- Baek, J.-H.; Lee, S.C.; Jin, H.; Kim, J.-W.; Ahn, H.S.; Nam, C.H. Poor outcomes of revision total knee arthroplasty in patients with septic loosening compared to patients with aseptic loosening. J. Orthop. Surg. 2021, 16, 624. [Google Scholar] [CrossRef]
- Lang, N.W.; Hobusch, G.M.; Funovics, P.T.; Windhager, R.; Hofstaetter, J.G. What sports activity levels are achieved in patients with modular tumor endoprostheses of osteosarcoma about the knee? Clin. Orthop. 2015, 473, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Moradi, B.; Zahlten-Hinguranage, A.; Lehner, B.; Zeifang, F. The impact of pathological fractures on therapy outcome in patients with primary malignant bone tumours. Int. Orthop. 2010, 34, 1017–1023. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Wang, Y.; Wang, D.; Han, G.; Jia, J.; Xu, M.; Bi, W. Survival and prognostic factors in Chinese patients with osteosarcoma: 13-year experience in 365 patients treated at a single institution. Pathol. Res. Pract. 2017, 213, 119–125. [Google Scholar] [CrossRef] [PubMed]


| Survival | Non-Survival | p | |
|---|---|---|---|
| Number | 20 | 12 | - |
| Follow-up in years | 8.12 ± 6.8 | 1.1 ± 0.9 | <0.001 |
| Age | 39 ± 23.2 | 67 ± 6.9 | 0.002 |
| Sex male | 10 | 6 | 0.999 |
| Secondary tumor type/metastasis | 3 | 8 | 0.008 |
| Pathological fracture | 2 | 5 | 0.010 |
| Arthroplasty type | |||
| Distal femur | 13 | 8 | 0.923 |
| Proximal tibia | 6 | 3 | 0.999 |
| Total | 1 | 1 | 0.999 |
| Revision surgery | |||
| Infection rate | 7 | 1 | 0.107 |
| Loosening | 3 | 0 | 0.270 |
| Cancer recurrence local | 0 | 0 | - |
| Amputations | 1 | 1 | 0.999 |
| Average surgeries following primary implantation | 3.0 ± 3.7 | 0.6 ± 1.3 | 0.039 |
| ASA | 2.25 ± 0.44 | 2.3 ± 0.48 | 0.716 |
| CCI | 3.4 ± 2.46 | 8.4 ± 1.98 | <0.001 |
| Proximal Tibia (A1) | Distal Femur (A2) | p-Value | No-Revision (B1) | Revision (B2) | p-Value | Age < 29 (C1) | Age > 29 (C2) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Number | 13 | 6 | 9 | 11 | 10 | 10 | |||
| KOOS | 50.2 ± 17.8 | 63.4 ± 11.7 | 0.109 | 49.5 ± 16.6 | 60 ± 16.1 | 0.129 | 60.9 ± 14.3 | 44.6 ± 17.3 | 0.041 |
| MSTS | 20.0 ± 6.2 | 20.0 ± 6.2 | 0.879 | 20.7 ± 7.0 | 19.7 ± 5.2 | 0.565 | 24.0 ± 3.2 | 16.4 ± 5.7 | 0.004 |
| OKS | 31.4 ± 11.2 | 35.8 ± 8.12 | 0.629 | 31.4 ± 10.7 | 34.1 ± 9.97 | 0.620 | 37.1 ± 9.3 | 27.3 ± 8.6 | 0.032 |
| KSS A | 82.5 ± 11.9 | 69 ± 15.1 | 0.819 | 82.5 ± 11.2 | 80.5 ± 14.6 | 0.906 | 85.6 ± 9.2 | 77 ± 14.6 | 0.264 |
| KSS B | 76.2 ± 30.7 | 69 ± 31.7 | 0.950 | 75.5 ± 34.7 | 72.5 ± 26.5 | 0.661 | 91.4 ± 14.4 | 58.1 ± 25.6 | 0.010 |
| Number of Patients (n) | 20 | Score in % |
|---|---|---|
| KOOS ± SD (Range) | 54.7 ± 16.7 (25–76) | 54.7 |
| OKS | 32.7 ± 10.1 (14–46) | 68.1 |
| MSTS | 20.2 ± 5.9 (6–29) | 67.3 |
| KSS A | 82.7 ± 11.9 (55–96) | 82.7 |
| KSS B | 74.1 ± 30.1 (0–100) | 74.1 |
| SF-36 overall | 63 ± 21 (17–89) | 63 |
| KPS | 79 ± 18.8 (40–100) | 79 |
| SF-36 | Proximal Tibia | Distal Femur | p-Value | No-Revision | Revision | p-Value | Age < 29 | Age > 29 | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| SF-36 overall | 0.60 ± 0.25 | 0.70 ± 0.08 | 0.655 | 0.64 ± 0.22 | 0.62 ± 0.21 | 0.897 | 68 ± 0.24 | 58 ± 0.19 | 0.150 |
| PF | 0.52 ± 0.32 | 0.58 ± 0.29 | 0.750 | 0.56 ± 0.36 | 0.55 ± 0.27 | 0.839 | 0.74 ± 0.23 | 0.37 ± 0.27 | 0.007 |
| PR | 0.56 ± 0.48 | 0.75 ± 0.31 | 0.470 | 0.67 ± 0.41 | 0.55 ± 0.45 | 0.689 | 0.68 ± 0.43 | 0.53 ± 0.45 | 0.543 |
| BP | 0.66 ± 0.32 | 0.77 ± 0.23 | 0.524 | 0.63 ± 0.33 | 0.74 ± 0.24 | 0.404 | 0.79 ± 0.25 | 0.59 ± 0.31 | 0.278 |
| GH | 0.48 ± 0.28 | 0.68 ± 0.19 | 0.217 | 0.61 ± 0.28 | 0.52 ± 0.26 | 0.380 | 0.61 ± 0.28 | 0.51 ± 0.27 | 0.323 |
| SR | 0.75 ± 0.27 | 0.83 ± 0.12 | 0.883 | 0.81 ± 0.23 | 0.77 ± 0.24 | 0.836 | 0.85 ± 0.24 | 0.73 ± 0.23 | 0.113 |
| EMO | 0.74 ± 0.43 | 0.89 ± 0.27 | 0.410 | 0.78 ± 0.37 | 0.79 ± 0.40 | 0.928 | 0.70 ± 0.43 | 0.87 ± 0.32 | 0.336 |
| MH | 0.46 ± 0.09 | 0.54 ± 0.08 | 0.228 | 0.50 ± 0.125 | 0.50 ± 0.11 | 0.999 | 0.53 ± 0.14 | 0.48 ± 0.08 | 0.635 |
| KPS | 78.4 ± 20.3 | 78.3 ± 17.2 | 0.842 | 82.2 ± 20.5 | 76.3 ± 18 | 0.310 | 90 ± 9.4 | 68 ± 19.6 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khakzad, T.; Putzier, M.; Thielscher, L.; Taheri, N.; Wittenberg, S.; Paksoy, A.; Rau, D.; Märdian, S. Reconstructive Arthroplasty for Malignant Bone Tumors of the Knee—A Single-Center Experience of Functionality and Quality of Life. J. Clin. Med. 2025, 14, 6287. https://doi.org/10.3390/jcm14176287
Khakzad T, Putzier M, Thielscher L, Taheri N, Wittenberg S, Paksoy A, Rau D, Märdian S. Reconstructive Arthroplasty for Malignant Bone Tumors of the Knee—A Single-Center Experience of Functionality and Quality of Life. Journal of Clinical Medicine. 2025; 14(17):6287. https://doi.org/10.3390/jcm14176287
Chicago/Turabian StyleKhakzad, Thilo, Michael Putzier, Leonard Thielscher, Nima Taheri, Silvan Wittenberg, Alp Paksoy, Daniel Rau, and Sven Märdian. 2025. "Reconstructive Arthroplasty for Malignant Bone Tumors of the Knee—A Single-Center Experience of Functionality and Quality of Life" Journal of Clinical Medicine 14, no. 17: 6287. https://doi.org/10.3390/jcm14176287
APA StyleKhakzad, T., Putzier, M., Thielscher, L., Taheri, N., Wittenberg, S., Paksoy, A., Rau, D., & Märdian, S. (2025). Reconstructive Arthroplasty for Malignant Bone Tumors of the Knee—A Single-Center Experience of Functionality and Quality of Life. Journal of Clinical Medicine, 14(17), 6287. https://doi.org/10.3390/jcm14176287








