Topographic and Anatomical Landmarks of Key Points in Embryologically Guided Surgery for Locally Advanced Gastric Cancer Using Computer-Assisted 3D Navigation
Abstract
1. Introduction
Study Aim and Objectives
2. Materials and Methods
2.1. Study Design
- Age ≥ 18 years;
- ECOG performance status ≤ 2;
- Histologically confirmed gastric adenocarcinoma confirmed by preoperative esophagogastroduodenoscopy with biopsy;
- PET-CT approved by the multidisciplinary tumor board;
- Written informed consent obtained.
- Diffuse peritoneal carcinomatosis (parietal or visceral);
- Synchronous malignant tumors;
- ASA > 3;
- Pregnancy and breast-feeding patients;
- Severe mental disorders.
2.2. Statistical Analysis
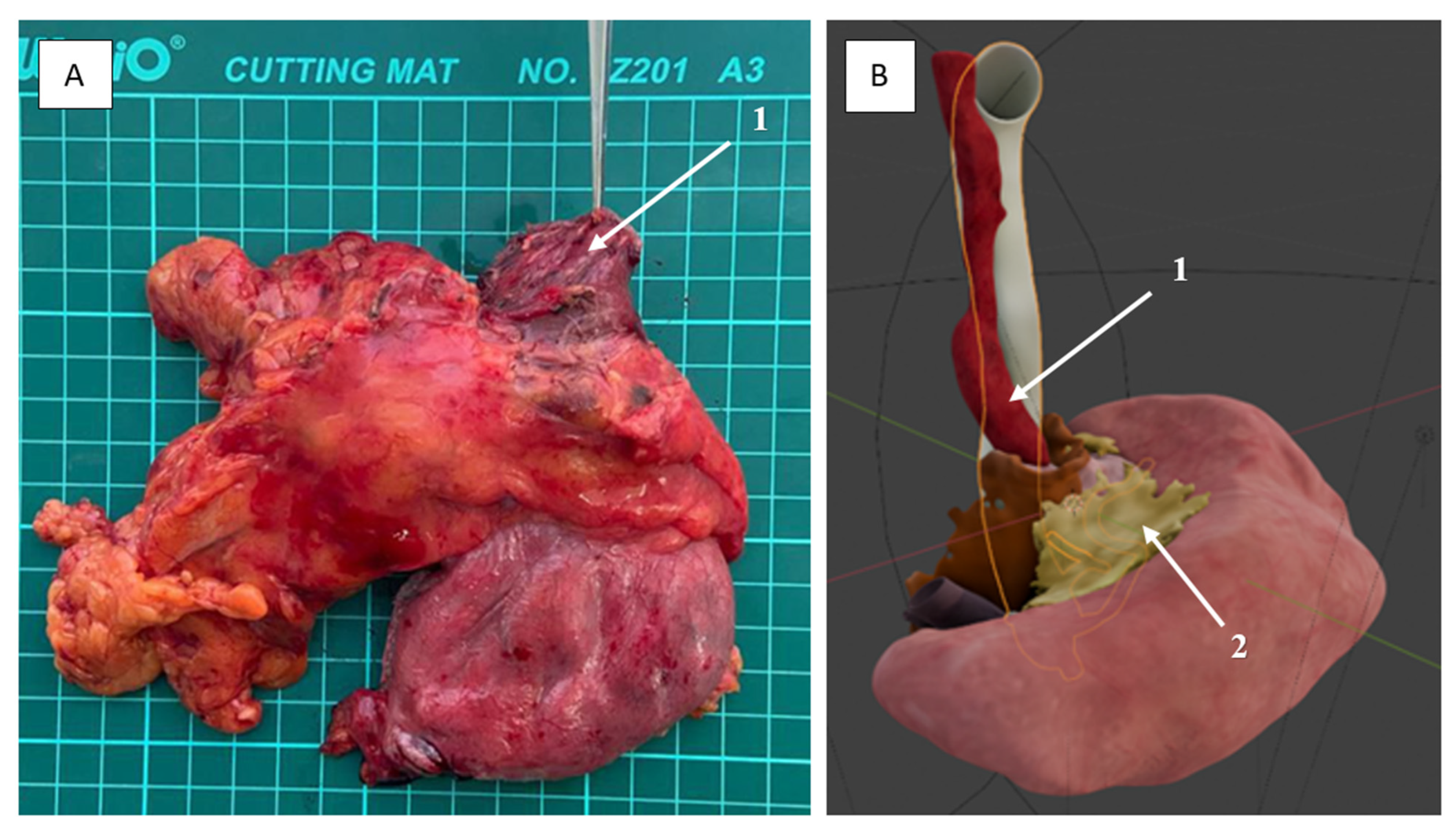
2.3. Technical Aspects of the Surgical Procedures
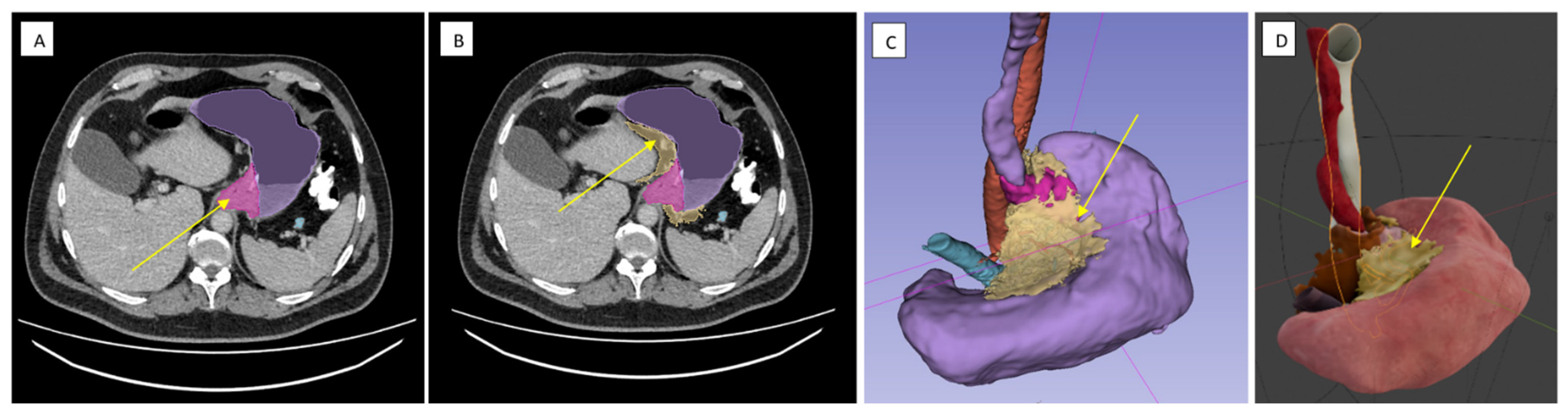
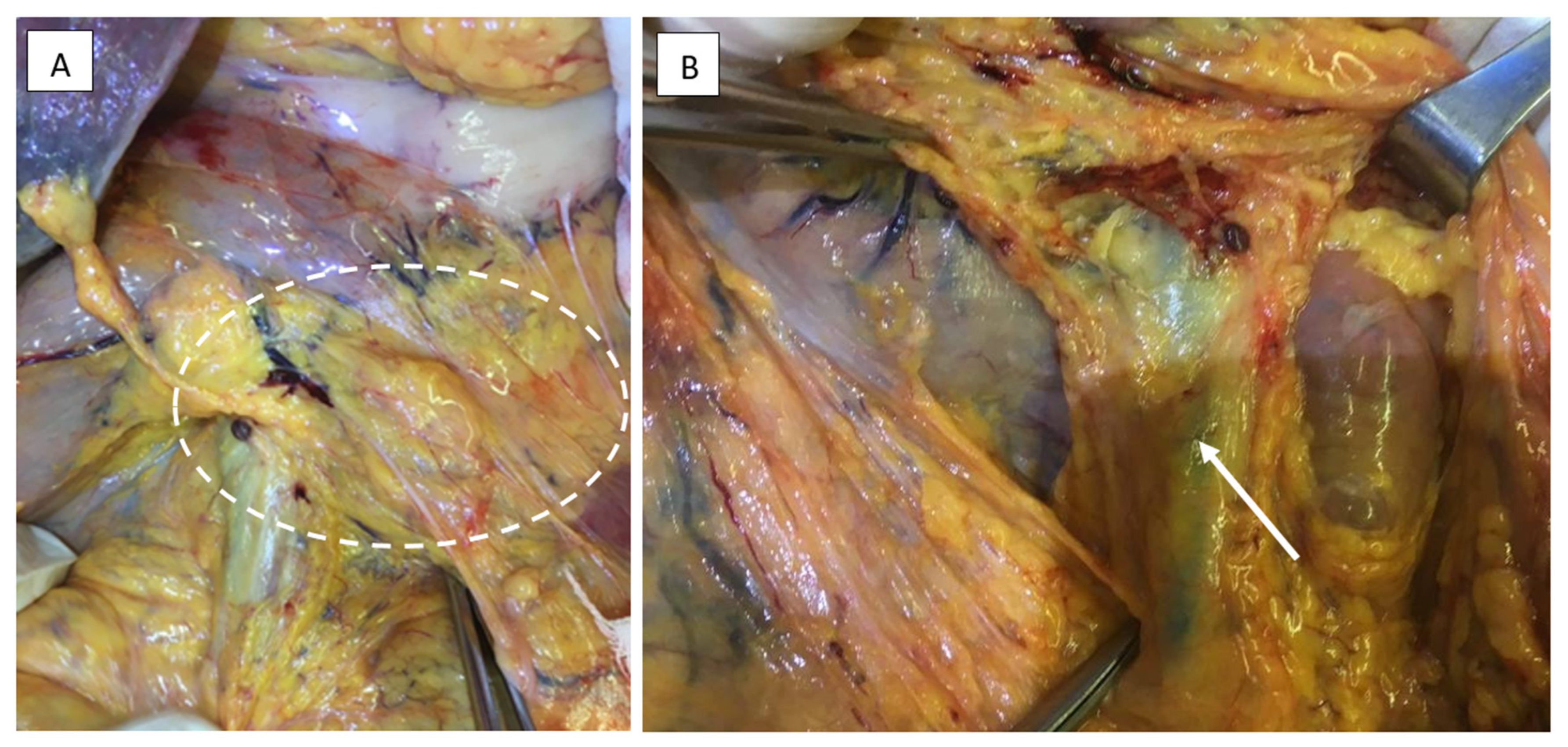
2.3.1. Surgical Dissection Planes and Landmarks: Critical Steps of Gastrectomy in the Embryonic Plane
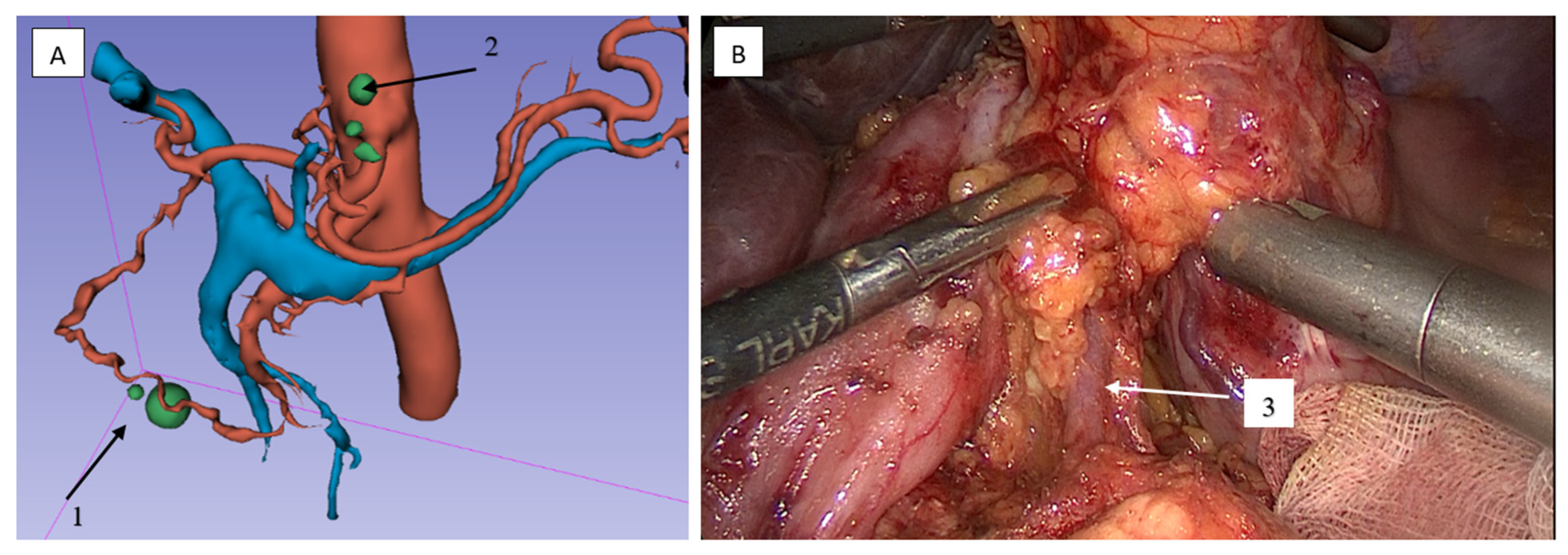
2.3.2. Lymphadenectomy of the Greater Omentum and Left Gastroepiploic Vascular Territory (Stations 4sb and 4d)
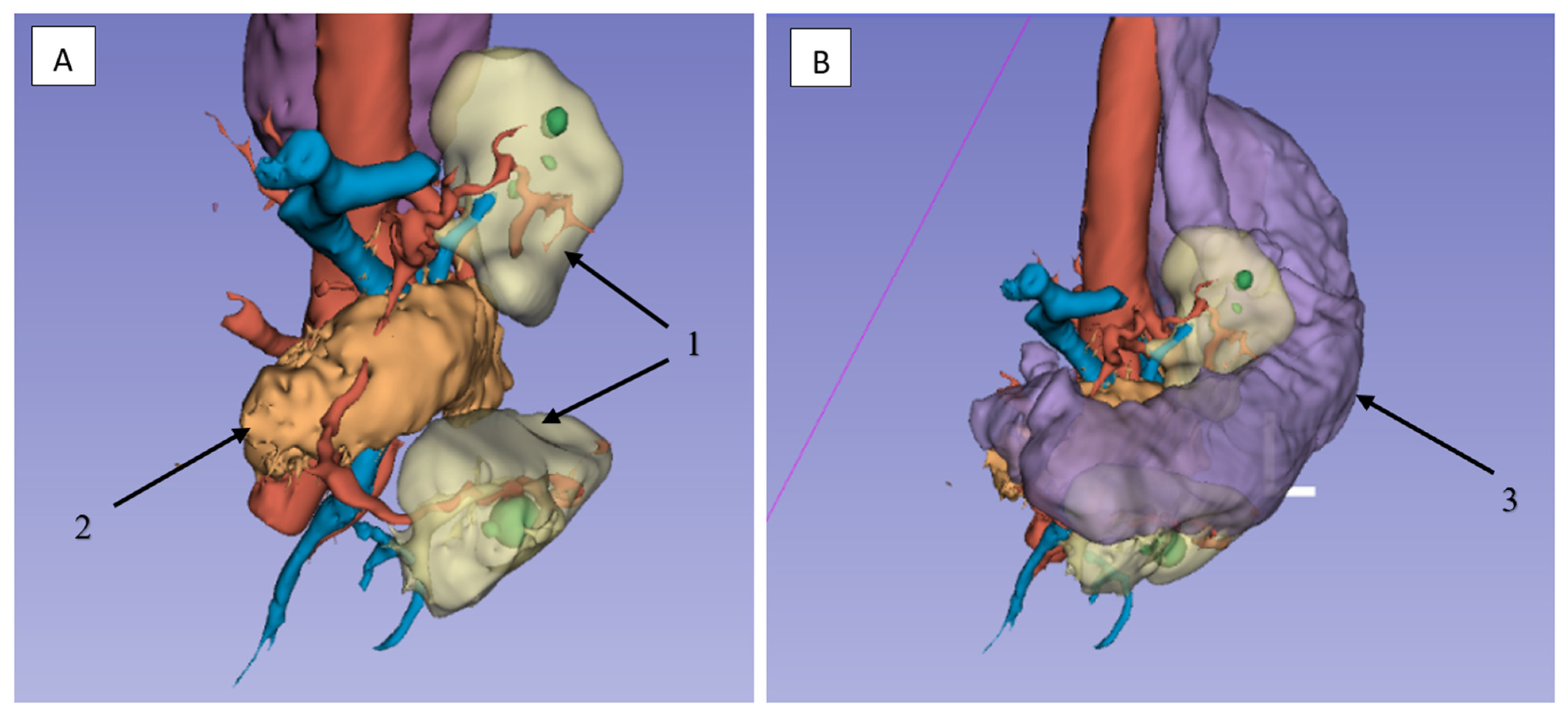
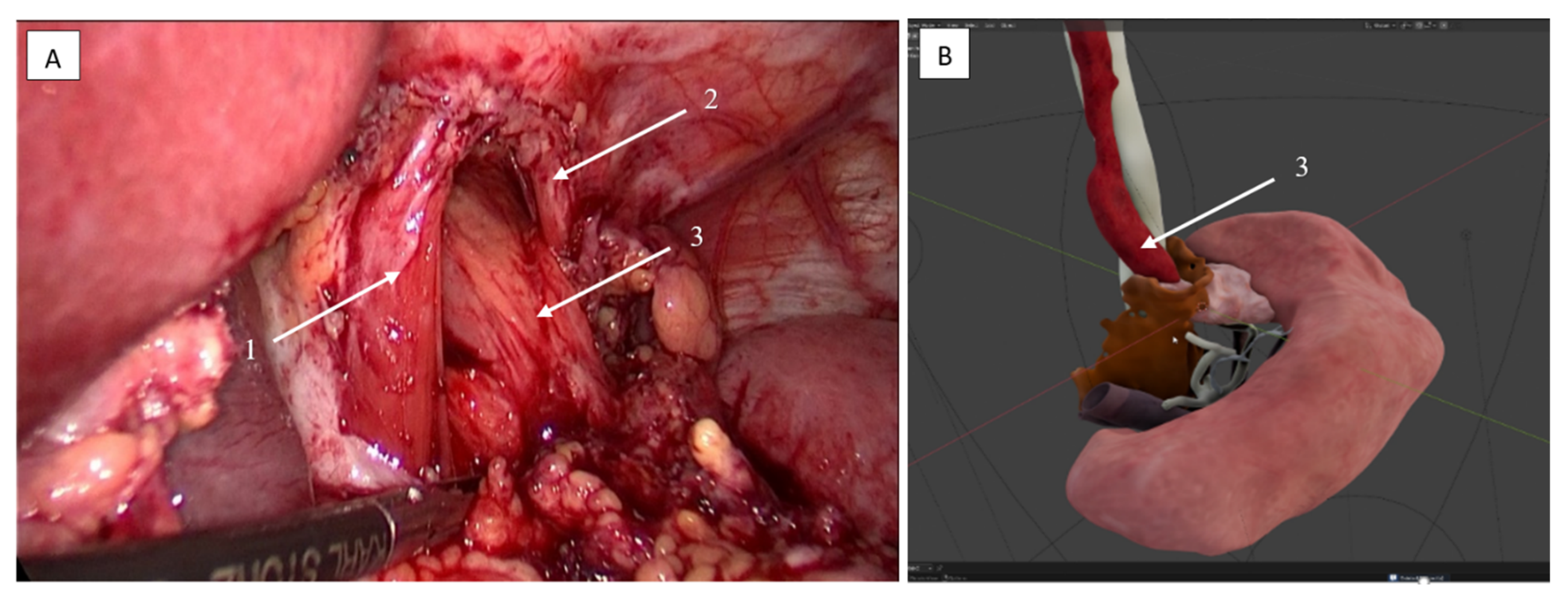
2.3.3. Lymphadenectomy of Stations 11p/11d and 10 (Splenic Artery and Hilar Nodes)
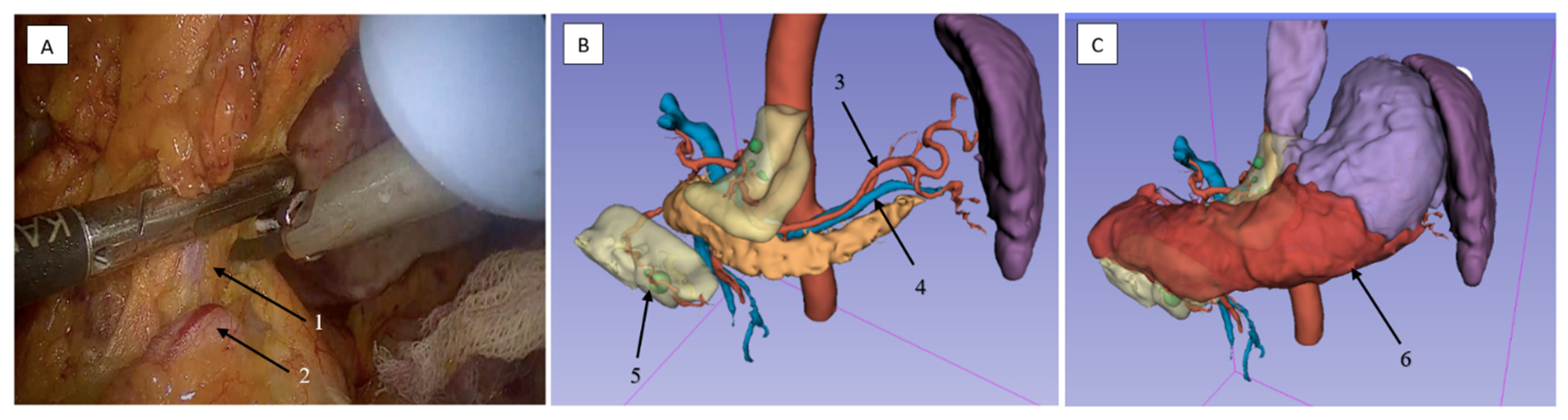
2.3.4. Lymphadenectomy of Stations 4sb and 6 Was Performed
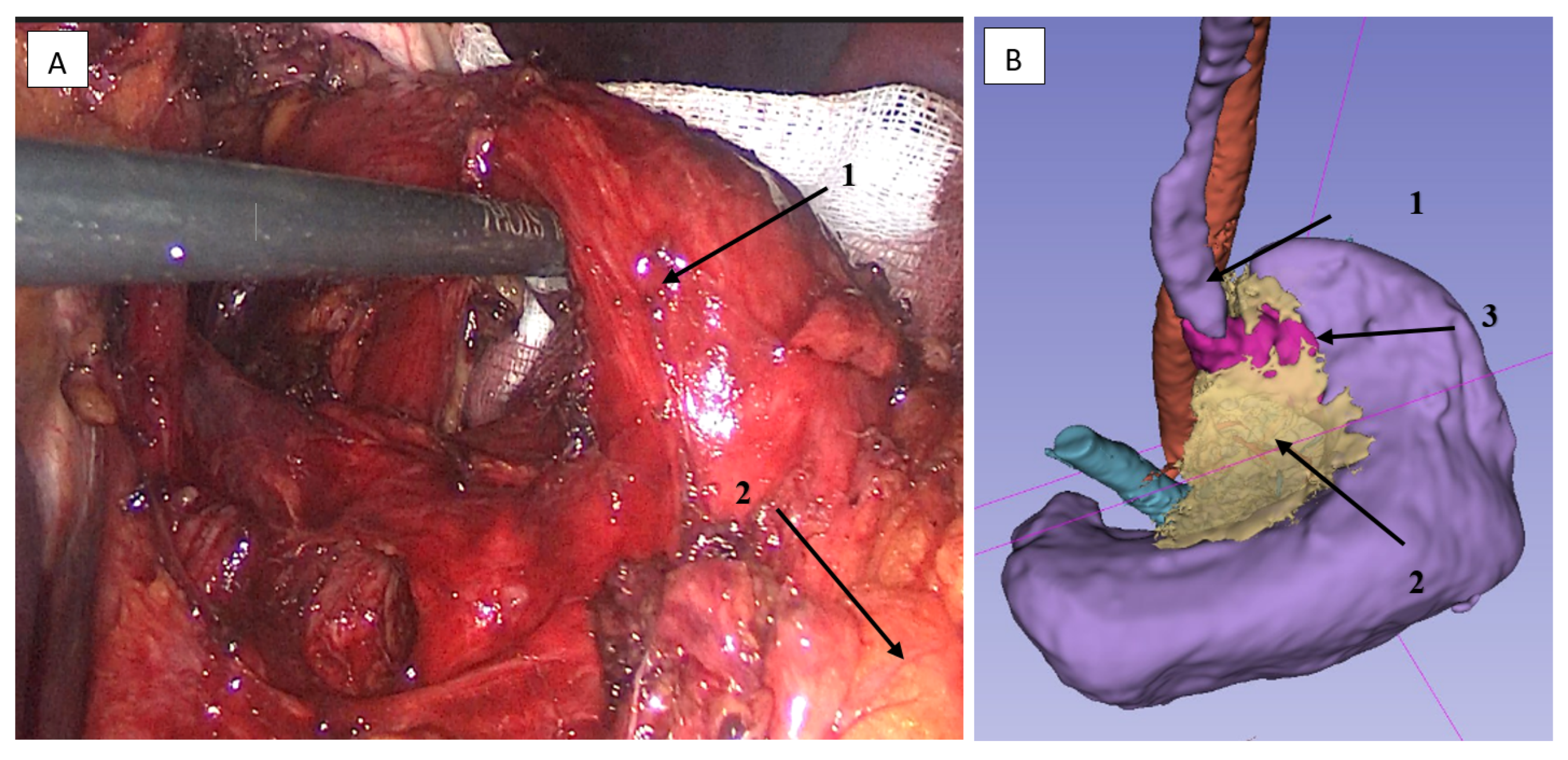
2.3.5. Lymphadenectomy of Stations 5 (Suprapyloric) and 12a (Hepatoduodenal Ligament)
2.4. Clinical Outcomes
- Stage IIIA (T2-4aN1-3M0): 16 patients (20.5%).
- Stage IIIB (T3-4bN0-3M0): 16 patients (20.5%).
- Stage IIIC (T4a-4bN2-3M0): 8 patients (10.3%).
- Stage IV (T × N × M1): 38 patients (48.7%).
2.5. Artificial Intelligence (AI) Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-Dimensional |
| ASA | American Society of Anesthesiologists |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| CT | Computed Tomography |
| DICOM | Digital Imaging and Communications in Medicine |
| ECOG | Eastern Cooperative Oncology Group |
| G1/G2/G3/G4 | Histological Grade (differentiation) |
| HU | Hounsfield Units |
| ICG | Indocyanine Green |
| ICU | Intensive Care Unit |
| JCOG | Japan Clinical Oncology Group |
| KLASS | Korean Laparoendoscopic Gastrointestinal Surgery Study Group |
| NCCN | National Comprehensive Cancer Network |
| NYHA | New York Heart Association |
| PE | Pulmonary Embolism |
| R0/R1/R2 | Resection Margins (R0: no residual tumor, R1: microscopic residual, R2: macroscopic residual) |
| TNM | Tumor, Node, Metastasis (staging system) |
| VR | Virtual Reality |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, H. Illustrated Abdominal Surgery: Based on Embryology and Anatomy of the Digestive System; Springer: Singapore, 2020; pp. 1–515. [Google Scholar] [CrossRef]
- Chernikovskiy, I.L.; Savanovich, N.V.; Smirnov, A.A.; Gavrilyukov, A.V.; Oganesyan, O.V. Топографическая Анатомия и Онкологическая Хирургия Ободочной Кишки: Новое Или Хорошо Забытое Старое? Хирургия и онкология 2017, 7, 49–55. [Google Scholar] [CrossRef][Green Version]
- Moore, K.L.; Persaud, T.V.N.; Torchia, M.G. Developing Human: Clinically Oriented Embryolog. In The Developing Human, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2016; p. 243. [Google Scholar]
- Nakamura, T.; Yamada, S.; Funatomi, T.; Takakuwa, T.; Shinohara, H.; Sakai, Y. Three-Dimensional Morphogenesis of the Omental Bursa from Four Recesses in Staged Human Embryos. J. Anat. 2020, 237, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, H.; Kurahashi, Y.; Haruta, S.; Ishida, Y.; Sasako, M. Universalization of the Operative Strategy by Systematic Mesogastric Excision for Stomach Cancer with That for Total Mesorectal Excision and Complete Mesocolic Excision Colorectal Counterparts. Ann. Gastroenterol. Surg. 2018, 2, 28–36. [Google Scholar] [CrossRef]
- Kumamoto, T.; Kurahashi, Y.; Haruta, S.; Niwa, H.; Nakanishi, Y.; Ozawa, R.; Okumura, K.; Ishida, Y.; Shinohara, H. Laparoscopic Modified Lymphadenectomy in Gastric Cancer Surgery Using Systematic Mesogastric Excision: A Novel Technique Based on a Concept. Langenbeck’s Arch. Surg. 2019, 404, 369–374. [Google Scholar] [CrossRef]
- Khatkov, I.E.; Izrailov, R.E.; Vasnev, O.S.; Pomortsev, B.A.; Semenov, N.E.; Bystrovskaya, E.V.; Schadrova, V.V. Laparoscopic Gastrectomy for Locally Advanced Gastric Cancer. Endosc. Surg. 2018, 24, 8–12. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, L.; Liao, M.; Chen, B.; Long, J.; Wu, W.; Deng, G. Laparoscopic versus Open Gastrectomy for Gastric Cancer. World J. Surg. Oncol. 2020, 18, 20. [Google Scholar] [CrossRef]
- Nusrath, S.; Rao Thammineedi, S.; Patnaik, S.C.; Saksena, A.R. ICG Fluorescence Navigation Surgery in Gastric Cancer: Role and Relevance. Indian J. Surg. Oncol. 2021, 12, 711–712. [Google Scholar] [CrossRef]
- Morales-Conde, S.; Licardie, E.; Alarcón, I.; Balla, A. Indocyanine Green (ICG) Fluorescence Guide for the Use and Indications in General Surgery: Recommendations Based on the Descriptive Review of the Literature and the Analysis of Experience. Cirugía Española 2022, 100, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.H.; Zhao, L.L.; Wu, H.L.; Zhao, D.B.; Chen, Y.T. Artificial Intelligence in Gastric Cancer: Application and Future Perspectives. World J. Gastroenterol. 2020, 26, 5408–5419. [Google Scholar] [CrossRef]
- Awiwi, M.O.; Ramanan, R.V.; Elshikh, M.; Vikram, R. Imaging of Gastric Carcinoma. Part One: Diagnosis and Staging. J. Gastrointest. Abdom. Radiol. 2021, 4, 194–205. [Google Scholar] [CrossRef]
- Ma, T.; Li, X.; Zhang, T.; Duan, M.; Ma, Q.; Cong, L.; Huang, Z.; Wang, X.; Chen, Y. Effect of Visceral Adipose Tissue on the Accuracy of Preoperative T-Staging of Gastric Cancer. Eur. J. Radiol. 2022, 155, 110488. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Shin, S.S.; Heo, S.H.; Lim, H.S.; Lim, N.Y.; Park, Y.K.; Jeong, Y.Y.; Kang, H.K. The Role of Three-Dimensional Multidetector CT Gastrography in the Preoperative Imaging of Stomach Cancer: Emphasis on Detection and Localization of the Tumor. Korean J. Radiol. 2015, 16, 80–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chernousov, A.F.; Khorobrykh, T.V.; Abdulhakimov, N.M. Laparoscopic Surgery for Treatment of Complicated Forms of Gastric Cancer. P.A. Herzen J. Oncol. 2020, 9, 18–24. [Google Scholar] [CrossRef]
- Ryu, K.W.; Park, Y.S.; Kwon, O.K.; Oh, J.; Lee, H.H.; Kong, S.H.; Son, T.; Hur, H.; Jee, Y.S.; Yoon, H.M.; et al. Korean Practice Guideline for Gastric Cancer 2018: An Evidence-Based, Multi-Disciplinary Approach. J. Gastric Cancer 2019, 19, 1–48. [Google Scholar] [CrossRef]
- Lee, H.J.; Hyung, W.J.; Yang, H.K.; Han, S.U.; Park, Y.K.; An, J.Y.; Kim, W.; Kim, H.I.; Kim, H.H.; Ryu, S.W.; et al. Short-Term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy with D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann. Surg. 2019, 270, 983–991. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2018 (5th Edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- Ajani, J.A.; D′Amico, T.A.; Bentrem, D.J.; Corvera, C.U.; Das, P.; Enzinger, P.C.; Enzler, T.; Gerdes, H.; Gibson, M.K.; Grierson, P.; et al. Gastric Cancer, Version 2.2025, NCCN Clinical Practice Guidelines In Oncology. J. Natl. Compr. Canc. Netw. 2025, 23, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef]
- Congdon, E.D.; Blumberg, R.; Henry, W. Fasciae of Fusion and Elements of the Fused Enteric Mesenteries in the Human Adult. Am. J. Anat. 1942, 70, 251–279. [Google Scholar] [CrossRef]
- Heald, R.J.; Husband, E.M.; Ryall, R.D.H. The Mesorectum in Rectal Cancer Surgery—the Clue to Pelvic Recurrence? Br. J. Surg. 1982, 69, 613–616. [Google Scholar] [CrossRef]
- Karachun, А.М.; Kashchenko, V.A.; Pelipas, Y.V. Technical aspects of laparoscopic gastrectomy for gastric cancer. Nauchno-prakticheskiy zhurnal federal’nogo mediko-biologicheskogo agentstva. Klin. Bol′nitsa 2016, 16, 6–20. [Google Scholar]
- Pilat, T.L.; Shaymardanov, I.V.; Novikov, G.; Hidiyatov, I.R.; Shaikhutdinov, N.G.; Gazizov, R.A.; Battalov, I.M. Эффективность Применения Детоксикационного Энтерального Лечебного Питания у Онкологических Паллиативных Пациентов. Медицинский Совет 2024, 21, 83–95. [Google Scholar] [CrossRef]
- Jongerius, E.J.; Boerma, D.; Seldenrijk, K.A.; Meijer, S.L.; Scheepers, J.J.G.; Smedts, F.; Lagarde, S.M.; Balague Ponz, O.; van Berge Henegouwen, M.I.; van Sandick, J.W.; et al. Role of Omentectomy as Part of Radical Surgery for Gastric Cancer. Br. J. Surg. 2016, 103, 1497–1503. [Google Scholar] [CrossRef]
- Sano, T.; Sasako, M.; Mizusawa, J.; Yamamoto, S.; Katai, H.; Yoshikawa, T.; Nashimoto, A.; Ito, S.; Kaji, M.; Imamura, H.; et al. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann. Surg. 2017, 265, 277–283. [Google Scholar] [CrossRef]
- Katai, H.; Sasako, M.; Fukuda, H.; Nakamura, K.; Hiki, N.; Saka, M.; Yamaue, H.; Yoshikawa, T.; Kojima, K. Safety and Feasibility of Laparoscopy-Assisted Distal Gastrectomy with Suprapancreatic Nodal Dissection for Clinical Stage i Gastric Cancer: A Multicenter Phase II Trial (JCOG 0703). Gastric Cancer 2010, 13, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Hyung, W.J.; Cho, G.S.; Kim, M.C.; Han, S.U.; Kim, W.; Ryu, S.W.; Lee, H.J.; Song, K.Y. Morbidity and Mortality of Laparoscopic Gastrectomy versus Open Gastrectomy for Gastric Cancer: An Interim Report-a Phase III Multicenter, Prospective, Randomized Trial (KLASS Trial). Ann. Surg. 2010, 251, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramos, D.; Miralles-Tena, J.M.; Cuesta, M.A.; Escrig-Sos, J.; van der Peet, D.; Hoashi, J.S.; Salvador-Sanchis, J.L. Laparoscopy versus Open Surgery for Advanced and Respectable Gastric Cancer: A Meta-Analysis. Rev. Esp. Enfermedades Dig. 2011, 103, 133–141. [Google Scholar] [CrossRef]
- Chan, B.Y.O.; Yau, K.K.W.; Chan, C.K.O. Totally Laparoscopic versus Open Gastrectomy for Advanced Gastric Cancer: A Matched Retrospective Cohort Study. Hong Kong Med. J. 2019, 25, 30–37. [Google Scholar] [CrossRef]
- Qi, X.; Liu, Y.; Wang, W.; Cai, D.; Li, W.; Hui, J.; Liu, C.; Zhao, Y.; Li, G. Management of Advanced Gastric Cancer: An Overview of Major Findings from Meta-Analysis. Oncotarget 2016, 7, 78180–78205. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Doki, Y.; Mizusawa, J.; Terashima, M.; Katai, H.; Yoshikawa, T.; Kimura, Y.; Takiguchi, S.; Nishida, Y.; Fukushima, N.; et al. Bursectomy versus Omentectomy Alone for Resectable Gastric Cancer (JCOG1001): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet Gastroenterol. Hepatol. 2018, 3, 460–468. [Google Scholar] [CrossRef]
- Chang, J.S.; Kim, K.H.; Yoon, H.I.; Hyung, W.J.; Rha, S.Y.; Kim, H.S.; Lee, Y.C.; Lim, J.S.; Noh, S.H.; Koom, W.S. Locoregional Relapse after Gastrectomy with D2 Lymphadenectomy for Gastric Cancer. Br. J. Surg. 2017, 104, 877–884. [Google Scholar] [CrossRef]
- Peparini, N.; Caronna, R.; Chirletti, P. The “Meso” of the Rectum and the “Meso” of the Pancreas: Similar Terms but Distinct Concepts in Surgical Oncology. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 548–551. [Google Scholar] [CrossRef]
- Netter, F.H. Netter Atlas of Human Anatomy: Classic Regional Approach-Ebook; Elsevier Health Sciences: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Nikolaev, A.V. Topographic Anatomy and Operative Surgery, 3rd ed.; Geotar: Moscow, Russia, 2019. [Google Scholar]
- Inaki, N.; Etoh, T.; Ohyama, T.; Uchiyama, K.; Katada, N.; Koeda, K.; Yoshida, K.; Takagane, A.; Kojima, K.; Sakuramoto, S.; et al. A Multi-Institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J. Surg. 2015, 39, 2734–2741. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Katai, H.; Mizusawa, J.; Yoshikawa, T.; Ando, M.; Terashima, M.; Ito, S.; Takagi, M.; Takagane, A.; Ninomiya, M.; et al. A Phase III Study of Laparoscopy-Assisted versus Open Distal Gastrectomy with Nodal Dissection for Clinical Stage IA/IB Gastric Cancer (JCOG0912). Jpn. J. Clin. Oncol. 2013, 43, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, K.; Yang, H.K.; Mizusawa, J.; Kim, Y.W.; Terashima, M.; Han, S.U.; Iwasaki, Y.; Hyung, W.J.; Takagane, A.; Park, D.J.; et al. Gastrectomy plus Chemotherapy versus Chemotherapy Alone for Advanced Gastric Cancer with a Single Non-Curable Factor (REGATTA): A Phase 3, Randomised Controlled Trial. Lancet Oncol. 2016, 17, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rudloff, U.; Langan, R.C.; Mullinax, J.E.; Beane, J.D.; Steinberg, S.M.; Beresnev, T.; Webb, C.C.; Walker, M.; Toomey, M.A.; Schrump, D.; et al. Impact of Maximal Cytoreductive Surgery plus Regional Heated Intraperitoneal Chemotherapy (HIPEC) on Outcome of Patients with Peritoneal Carcinomatosis of Gastric Origin: Results of the GYMSSA Trial. J. Surg. Oncol. 2014, 110, 275–284. [Google Scholar] [CrossRef]
- Kapuria, S.; Bonyun, J.; Kulkarni, Y.; Ikoma, N.; Chinchali, S.; Alambeigi, F. Robot-enabled machine learning-based diagnosis of gastric cancer polyps using partial surface tactile imaging. In Proceedings of the 2024 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Abu Dhabi, United Arab Emirates, 14–18 October 2024; pp. 2360–2365. [Google Scholar]

| N = 78 (%) | |
|---|---|
| Mean age, years (confidence interval—CI) | 60 (58–62) |
| Gender Distribution | n (%) |
| Male | 53 (67.9) |
| Female | 25 (32.1) |
| ASA Physical Status | n (%) |
| ASA I | 15 (19.2) |
| ASA II | 22 (28.2) |
| ASA III | 41 (52.6) |
| ECOG Performance Status | n (%) |
| 0 | 11 (14.1) |
| 1 | 52 (66.7) |
| 2 | 15 (19.2) |
| Comorbidity Distribution | n (%) |
| Cardiovascular comorbidities (hypertension, coronary artery disease) stratified using NYHA guidelines | 72 (92.3) |
| Type 2 diabetes mellitus | 20 (25.6) |
| Peptic ulcer disease (gastric and duodenal ulcers) | 9 (11.5) |
| Chronic obstructive pulmonary disease | 7 (9.0) |
| Mean BMI (CI) | 27.1 (23.4–33.2) |
| Tumor Localization | n (%) |
| Gastric cardia | 4 (5.1) |
| Gastric body | 4 (5.1) |
| Antrum/pyloric region | 48 (61.5) |
| Subtotal involvement | 15 (19.2) |
| Total gastric involvement | 7 (9.0) |
| Life-Threatening Complications Requiring Emergency Intervention | n (%) |
| Bleeding | 22 (28.2) |
| Stenosis | 41 (52.6) |
| Combined gastric outlet obstruction and recurrent bleeding | 9 (11.5) |
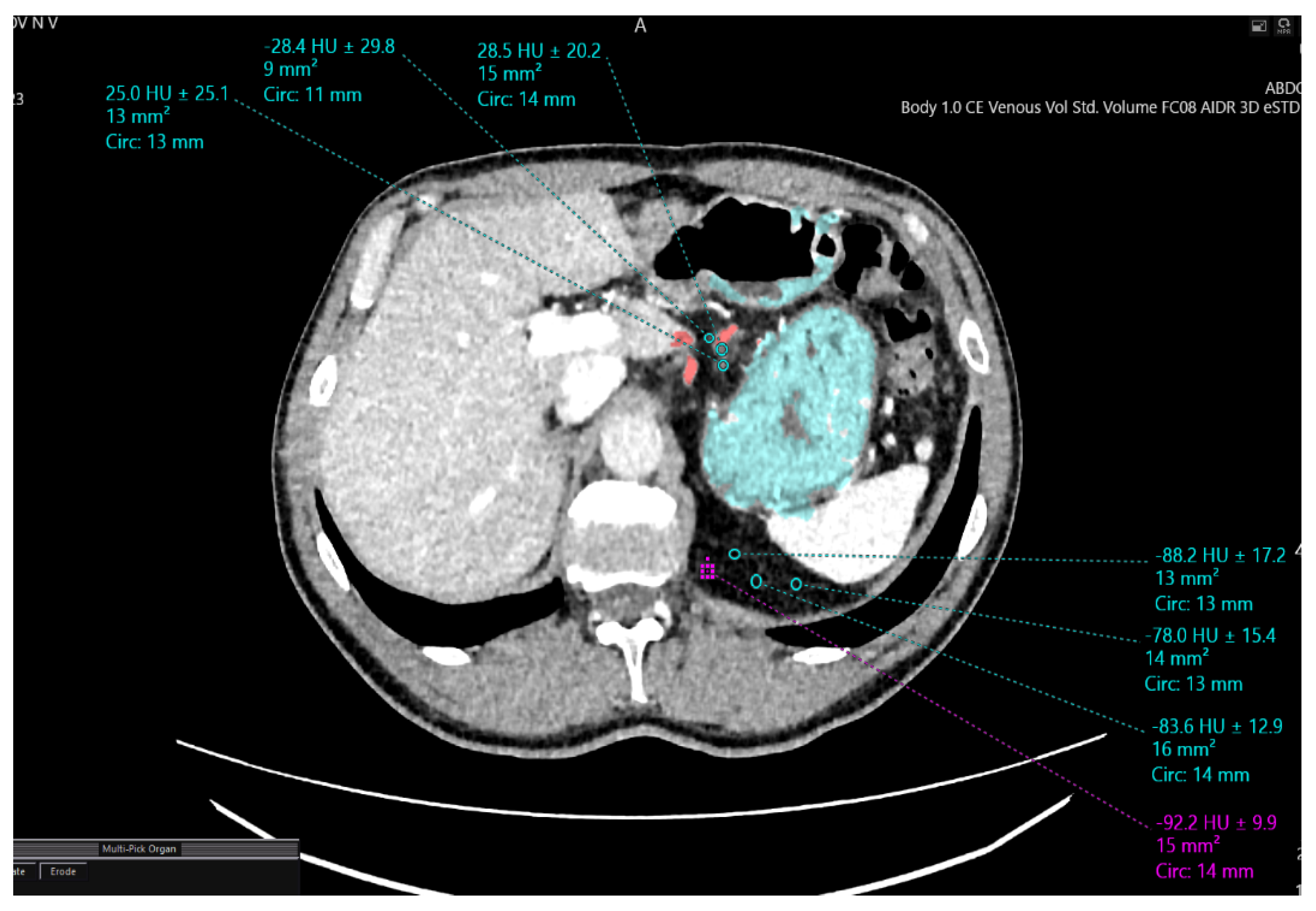
| Densitometric Density Points | Mesogastric Layer Along the Left Gastric Artery (HU) | Mesogastric Layer in the Region of Retrogastric Tissue (HU) |
|---|---|---|
| 1 | −88.2 | −28.2 |
| 2 | −78.8 | −26.6 |
| 3 | −92.9 | −27.7 |
| Surgical Procedure | Cases, n (%) | |
|---|---|---|
| Total gastrectomy with abdominal esophageal resection | 22 (28.2%) | |
| Distal subtotal gastrectomy | Billroth I reconstruction | 39 (50.0%) |
| Billroth II reconstruction | 9 (11.5%) | |
| Proximal gastrectomy with abdominal and lower thoracic esophageal resection | 8 (10.3%) | |
| Locally Invaded Structures | Cases, n (%) | |
| Abdominal esophagus | 11 (14.1%) | |
| Greater/lesser omentum | 46 (59.0%) | |
| Pancreatic capsule | 4 (5.1%) | |
| Splenic hilum (direct invasion) | 4 (5.1%) | |
| Duodenum | 7 (9.0%) | |
| Indicator | Number of Observations (N = 78), n (%) |
|---|---|
| Mean duration of surgery, min (CI) | 259 (251–266) |
| Mean intraoperative blood loss, mL (CI) | 185 (177–192) |
| Number of conversions to laparotomy, n (%) | 6 (7.7) |
| Mean duration of ICU stay, days (CI) | 2 (1–2) |
| Mean duration of narcotic analgesia, days (CI) | 2 (2–3) |
| Mean hospital stay, days (CI) | 8 (7–9) |
| Total number of postoperative complications, n (%) | 28 (35.9) |
| Postoperative mortality, n (%) | 4 (5.1) |
| Mean number of removed lymph nodes (CI) | 22 (21–22) |
| Radicality, n (%) | |
| R0 | 58 (74.4) |
| R1 | 16 (20.5) |
| R2 | 4 (5.1) |
| Tumor type, n (%) | |
| Adenocarcinoma | 65 (83.3) |
| Signet ring cell carcinoma | 13 (16.7) |
| Degree of histological differentiation, n (%) | |
| High grade (G1) | 9 (11.5) |
| Moderate grade (G2) | 14 (17.9) |
| Low grade (G3) | 33 (42.3) |
| Undifferentiated carcinoma (G4) | 22 (28.2) |
| TNM stage, n (%) | |
| IIIA | 16 (20.5) |
| IIIB | 16 (20.5) |
| IIIC IV | 8 (10.3) 38 (48.7) |
| Indicator | Method | Tested Characteristic | p-Value |
|---|---|---|---|
| Duration of surgery | Mann–Whitney U test | Difference between groups | 0.047 |
| Conversion to laparotomy | 0.070 | ||
| Postoperative mortality | 0.189 | ||
| Number of removed lymph nodes | <0.00001 | ||
| Duration of surgery | Brown–Forsythe test | Difference in variances | 0.365 |
| Conversion to laparotomy | 0.068 | ||
| Postoperative mortality | 0.187 | ||
| Number of removed lymph nodes | 0.564 | ||
| Radicality R0 vs. R1 | Fisher’s exact test | Categorical association | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khorobrykh, T.; Agadzhanov, V.; Grachalov, A.; Ivashov, I.; Spartak, A.; Romanovskii, A.; Drach, Y.; Kharkov, D. Topographic and Anatomical Landmarks of Key Points in Embryologically Guided Surgery for Locally Advanced Gastric Cancer Using Computer-Assisted 3D Navigation. J. Clin. Med. 2025, 14, 6282. https://doi.org/10.3390/jcm14176282
Khorobrykh T, Agadzhanov V, Grachalov A, Ivashov I, Spartak A, Romanovskii A, Drach Y, Kharkov D. Topographic and Anatomical Landmarks of Key Points in Embryologically Guided Surgery for Locally Advanced Gastric Cancer Using Computer-Assisted 3D Navigation. Journal of Clinical Medicine. 2025; 14(17):6282. https://doi.org/10.3390/jcm14176282
Chicago/Turabian StyleKhorobrykh, Tatiana, Vadim Agadzhanov, Anton Grachalov, Ivan Ivashov, Alexey Spartak, Artem Romanovskii, Yaroslav Drach, and Daniil Kharkov. 2025. "Topographic and Anatomical Landmarks of Key Points in Embryologically Guided Surgery for Locally Advanced Gastric Cancer Using Computer-Assisted 3D Navigation" Journal of Clinical Medicine 14, no. 17: 6282. https://doi.org/10.3390/jcm14176282
APA StyleKhorobrykh, T., Agadzhanov, V., Grachalov, A., Ivashov, I., Spartak, A., Romanovskii, A., Drach, Y., & Kharkov, D. (2025). Topographic and Anatomical Landmarks of Key Points in Embryologically Guided Surgery for Locally Advanced Gastric Cancer Using Computer-Assisted 3D Navigation. Journal of Clinical Medicine, 14(17), 6282. https://doi.org/10.3390/jcm14176282






