Comparison of Hemodynamic Management by Hypotension Prediction Index or Goal-Directed Therapy in Radical Cystectomies: A Prospective Observational Study
Abstract
1. Introduction
2. Methods
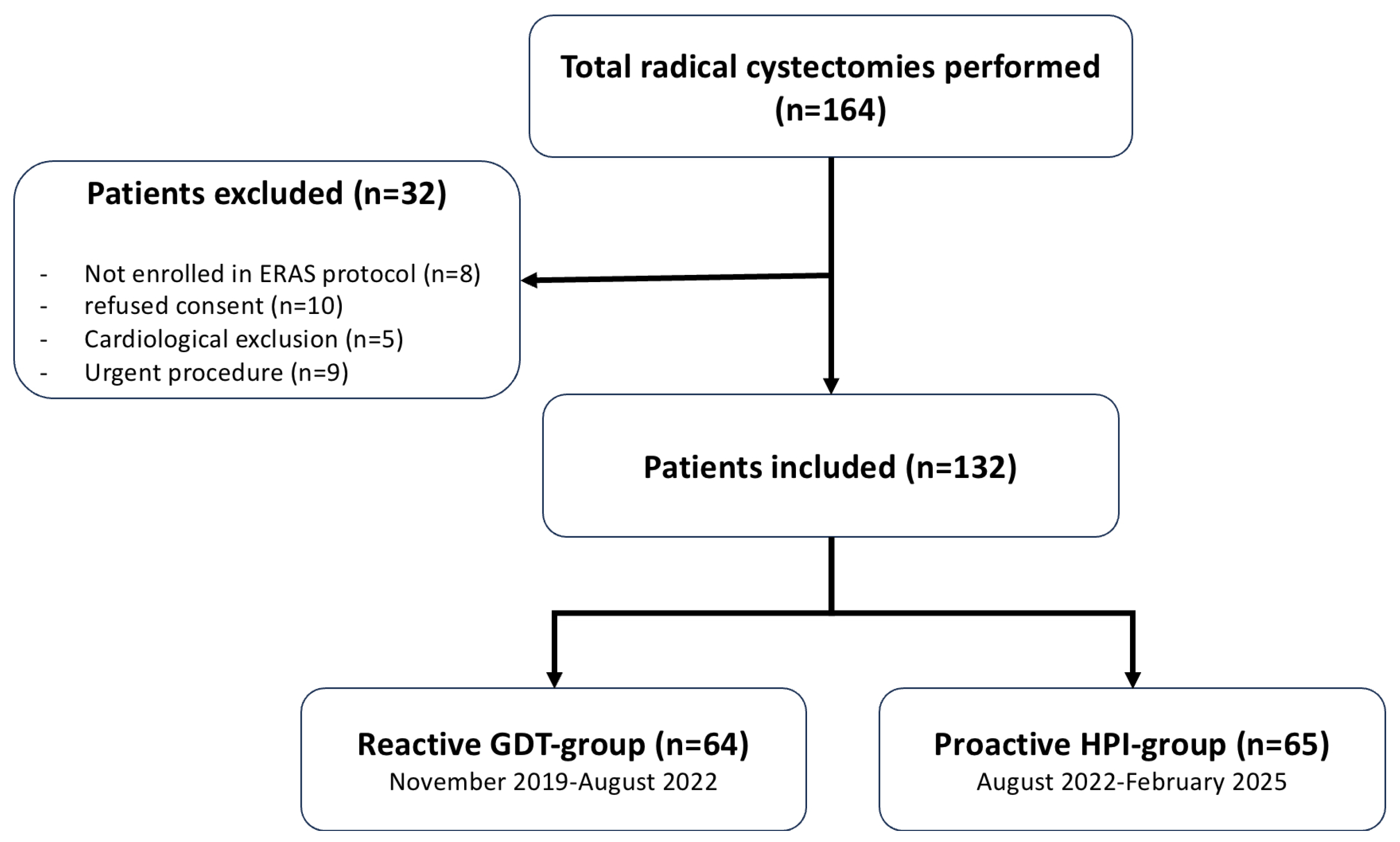
2.1. Study Design and Patients
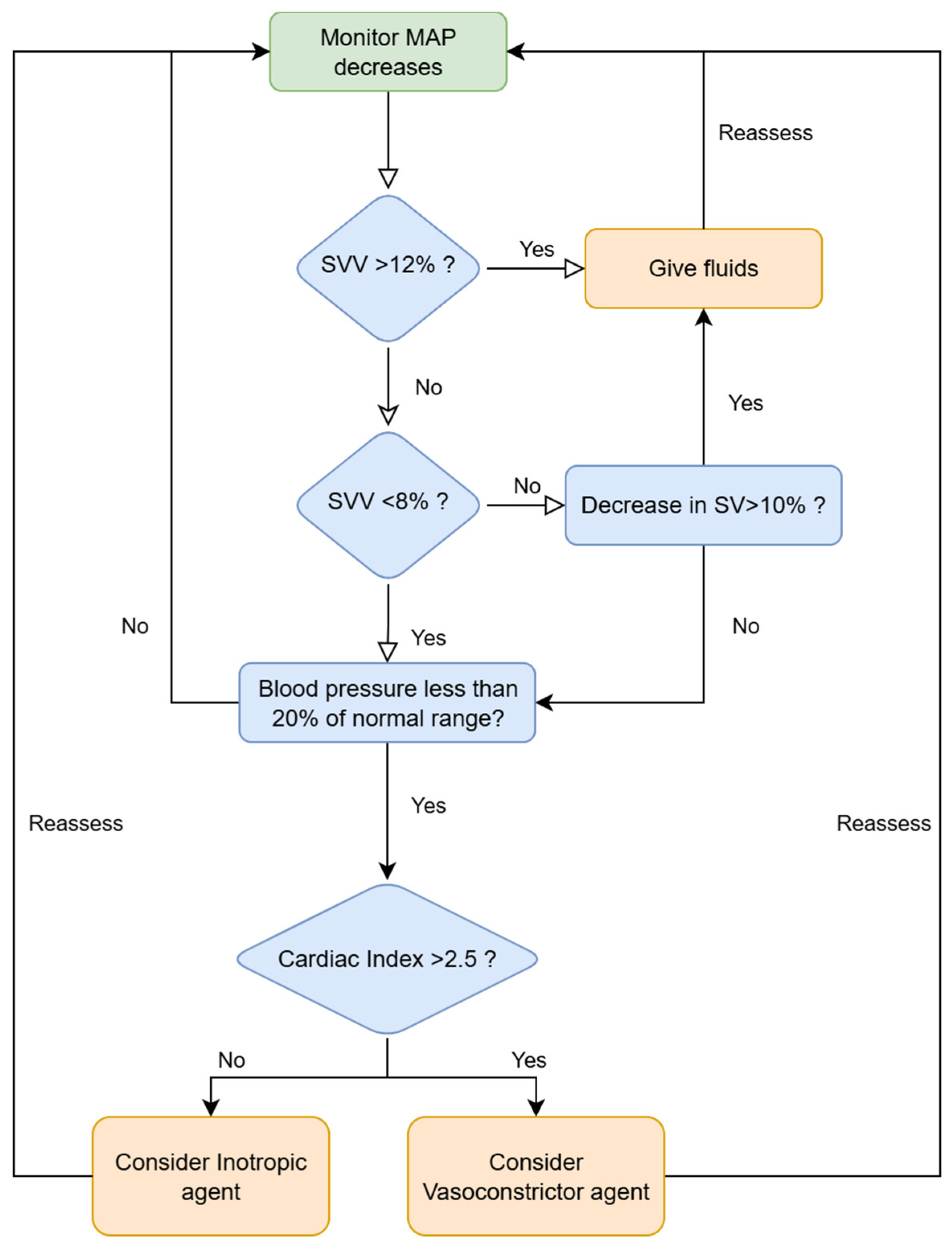
2.2. ERAS Protocol and Intraoperative Procedures
2.3. Data Collection
2.4. Aim of the Study
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wesselink, E.M.; Kappen, T.H.; Torn, H.M.; Slooter, A.J.C.; van Klei, W.A. Intraoperative Hypotension and the Risk of Postoperative Adverse Outcomes: A Systematic Review. Br. J. Anaesth. 2018, 121, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Devereaux, P.J.; Garg, A.X.; Kurz, A.; Turan, A.; Rodseth, R.N.; Cywinski, J.; Thabane, L.; Sessler, D.I. Relationship between Intraoperative Mean Arterial Pressure and Clinical Outcomes after Noncardiac Surgery: Toward an Empirical Definition of Hypotension. Anesthesiology 2013, 119, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, V.; Maheshwari, K.; Yang, D.; Mascha, E.J.; Singh, A.; Sessler, D.I.; Kurz, A. Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery: A Retrospective Cohort Analysis. Anesthesiology 2017, 126, 47–65. [Google Scholar] [CrossRef]
- Larsson, A.; Sjöquist, J. Novel Latex Agglutination Method with Chicken Anti-Protein A for Detection of Staphylococcus Aureus Infections. J. Clin. Microbiol. 1989, 27, 2856–2857. [Google Scholar] [CrossRef]
- Monk, T.G.; Bronsert, M.R.; Henderson, W.G.; Mangione, M.P.; Sum-Ping, S.T.J.; Bentt, D.R.; Nguyen, J.D.; Richman, J.S.; Meguid, R.A.; Hammermeister, K.E. Association between Intraoperative Hypotension and Hypertension and 30-Day Postoperative Mortality in Noncardiac Surgery. Anesthesiology 2015, 123, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Hallqvist, L.; Mårtensson, J.; Granath, F.; Sahlén, A.; Bell, M. Intraoperative Hypotension Is Associated with Myocardial Damage in Noncardiac Surgery: An Observational Study. Eur. J. Anaesthesiol. 2016, 33, 450–456. [Google Scholar] [CrossRef]
- Bijker, J.B.; Persoon, S.; Peelen, L.M.; Moons, K.G.M.; Kalkman, C.J.; Kappelle, L.J.; van Klei, W.A. Intraoperative Hypotension and Perioperative Ischemic Stroke after General Surgery: A Nested Case-Control Study. Anesthesiology 2012, 116, 658–664. [Google Scholar] [CrossRef]
- Sessler, D.I.; Khanna, A.K. Perioperative Myocardial Injury and the Contribution of Hypotension. Intensive Care Med. 2018, 44, 811–822. [Google Scholar] [CrossRef]
- van Waes, J.A.R.; van Klei, W.A.; Wijeysundera, D.N.; van Wolfswinkel, L.; Lindsay, T.F.; Beattie, W.S. Association between Intraoperative Hypotension and Myocardial Injury after Vascular Surgery. Anesthesiology 2016, 124, 35–44. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Cecconi, M.; Rhodes, A. A Systematic Review and Meta-Analysis on the Use of Preemptive Hemodynamic Intervention to Improve Postoperative Outcomes in Moderate and High-Risk Surgical Patients. Anesth. Analg. 2011, 112, 1392–1402. [Google Scholar] [CrossRef]
- Brienza, N.; Giglio, M.T.; Marucci, M.; Fiore, T. Does Perioperative Hemodynamic Optimization Protect Renal Function in Surgical Patients? A Meta-Analytic Study. Crit. Care Med. 2009, 37, 2079–2090. [Google Scholar] [CrossRef]
- Giglio, M.T.; Marucci, M.; Testini, M.; Brienza, N. Goal-Directed Haemodynamic Therapy and Gastrointestinal Complications in Major Surgery: A Meta-Analysis of Randomized Controlled Trials. Br. J. Anaesth. 2009, 103, 637–646. [Google Scholar] [CrossRef]
- Dalfino, L.; Giglio, M.T.; Puntillo, F.; Marucci, M.; Brienza, N. Haemodynamic Goal-Directed Therapy and Postoperative Infections: Earlier Is Better. A Systematic Review and Meta-Analysis. Crit. Care 2011, 15, R154. [Google Scholar] [CrossRef] [PubMed]
- Cerantola, Y.; Valerio, M.; Persson, B.; Jichlinski, P.; Ljungqvist, O.; Hubner, M.; Kassouf, W.; Muller, S.; Baldini, G.; Carli, F.; et al. Guidelines for Perioperative Care after Radical Cystectomy for Bladder Cancer: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations. Clin. Nutr. 2013, 32, 879–887. [Google Scholar] [CrossRef]
- Brusasco, C.; Di Domenico, A.; Ennas, M.; Benelli, A.; Dotta, F.; Tosi, M.; Manfredi, M.; Calcagno, T.; Campodonico, F.; Germinale, F.; et al. Application of a Protocol for Enhanced Recovery after Radical Cystectomy: A before-and-after Cohort Study. World J. Urol. 2023, 41, 2273–2280. [Google Scholar] [CrossRef]
- Makaryus, R.; Miller, T.E.; Gan, T.J. Current Concepts of Fluid Management in Enhanced Recovery Pathways. Br. J. Anaesth. 2018, 120, 376–383. [Google Scholar] [CrossRef]
- Wijnberge, M.; Geerts, B.F.; Hol, L.; Lemmers, N.; Mulder, M.P.; Berge, P.; Schenk, J.; Terwindt, L.E.; Hollmann, M.W.; Vlaar, A.P.; et al. Effect of a Machine Learning-Derived Early Warning System for Intraoperative Hypotension vs Standard Care on Depth and Duration of Intraoperative Hypotension During Elective Noncardiac Surgery: The HYPE Randomized Clinical Trial. JAMA 2020, 323, 1052–1060. [Google Scholar] [CrossRef]
- Cella, L.; Basile, G.; Moretto, S.; Paciotti, M.; Hurle, R.; Lughezzani, G.; Avolio, P.P.; Piccolini, A.; Mancon, S.; Lazzeri, M.; et al. Robotic Assisted vs Open Radical Cystectomy: An Updated Systematic Review and Meta-Analysis. J. Robot. Surg. 2024, 18, 277. [Google Scholar] [CrossRef]
- Maheshwari, K.; Turan, A.; Mao, G.; Yang, D.; Niazi, A.K.; Agarwal, D.; Sessler, D.I.; Kurz, A. The Association of Hypotension during Non-Cardiac Surgery, before and after Skin Incision, with Postoperative Acute Kidney Injury: A Retrospective Cohort Analysis. Anaesthesia 2018, 73, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Tsoumpa, M.; Kyttari, A.; Matiatou, S.; Tzoufi, M.; Griva, P.; Pikoulis, E.; Riga, M.; Matsota, P.; Sidiropoulou, T. The Use of the Hypotension Prediction Index Integrated in an Algorithm of Goal Directed Hemodynamic Treatment during Moderate and High-Risk Surgery. J. Clin. Med. 2021, 10, 5884. [Google Scholar] [CrossRef] [PubMed]
- de Keijzer, I.N.; Vos, J.J.; Scheeren, T.W.L. Hypotension Prediction Index: From Proof-of-Concept to Proof-of-Feasibility. J. Clin. Monit. Comput. 2020, 34, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Murabito, P.; Astuto, M.; Sanfilippo, F.; La Via, L.; Vasile, F.; Basile, F.; Cappellani, A.; Longhitano, L.; Distefano, A.; Li Volti, G. Proactive Management of Intraoperative Hypotension Reduces Biomarkers of Organ Injury and Oxidative Stress during Elective Non-Cardiac Surgery: A Pilot Randomized Controlled Trial. J. Clin. Med. 2022, 11, 392. [Google Scholar] [CrossRef]
- Rellum, S.R.; Noteboom, S.H.; van der Ster, B.J.P.; Schuurmans, J.; Kho, E.; Vlaar, A.P.J.; Schenk, J.; Veelo, D.P. The Hypotension Prediction Index versus Mean Arterial Pressure in Predicting Intraoperative Hypotension: A Clinical Perspective. Eur. J. Anaesthesiol. EJA 2025, 42, 527. [Google Scholar] [CrossRef]
- Mulder, M.P.; Harmannij-Markusse, M.; Fresiello, L.; Donker, D.W.; Potters, J.-W. Hypotension Prediction Index Is Equally Effective in Predicting Intraoperative Hypotension during Noncardiac Surgery Compared to a Mean Arterial Pressure Threshold: A Prospective Observational Study. Anesthesiology 2024, 141, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Michard, F.; Biais, M.; Futier, E.; Romagnoli, S. Mirror, Mirror on the Wall, Who Is Going to Become Hypotensive? Eur. J. Anaesthesiol. 2023, 40, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.B.; Kaye, A.D.; Tong, Y.; Belani, K.; Urman, R.D.; Hoffman, C.; Liu, H. Goal-Directed Fluid Therapy in the Perioperative Setting. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, S29–S34. [Google Scholar] [CrossRef]
- Zhu, A.C.-C.; Agarwala, A.; Bao, X. Perioperative Fluid Management in the Enhanced Recovery after Surgery (ERAS) Pathway. Clin. Colon. Rectal. Surg. 2019, 32, 114–120. [Google Scholar] [CrossRef]
- Simmons, J.W.; Dobyns, J.B.; Paiste, J. Enhanced Recovery After Surgery: Intraoperative Fluid Management Strategies. Surg. Clin. N. Am. 2018, 98, 1185–1200. [Google Scholar] [CrossRef]
- Cannesson, M.; Ramsingh, D.; Rinehart, J.; Demirjian, A.; Vu, T.; Vakharia, S.; Imagawa, D.; Yu, Z.; Greenfield, S.; Kain, Z. Perioperative Goal-Directed Therapy and Postoperative Outcomes in Patients Undergoing High-Risk Abdominal Surgery: A Historical-Prospective, Comparative Effectiveness Study. Crit. Care 2015, 19, 261. [Google Scholar] [CrossRef]
- Ripollés-Melchor, J.; Ruiz-Escobar, A.; Fernández-Valdes-Bango, P.; Lorente, J.V.; Jiménez-López, I.; Abad-Gurumeta, A.; Carrasco-Sánchez, L.; Monge-García, M.I. Hypotension Prediction Index: From Reactive to Predictive Hemodynamic Management, the Key to Maintaining Hemodynamic Stability. Front. Anesthesiol. 2023, 2, 1138175. [Google Scholar] [CrossRef]
- Sidiropoulou, T.; Tsoumpa, M.; Griva, P.; Galarioti, V.; Matsota, P. Prediction and Prevention of Intraoperative Hypotension with the Hypotension Prediction Index: A Narrative Review. J. Clin. Med. 2022, 11, 5551. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, C.D.; Wischermann, J.M.; Fassbender, P.; Bischoff, P.; Frey, U.H. Hemodynamic Monitoring with Hypotension Prediction Index versus Arterial Waveform Analysis Alone and Incidence of Perioperative Hypotension. Acta Anaesthesiol. Scand. 2021, 65, 1404–1412. [Google Scholar] [CrossRef]
- van der Ven, W.H.; Veelo, D.P.; Wijnberge, M.; van der Ster, B.J.P.; Vlaar, A.P.J.; Geerts, B.F. One of the First Validations of an Artificial Intelligence Algorithm for Clinical Use: The Impact on Intraoperative Hypotension Prediction and Clinical Decision-Making. Surgery 2021, 169, 1300–1303. [Google Scholar] [CrossRef]
- Schneck, E.; Schulte, D.; Habig, L.; Ruhrmann, S.; Edinger, F.; Markmann, M.; Habicher, M.; Rickert, M.; Koch, C.; Sander, M. Hypotension Prediction Index Based Protocolized Haemodynamic Management Reduces the Incidence and Duration of Intraoperative Hypotension in Primary Total Hip Arthroplasty: A Single Centre Feasibility Randomised Blinded Prospective Interventional Trial. J. Clin. Monit. Comput. 2020, 34, 1149–1158. [Google Scholar] [CrossRef]
- Šribar, A.; Jurinjak, I.S.; Almahariq, H.; Bandić, I.; Matošević, J.; Pejić, J.; Peršec, J. Hypotension Prediction Index Guided versus Conventional Goal Directed Therapy to Reduce Intraoperative Hypotension during Thoracic Surgery: A Randomized Trial. BMC Anesthesiol. 2023, 23, 101. [Google Scholar] [CrossRef]
- Ranucci, M.; Barile, L.; Ambrogi, F.; Pistuddi, V.; Surgical and Clinical Outcome Research (SCORE) Group. Discrimination and Calibration Properties of the Hypotension Probability Indicator during Cardiac and Vascular Surgery. Minerva Anestesiol. 2019, 85, 724–730. [Google Scholar] [CrossRef]
- Ripollés-Melchor, J.; Valbuena-Bueno, M.A.; Fernández-Valdés-Bango, P.; Rodríguez-Herrero, A.; Tomé-Roca, J.L.; Olvera-García, M.; García-López, D.; Ruiz-Escobar, A.; Carrasco-Sánchez, L.; Abad-Gurumeta, A.; et al. Characterization of Intraoperative Hemodynamic Instability in Patients Undergoing General Anesthesia. Front. Anesthesiol. 2024, 3, 1405405. [Google Scholar] [CrossRef]
- Shirmohamadi, E.; Dolama, R.H.; Mohammadzadeh, N.; Ebrahimi, N.; Ghasemloo, N. Intraoperative Hypotension Prediction in Cardiac and Noncardiac Procedures: Is HPI Truly Worthwhile? A Systematic Review and Meta-Analysis. BMC Anesthesiol. 2025, 25, 388. [Google Scholar] [CrossRef]
- Wildhaber, P.O.; Wanner, P.M.; Wulff, D.U.; Schnider, T.W.; Filipovic, M. Impact of Staff Education on the Burden of Hypotension during Major Noncardiac Surgery. Br. J. Anaesth. 2024, 133, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Kumar, S.S.; Shah, N.J.; Penning, D.; Weinstein, M.; Malhotra, G.; Rose, S.; Drover, D.; Pennington, M.W.; Domino, K.; et al. AcumenTM Hypotension Prediction Index Guidance for Prevention and Treatment of Hypotension in Noncardiac Surgery: A Prospective, Single-Arm, Multicenter Trial. Perioper. Med. 2024, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-J.; Cheng, Y.-J.; Han, Y.-Y.; Hsiao, P.-N.; Lin, P.-L.; Chiu, C.-T.; Lee, J.-M.; Tien, Y.-W.; Chien, K.-L. Hypotension Prediction Index for Prevention of Intraoperative Hypotension in Patients Undergoing General Anesthesia: A Randomized Controlled Trial. Perioper. Med. 2024, 13, 57. [Google Scholar] [CrossRef]
- Solares, G.J.; Garcia, D.; Monge Garcia, M.I.; Crespo, C.; Rabago, J.L.; Iglesias, F.; Larraz, E.; Zubizarreta, I.; Rabanal, J.M. Real-World Outcomes of the Hypotension Prediction Index in the Management of Intraoperative Hypotension during Non-Cardiac Surgery: A Retrospective Clinical Study. J. Clin. Monit. Comput. 2023, 37, 211–220. [Google Scholar] [CrossRef]
- Reddy, V.S.; Stout, D.M.; Fletcher, R.; Barksdale, A.; Parikshak, M.; Johns, C.; Gerdisch, M. Advanced Artificial Intelligence-Guided Hemodynamic Management within Cardiac Enhanced Recovery after Surgery Pathways: A Multi-Institution Review. JTCVS Open 2023, 16, 480–489. [Google Scholar] [CrossRef]
- Pilakouta Depaskouale, M.A.; Archonta, S.A.; Katsaros, D.M.; Paidakakos, N.A.; Dimakopoulou, A.N.; Matsota, P.K. Beyond the Debut: Unpacking Six Years of Hypotension Prediction Index Software in Intraoperative Hypotension Prevention—A Systematic Review and Meta-Analysis. J. Clin. Monit. Comput. 2024, 38, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, I.; Firouzabadi, S.R.; Hosseinpour, M.; Akhlaghpasand, M.; Hajikarimloo, B.; Tavanaei, R.; Izadi, A.; Zeraatian-Nejad, S.; Eghbali, F. Predictive Ability of Hypotension Prediction Index and Machine Learning Methods in Intraoperative Hypotension: A Systematic Review and Meta-Analysis. J. Transl. Med. 2024, 22, 725. [Google Scholar] [CrossRef] [PubMed]
- Brusasco, C.; Tavazzi, G.; Cucciolini, G.; Di Nicolò, P.; Wong, A.; Di Domenico, A.; Germinale, F.; Dotta, F.; Micali, M.; Coccolini, F.; et al. Perioperative Renal Ultrasonography of Arterio-to-Venous Coupling Predicts Postoperative Complications after Major Laparoscopic Urologic Surgery. J. Clin. Med. 2023, 12, 5013. [Google Scholar] [CrossRef] [PubMed]
- Corradi, F.; Via, G.; Tavazzi, G. What’s New in Ultrasound-Based Assessment of Organ Perfusion in the Critically Ill: Expanding the Bedside Clinical Monitoring Window for Hypoperfusion in Shock. Intensive Care Med. 2020, 46, 775–779. [Google Scholar] [CrossRef]
- Corradi, F.; Tavazzi, G. The Doppler Combined Assessment of Splanchnic Perfusion and Congestion in Cardiogenic Shock: A Physiological Approach. Intensive Care Med. 2025, 51, 1168–1171. [Google Scholar] [CrossRef]
- Bidar, F.; Peillon, L.; Bodinier, M.; Venet, F.; Monneret, G.; Lukaszewicz, A.-C.; Llitjos, J.-F.; Textoris, J.; Rimmelé, T. Immune Profiling of Critically Ill Patients with Acute Kidney Injury during the First Week after Various Types of Injuries: The REALAKI Study. Crit. Care 2024, 28, 227. [Google Scholar] [CrossRef]

| GDT Group (n.67) | HPI Group (n.65) | p-Value | |
|---|---|---|---|
| Age (yr) | 74 [66–78] | 74 [67–79] | 0.742 |
| Sex, m/f (n) | 56/11 | 55/10 | 0.910 |
| BMI (kg/m2) | 25 [23–28] | 26 [23–29] | 0.158 |
| ASA physical status | |||
| I | 0 | 0 | |
| II | 39 | 35 | |
| III | 29 | 29 | 0.563 |
| IV | 0 | 1 | |
| Previous medical history (n) | |||
| Chronic congestive heart failure | 3 | 1 | 0.62 |
| Coronary disease | 7 | 4 | 0.532 |
| Atrial fibrillation and/or valvopathy | 7 | 6 | 0.535 |
| Smoking | 27 | 19 | 0.274 |
| COPD | 13 | 7 | 0.227 |
| Diabetes | 12 | 10 | 0.817 |
| Previous cerebrovascular event | 3 | 5 | 0.486 |
| Chronic AKI | 21 | 13 | 0.168 |
| Preoperative hemoglobin (g/dL) | 13.2 [11.8–14.3] | 12.8 [11.3–14.3] | 0.317 |
| Preoperative creatinine (mg/dL) | 1.1 [0.88–1.3] | 1.1 [0.9–1.4] | 0.636 |
| Preoperative eGFR (mL/min/1.73 m2) | 64.1 [46.9–74.9] | 60.1 [45.1–80.1] | 0.357 |
| Video laparoscopic or robotic surgery (n) | 25 | 37 | 0.06 |
| Type of surgery (n) | |||
| Open surgery | 42 | 28 | |
| Video laparoscopic | 25 | 17 | |
| Robotic surgery | 0 | 20 | |
| Duration of surgery (min) | 205 [180–240] | 240 [195–318] | 0.07 |
| Type of urinary diversion (n) | |||
| Orthotopic | 13 | 5 | |
| Bricker | 31 | 27 | |
| Ureterocutaneostomy | 23 | 33 |
| GDT Group (n.67) | HPI Group (n.65) | p-Value | |
|---|---|---|---|
| Patients requiring noradrenaline (n) | 14 | 10 | 0.503 |
| Crystalloids infused during surgery (mL) | 2750 [1700–2500] | 1700 [1450–2200] | 0.036 |
| Blood loss during surgery (mL) | 400 [213–600] | 400 [200–750] | 0.594 |
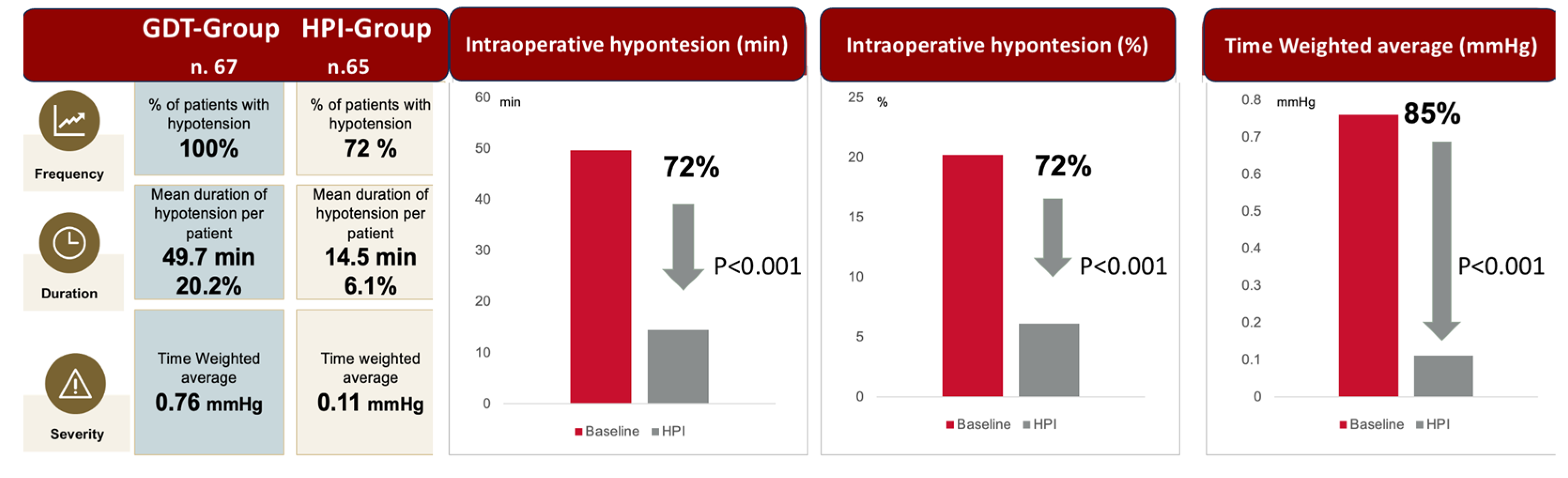
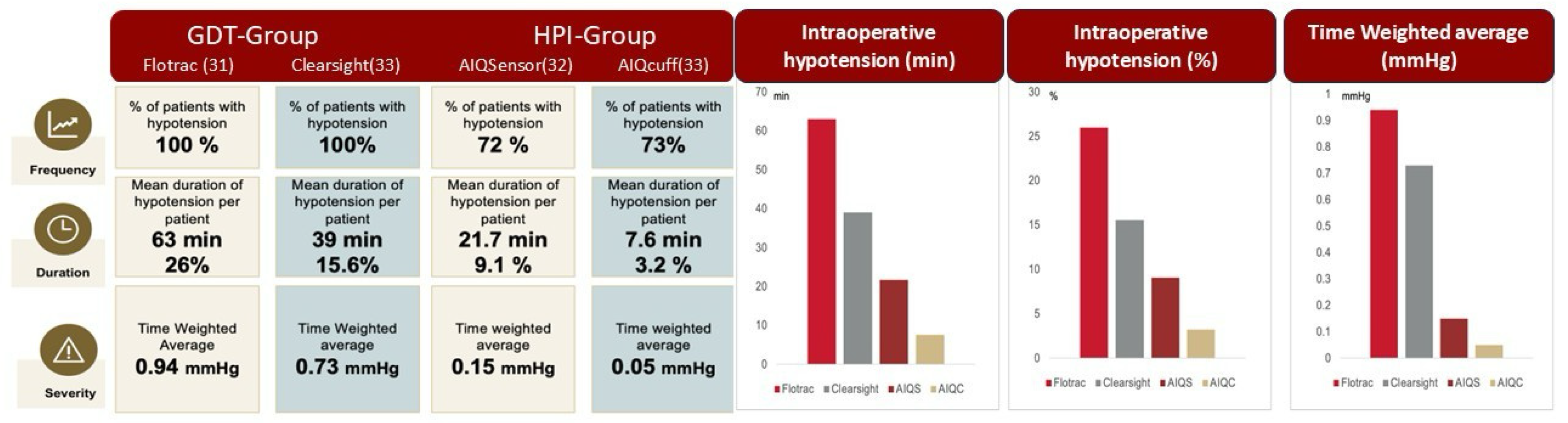
| Total hypotensive events (n) | 633 | 225 | <0.001 |
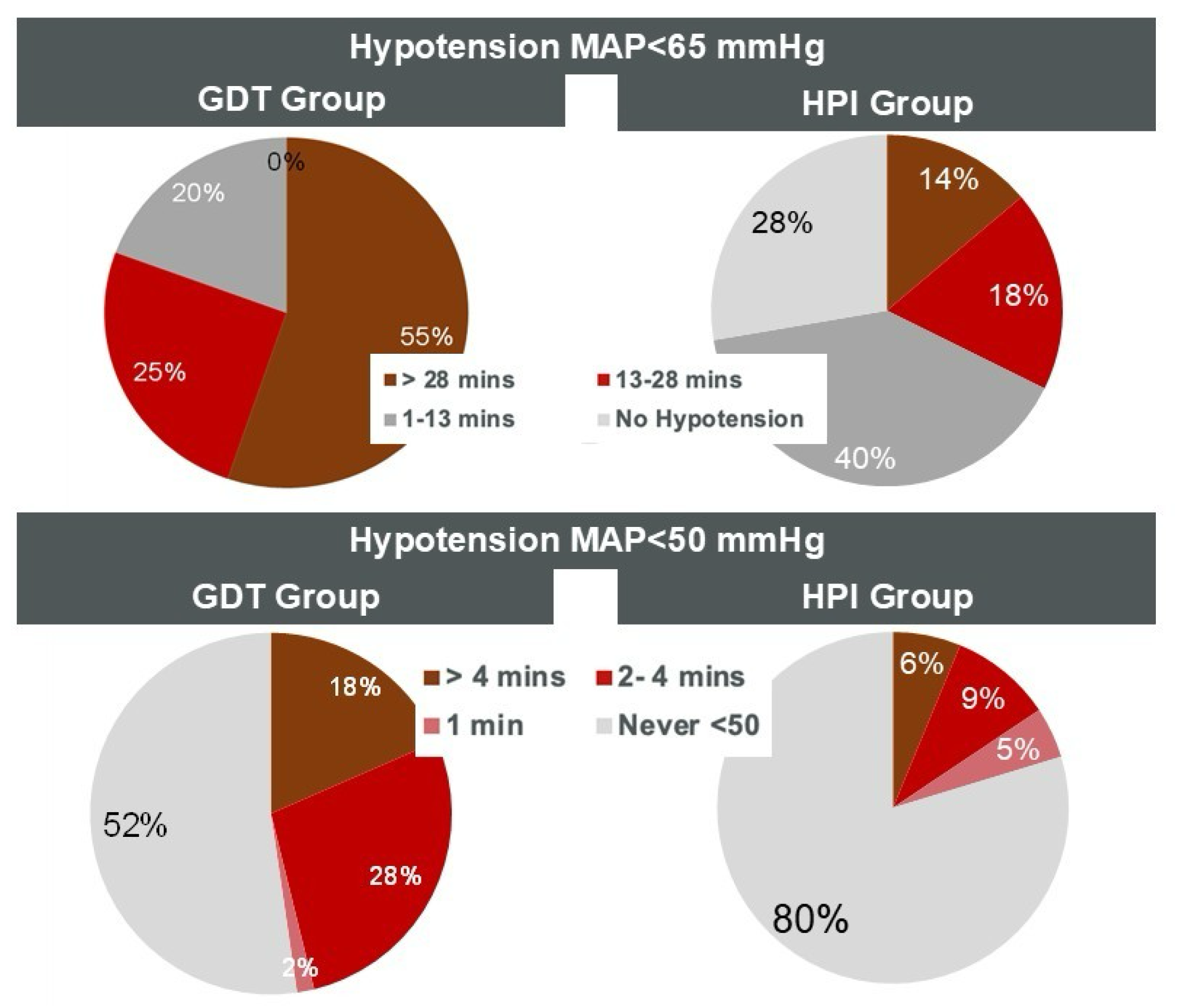
| Time-weighted average of MAP < 65 mmHg | 0.76 [0.4–1.75] | 0.11 [0.01–0.34] | <0.001 |
| Patients with hypotensive events (n) | 67 | 47 | 0.03 |
| Hypotensive events < 65 mmHg duration (min) | 3 [2–6] | 2 [1–4] | <0.001 |
| Total time in hypotension < 65 mmHg (min) | 32 [14.8–64.7] | 3.3 [0–21] | <0.001 |
| Hypotension < 65 mmHg (% of surgery time) | 14.1 [6.8–30.1] | 1.6 [0–7.9] | <0.001 |
| Time-weighted average for MAP < 50 mmHg | 0.06 [0–0.14] | 0.02 [0–0.6] | <0.001 |
| Patients with hypotensive events < 50 mmHg (n) | 33 | 9 | <0.001 |
| Hypotension < 50 mmHg (% of surgery time) | 1.2 [0.1–3.1] | 0.3 [0.1–0.84] | <0.001 |
| Hemoglobin at discharge (g/dL) | 10.3 [9.4–11.3] | 8.3 [9.6–11.8] | 0.142 |
| Creatinine at discharge (mg/dL) | 1.1 [0.8–1.3] | 1.0 [0.8–1.45] | 0.614 |
| eGFR at discharge (mL/min/1.73 m2) | 64.9 [45.5–84.1] | 64.6 [44.1–93.3] | 0.267 |
| Intensive care unit admittance (n) | 12 | 4 | 0.05 |
| Total major postoperative complications (n) | 28 | 23 | 0.593 |
| Postoperative acute kidney injury (n) | 20 | 18 | 0.512 |
| Kidgo 1 | 12 | 8 | 0.472 |
| Kidgo 2 | 3 | 4 | 0.712 |
| Kidgo 3 | 5 | 6 | 0.759 |
| Postoperative cardiovascular complications (n) | 0 | 0 | 0.999 |
| Infectious postoperative complications (n) | 26 | 10 | 0.003 |
| Reintervention (n) | 6 | 6 | 0.999 |
| Anastomotic dehiscence or urinary leakage (n) | 5 | 6 | 0.76 |
| In-hospital length of stay (days) | 10 [8–15] | 7 [6–9] | <0.001 |
| Readmission at 30 days from surgery (n) | 16 | 13 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brusasco, C.; Micali, M.; Cucciolini, G.; Filolli, D.; Gandini, M.; Lattuada, M.; Introini, C.; Corradi, F. Comparison of Hemodynamic Management by Hypotension Prediction Index or Goal-Directed Therapy in Radical Cystectomies: A Prospective Observational Study. J. Clin. Med. 2025, 14, 6285. https://doi.org/10.3390/jcm14176285
Brusasco C, Micali M, Cucciolini G, Filolli D, Gandini M, Lattuada M, Introini C, Corradi F. Comparison of Hemodynamic Management by Hypotension Prediction Index or Goal-Directed Therapy in Radical Cystectomies: A Prospective Observational Study. Journal of Clinical Medicine. 2025; 14(17):6285. https://doi.org/10.3390/jcm14176285
Chicago/Turabian StyleBrusasco, Claudia, Marco Micali, Giada Cucciolini, Desjan Filolli, Michela Gandini, Marco Lattuada, Carlo Introini, and Francesco Corradi. 2025. "Comparison of Hemodynamic Management by Hypotension Prediction Index or Goal-Directed Therapy in Radical Cystectomies: A Prospective Observational Study" Journal of Clinical Medicine 14, no. 17: 6285. https://doi.org/10.3390/jcm14176285
APA StyleBrusasco, C., Micali, M., Cucciolini, G., Filolli, D., Gandini, M., Lattuada, M., Introini, C., & Corradi, F. (2025). Comparison of Hemodynamic Management by Hypotension Prediction Index or Goal-Directed Therapy in Radical Cystectomies: A Prospective Observational Study. Journal of Clinical Medicine, 14(17), 6285. https://doi.org/10.3390/jcm14176285







