Prevalence of Polypharmacy Among Patients with Chronic Liver Disease—A Narrative Literature Review
Abstract
1. Impact and Implications
2. Introduction
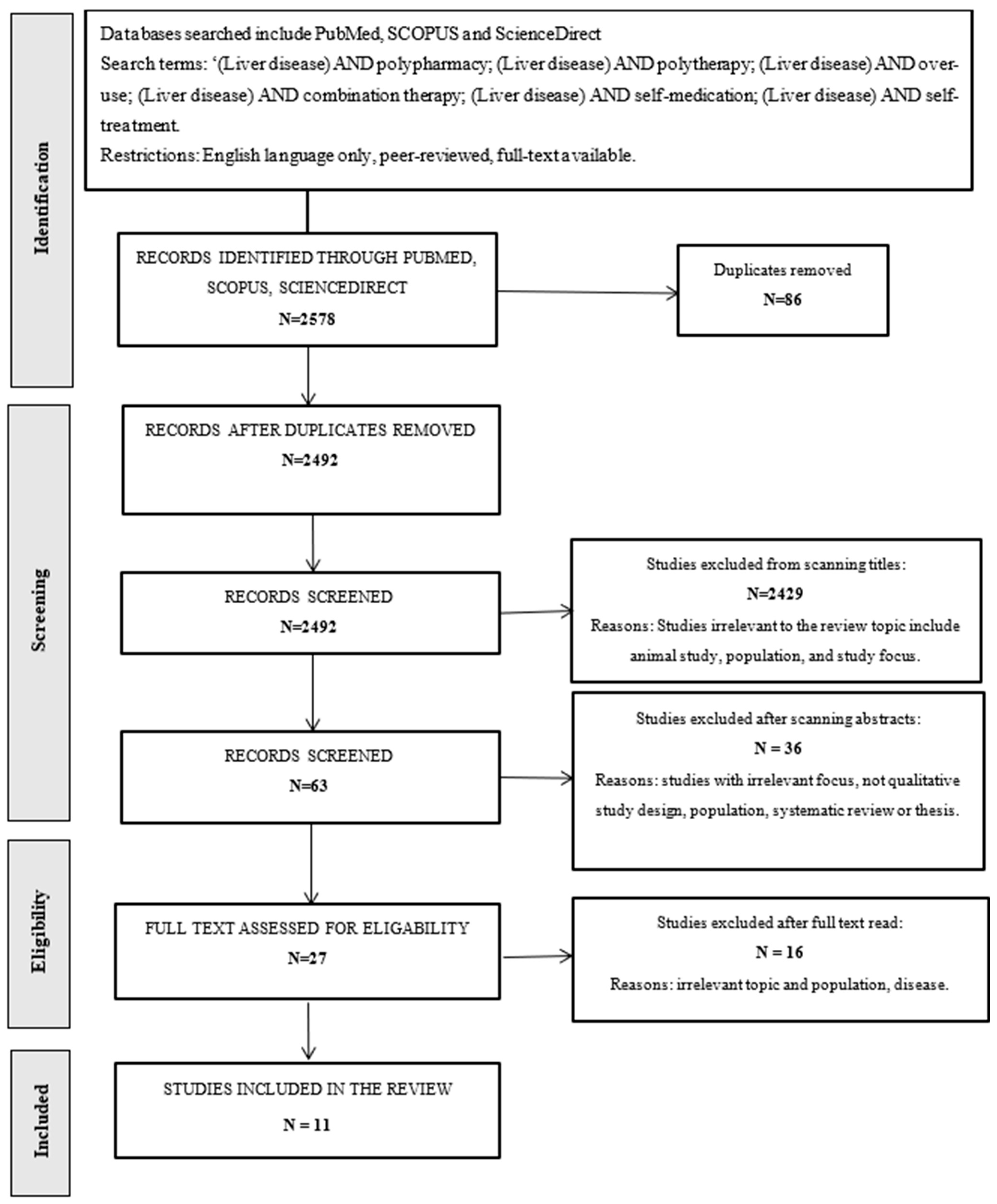
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Skat-Rørdam, J.; Lykkesfeldt, J.; Gluud, L.L.; Tveden-Nyborg, P. Mechanisms of drug induced liver injury. Cell. Mol. Life Sci. 2025, 82, 213. [Google Scholar] [CrossRef] [PubMed]
- Merrell, M.D.; Cherrington, N.J. Drug metabolism alterations in nonalcoholic fatty liver disease. Drug Metab. Rev. 2011, 43, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Massart, J.; Begriche, K.; Moreau, C.; Fromenty, B. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J. Clin. Transl. Res. 2017, 3 (Suppl. S1), 212–232. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Khov, N. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 6821–6825. [Google Scholar] [CrossRef]
- Verma, S.; Jensen, D.; Hart, J.; Mohanty, S.R. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 2013, 33, 1398–1405. [Google Scholar] [CrossRef]
- Fracanzani, A.L.; Valenti, L.; Bugianesi, E.; Andreoletti, M.; Colli, A.; Vanni, E.; Bertelli, C.; Fatta, E.; Bignamini, D.; Marchesini, G.; et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology 2008, 48, 792–798. [Google Scholar] [CrossRef]
- Badary, H.A.; Hashem, M.B.; El-Kassas, M. Drug-induced liver injury during the era of COVID-19 polypharmacy: A statement of account, lessons learned, and a proposed approach. Egypt. Liver J. 2024, 14, 75. [Google Scholar] [CrossRef]
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [Google Scholar] [CrossRef]
- Peter, T.; Hemavathi, G.; Kanishka, P.H.; Haritha, C.; Harshini, S.; Janani, K.; Wani, J.A. A Comprehensive Clinical Review on Geriatric Polypharmacy. Int. J. Pharm. Sci. Rev. Res. 2025, 18, 112–116. [Google Scholar] [CrossRef]
- URPL. Annual Report of the President of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products 2023. Available online: https://www.gov.pl/web/urpl/raport-roczny (accessed on 9 October 2024).
- Licata, A.; Minissale, M.G.; Calvaruso, V.; Craxì, A. A focus on epidemiology of drug-induced liver injury: Analysis of a prospective cohort. Eur. Rew. Med. Pharmacol. Sci. 2017, 21 (Suppl. S1), 112–121. [Google Scholar]
- Woroń, J.; Gryglewska, B.; Gupało, J.; Tymiński, R.; Lorkowska-Zawicka, B.; Wordliczek, J.; Kocot-Kępska, M.; Drygalski, T. Farmakoterapia jatrogenizacyjna w medycynie paliatywnej, nie tylko w populacji senioralnej—Lekceważony problem praktyczny w 10 odsłonach. Geriatria 2024, 18, 94–102. [Google Scholar]
- Amacher, D.E. Female gender as a susceptibility factor for drug-induced liver injury. Hum. Exp. Toxicol. 2014, 33, 928–939. [Google Scholar] [CrossRef]
- Naga, C.; Rajender, R.; Robert, F.; Huiman, B.; Jiezhun, G.; Paul, H.; Jawad, A.; Andrew, S.; Victor, N.; Jay, H. Idiosyncratic drug induced liver injury in African-Americans is associated with greater morbidity and mortality compared to caucasians: A randomized controlled trial. Am. J. Gastroenterol. 2017, 112, 1382–1388. [Google Scholar]
- Pauli-Magnus, C.; Stieger, B.; Meier, Y.; Kullak-Ublick, G.A.; Meier, P.J. Enterohepatic transport of bile salts and genetics of cholestasis. J. Hepatol. 2005, 43, 342–357. [Google Scholar] [CrossRef]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef]
- Noor, S.; Ismail, M.; Haider, I.; Khadim, F. Drug-Drug Interactions in Hepatitis Patients: Do these Interactions Matter in Clinical Perspectives? Ann. Hepatol. 2018, 17, 1001–1011. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Elzouki, A.-N.; Zahid, M.; Akbar, R.A.; Alfitori, G.B.; Purayil, S.C.; Imanullah, R.; Danjuma, M.I. Polypharmacy and drug interactions amongst cirrhotic patients discharged from a tertiary center: Results from a national quality improvement audit. Arab. J. Gastroenterol. 2020, 21, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Umetsu, R.; Uranishi, H.; Suzuki, H.; Nishibata, Y.; Kato, Y.; Ueda, N.; Sasaoka, S.; Hatahira, H.; Motooka, Y.; et al. Analysis of polypharmacy effects in older patients using Japanese Adverse Drug Event Report database. PLoS ONE 2017, 12, e0190102. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.C.; Egger, S.; Born, C.; Bravo, A.E.R.; Krähenbühl, S. Potential drug-drug interactions and adverse drug reactions in patients with liver cirrhosis. Eur. J. Clin. Pharmacol. 2012, 68, 179–188. [Google Scholar] [CrossRef]
- Hayward, K.L.; Patel, P.J.; Valery, P.C.; Horsfall, L.U.; Li, C.Y.; Wright, P.L.; Tallis, C.J.; Stuart, K.A.; Irvine, K.M.; Cottrell, W.N.; et al. Medication-Related Problems in Outpatients With Decompensated Cirrhosis: Opportunities for Harm Prevention. Hepatol. Commun. 2019, 3, 620–631. [Google Scholar] [CrossRef]
- Ruzicka, D.J.; Tetsuka, J.; Fujimoto, G.; Kanto, T. Comorbidities and co-medications in populations with and without chronic hepatitis C virus infection in Japan between 2015 and 2016. BMC Infect. Dis. 2018, 18, 237. [Google Scholar] [CrossRef]
- Alrasheed, M.; Guo, J.J.; Lin, A.C.; Wigle, P.R.; Hardee, A.; Hincapie, A.L. The effect of polypharmacy on quality of life in adult patients with nonalcoholic fatty liver disease in the United States. Qual. Life Res. 2022, 31, 2481–2491. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.J.; Hayward, K.L.; Rudra, R.; Horsfall, L.U.; Hossain, F.; Williams, S.; Johnson, T.; Brown, N.N.; Saad, N.; Clouston, A.D.; et al. Multimorbidity and polypharmacy in diabetic patients with NAFLD. Medicine 2017, 96, e6761. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.; Walker, A.J.; Irving, W.L. Comorbidities and medications of patients with chronic hepatitis C under specialist care in the UK. J. Med. Virol. 2017, 89, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Hayward, K.L.; Weersink, R.A.; Bernardes, C.M.; McIvor, C.; Rahman, T.; Skoien, R.; Clark, P.J.; Stuart, K.A.; Hartel, G.; Valery, P.C.; et al. Changing Prevalence of Medication Use in People with Cirrhosis: A Retrospective Cohort Study Using Pharmaceutical Benefits Scheme Data. Drugs Real World Outcomes 2023, 10, 605–618. [Google Scholar] [CrossRef]
- Farooq, J.; Sana, M.; Chetana, P.; Almuqbil, M.; Bhat, N.P.; Sultana, R.; Khaiser, U.; Asdaq, S.M.B.; Almalki, M.E.M.; Khormi, A.M.S.; et al. Polypharmacy in chronic liver disease patients: Implications for disease severity, drug-drug interaction, and quality of life. Saudi Pharm. J. 2023, 31, 101668. [Google Scholar] [CrossRef]
- Francis, P.; Navarro, V.J. Drug-Induced Hepatotoxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hu, X.; Liu, R.; Tang, L.; Mei, M.; Li, Y.; Tang, G.; Feng, J.; Chen, W.; Li, G. Physicians and hospital pharmacists’ knowledge, attitudes, and practices towards polypharmacy in older patients with chronic diseases. Sci. Rep. 2024, 14, 29885. [Google Scholar] [CrossRef]
- Yu, K.; Geng, X.; Chen, M.; Zhang, J.; Wang, B.; Ilic, K.; Tong, W. High Daily Dose and Being a Substrate of Cytochrome P450 Enzymes Are Two Important Predictors of Drug-Induced Liver Injury. Drug Metab. Dispos. 2014, 42, 744–750. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Małysz, M.; Więcek, A.; Piechaczek, M.; Pomierny-Chamioło, L. Drug-induced liver injury (DILI)—Mechanisms and diagnostic. Farm. Polska 2022, 78, 460–468. (In Polish) [Google Scholar] [CrossRef]
- Lewis, J.H.; Seeff, L.B. The Origins of the Modern-Day Study of Drug Hepatotoxicity: Focus on Hyman J. Zimmerman. Clin. Liver Dis. 2020, 15 (Suppl. S1), S25–S36. [Google Scholar] [CrossRef]
- Haque, T.; Sasatomi, E.; Hayashi, P.H. Drug-Induced Liver Injury: Pattern Recognition and Future Directions. Gut Liver 2016, 10, 27–36. [Google Scholar] [CrossRef]
- Mahin, D.; Lauren, M.; Behnaz, J.; Anees, B.; Zahra, G.; Julia, K.; Zia, C. Prevalence and factors associated with polypharmacy: A systematic review and meta-analysis. BMC Geriatr. 2022, 22, 601. [Google Scholar]
- Khezrian, M.; McNeil, C.J.; Murray, A.D.; Myint, P.K. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther. Adv. Drug Saf. 2020, 11. [Google Scholar] [CrossRef]
- Alrasheed, M.; Guo, J.J.; Lin, A.C.; Wigle, P.R.; Hardee, A.; Hincapie, A.L. Association between polypharmacy, patient-reported symptoms, and quality of life among nonalcoholic fatty liver disease patients in the United States. Drugs Ther. Perspect. 2022, 38, 490–498. [Google Scholar] [CrossRef]
- Zaij, S.; Maia, K.P.; Leguelinel-Blache, G.; Roux-Marson, C.; Kinowski, J.M.; Richard, H. Intervention of pharmacist included in multidisciplinary team to reduce adverse drug event: A qualitative systematic review. BMC Health Serv. Res. 2023, 23, 927. [Google Scholar] [CrossRef] [PubMed]
- Hayward, K.L.; Weersink, R.A. Improving Medication-Related Outcomes in Chronic Liver Disease. Hepatol. Commun. 2020, 4, 1562–1577. [Google Scholar] [CrossRef] [PubMed]
- Alqenae, F.A.; Steinke, D.; Keers, R.N. Prevalence and Nature of Medication Errors and Medication-Related Harm Following Discharge from Hospital to Community Settings: A Systematic Review. Drug Saf. 2020, 43, 517–537. [Google Scholar] [CrossRef]
- Elbeddini, A.; Yang, L.; Aly, A. A Case-Control Study: The Impact of Unintentional Discrepancies and Pharmacist Discharge Prescription Review on 30-Day Hospital Readmission. J. Prim. Care Community Health 2020, 11. [Google Scholar] [CrossRef]
- George, D.; Supramaniam, N.D.; Hamid, S.Q.A.; Hassali, M.A.; Lim, W.-Y.; Hss, A.-S. Effectiveness of a pharmacist-led quality improvement program to reduce medication errors during hospital discharge. Pharm. Pract. 2019, 17, 1501. [Google Scholar] [CrossRef] [PubMed]
- Waszyk-Nowaczyk, M.; Dymek, J.; Drozd, M.; Sierpniowska, O.; Stankiewicz, A.; Jędra, A.; Jasińska-Stroschein, M. New Medicine Service as support for medication adherence by chronically ill patients. Farm. Polska 2023, 79, 281–288. [Google Scholar] [CrossRef]
- Skowron, A.; Drozd, M.; Brukiewicz, P.; Dymek, J.; Gołda, A.; Żmudzka, E. Zalecenia Dotyczące Postępowania u Osób Objętych Świadczeniem Konsultacja w Przypadku Drobnej Dolegliwości. Naczelna Izba Aptekarska, Warszawa. 2024. Available online: https://www.nia.org.pl/wp-content/uploads/2024/01/Konsultacja_w_przypadku_drobnej_dolegliwosci_2024-01-30.pdf (accessed on 23 August 2025).
- Drozd, M.; Skowron, A.; Karolewicz, B.; Bułaś, L.; Machalska, J.; Dymek, J.; Gołda, A.; Jachowicz, M. Wytyczne Polskiego Towarzystwa Farmaceutycznego Dotyczące Prowadzenia Świadczenia Zdrowotnego—Przegląd Lekowy. Polskie Towarzystwo Farmaceutyczne, Warszawa. 2023. Available online: https://ptfarm.pl/pub/File/Sekcje/Sekcja_opieki_farmaceutycznej/WYTYCZNE_przeglad_lekowy_net.pdf (accessed on 23 August 2025).
- Bae-Shaaw, Y.H.; Eom, H.; Chun, R.F.; Fox, D.S. Real-world evidence on impact of a pharmacist-led transitional care program on 30- and 90-day readmissions after acute care episodes. Am. J. Health Pharm. 2020, 77, 535–545. [Google Scholar] [CrossRef]
- Becker, C.; Zumbrunn, S.; Beck, K.; Vincent, A.; Loretz, N.; Müller, J.; Amacher, S.A.; Schaefert, R.; Hunziker, S. Interventions to Improve Communication at Hospital Discharge and Rates of Readmission. JAMA Netw. Open 2021, 4, e2119346. [Google Scholar] [CrossRef]
- Heaton, P.C.; Frede, S.; Kordahi, A.; Lowery, L.; Moorhead, B.; Kirby, J.; Kunze, N.; Luder, H. Improving care transitions through medication therapy management: A community partnership to reduce readmissions in multiple health-systems. J. Am. Pharm. Assoc. 2019, 59, 319–328. [Google Scholar] [CrossRef]
- McKay, C.; Park, C.; Chang, J.; Brackbill, M.; Choi, J.-Y.; Lee, J.H.; Kim, S.H. Systematic Review and Meta-analysis of Pharmacist-Led Transitions of Care Services on the 30-Day All-Cause Readmission Rate of Patients with Congestive Heart Failure. Clin. Drug Investig. 2019, 39, 703–712. [Google Scholar] [CrossRef]
- Nazar, H.; Howard, C.; Nazar, Z.; Watson, N.W. A rapid review and narrative synthesis of hospital to community pharmacy transfer of care services in England. Int. J. Pharm. Pract. 2021, 29, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Odeh, M.; Scullin, C.; Fleming, G.; Scott, M.G.; Horne, R.; McElnay, J.C. Ensuring continuity of patient care across the healthcare interface: Telephone follow-up post-hospitalization. Br. J. Clin. Pharmacol. 2019, 85, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, S.; Smith, J.N.; Pontiggia, L.; Patel, R.V.; Day, S.C.; Grande, D.T. Impact of an interprofessional transition of care service on 30-day hospital reutilizations. J. Interprof. Care 2019, 33, 32–37. [Google Scholar] [CrossRef]
- Pourrat, X.; Leyrat, C.; Allenet, B.; Bouzige, B.; Develay, A.; Fraysse, M.; Garnier, V.; Halimi, J.; Roux-Marson, C.; Giraudeau, B. Effectiveness of a multicomponent pharmacist intervention at hospital discharge for drug-related problems: A cluster randomised cross-over trial. Br. J. Clin. Pharmacol. 2020, 86, 2441–2454. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.A.; Graham, J.H.; Maeng, D.; Tusing, L.; Zaleski, L.; Martin, R.; Seipp, R.; Citsay, B.; McDonald, B.; Bolesta, K.; et al. Reductions in 30-day readmission, mortality, and costs with inpatient–to–community pharmacist follow-up. J. Am. Pharm. Assoc. 2019, 59, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Górecka, A.; Drozd, M.; Olejniczak-Rabinek, M.; Bułaś, L.; Jasińska-Stroschein, M.; Machalska, J.; Matschay, A.; Waszyk-Nowaczyk, M. Wytyczne wykonywania Świadczenia Zdrowotnego—Poszpitalna Opieka Farmaceutyczna (POF) Polskie Towarzystwo Farmaceutyczne; Warszawa. 2025. Available online: https://ptfarm.pl/pub/File/opieka_farmaceutyczna/Poszpitalna%20Opieka%20Farmaceutyczna/Wytyczne_POF_net.pdf (accessed on 23 August 2025).
| Authors/Title | Objective/Evidence Type | Population/Results |
|---|---|---|
| Elzouki et al.: Polypharmacy and drug interactions amongst cirrhotic patients discharged from a tertiary center: Results from a national quality improvement audit. (2020) [20] | Audit the drug prescribed in patients with cirrhosis and analyze the quantity and severity of potential drug-drug interaction/Electronic Medical Records review | Adult patients, diagnosed with cirrhosis. Relevant data from 181 patients. Average drug utilization 7.8 ± 3.1 (range = 1–17). 102 (56.3%) patients used polypharmacy. The drug should be avoided in case of 3.3%, 16.6% of cases to consider therapy modification, 35.9% to monitor treatment. Utilization of polypharmacy was statistically significant in patients where drug should be avoided (83.3%, p = 0.03). |
| Abe et al.: Analysis of polypharmacy effects in older patients using Japanese adverse drug event report database. (2017) [21] | Examined AE profiles associated with polypharmacy and aging; association between polypharmacy and “renal disorder” or “hepatic disorder”/Database review | Older patients, hepatic, disorder. For hepatic disorder, the adjusted RORs (reported odds ratio) were as follows: 1.17 (1.14–1.20) for the number of administered drugs group (5–9) and 1.14 (1.11–1.18) for the number of administered drugs group (≥10). The adjusted RORs of hepatic disorder compared to those of renal disorder had lower adjusted RORs related to the increase in the number of administered drugs. |
| Franz et al.: Potential drug-drug interactions and adverse drug reactions in patients with liver cirrhosis. (2012) [22] | Assess risk for potential drug-drug interactions (pDDIs) and/or adverse drug reactions (ADRs) due to the severity of disease and comorbidities associated with polypharmacy/Electronic Medical Records review | Adult patients with cirrhosis. There were 400 patients with cirrhosis, 6 (1–10) diagnoses per patient; 60.7% of the diagnoses were not liver-associated; median number of drugs per patient: 5 (0–18), whereof 3 (0–16) were predominantly hepatically eliminated. Prescribed drugs were primarily indicated for gastrointestinal, cardiovascular, or nervous system disorders, reflecting the prevalent diagnoses. 28% patients had 200 ADRs; 21.5% patients had 132 pDDIs. 7 pDDIs were cause of 15 ADRs and 3 resulted in hospitalization. Patients with ADRs: older, more comorbidities, treated with more drugs, worse renal function, more pDDIs. |
| Hayward et al.: Medication-Related Problems in Outpatients With Decompensated Cirrhosis: Opportunities for Harm Prevention. (2019) [23] | Assessed the association between MRPs (medication related problems) and patient outcomes/Randomized clinical trial | Patients with a history of decompensated cirrhosis. There were 57 intervention patients; 375 MRPs identified; most prevalent MRP types: nonadherence (31.5%) and indication issues (29.1%); risk of potential harm associated with MRPs was low in 18.9% of instances, medium in 33.1%, and high in 48.0%, as categorized by a clinician panel using a risk matrix tool. Patients had a greater incidence rate of high-risk MRPs if they had a higher Child-Pugh score (incidence rate ratio [IRR], 1.31; 95% confidence interval [CI], 1.09–1.56); greater comorbidity burden (IRR, 1.15; 95% CI, 1.02–1.29); and were taking more medications (IRR, 1.12; 95% CI, 1.04–1.22). Pharmacist intervention resulted in the resolution of 58.9% of MRPs. |
| Noor et al.: Drug-drug interactions in hepatitis patients: Do these interactions matter in clinical perspectives? (2018) [18] | Explored frequency, levels, predictors, and clinical relevance of pDDIs (potential drug-drug interactions) in hospitalized hepatitis patients./Retrospective cohort study | Hepatitis patients. A total of 413 hepatitis patients, 55.2% reported pDDIs; total of 660 pDDIs; 35% patients—major pDDIs. Significant association for the presence of pDDIs with > 9 prescribed medicines (p < 0.001), hospitalization of >5 days (p = 0.03), and stroke as comorbidity (p = 0.05). Significantly higher odds of exposure to major-pDDIs in patients taking > 9 prescribed medicines (p < 0.001), hospitalization of > 5 days (p = 0.002), and stroke as comorbidity (p = 0.002). |
| Ruzicka et al.: Comorbidities and co-medications in populations with and without chronic hepatitis C virus infection in Japan between 2015 and 2016. (2018) [24] | To examine number and types of comorbidities and co-medications by age group in patients with and without chronic HCV/Retrospective, observational hospital database study | Patients with chronic HCV and non-HCV patients. There were 128,967 chronic HCV patients and 515,868 non-HCV patients. The most common comorbidities in chronic HCV patients were diseases of oesophagus, stomach and duodenum (41.7%), followed by hypertensive diseases (31.4%). Chronic HCV patients and older patients used more co-medications. 41.9% of chronic HCV patients and 26.0% of non-HCV patients used at least one co-medication supplied for ≥180 days or recorded in at least 6 consecutive months. Among chronic HCV patients, 19.0% used 1–3 co-medications, 11.0% used 4–6, and 11.9% used ≥7 co-medications, compared with 13.8, 6.5, and 5.8% of non-HCV patients. 16.2% of chronic HCV patients aged 80–84 years used ≥7 co-medications. The most common long-term co-medications in chronic HCV patients were proton pump inhibitors (prescribed to 31.9% of chronic HCV patients at least once during the study period). |
| Alrasheed et al.: The effect of polypharmacy on quality of life in adult patients with nonalcoholic fatty liver disease in the United States. (2022) [25] | To examine the association between polypharmacy and health-related quality of life (QoL) in NAFLD adult patients/Retrospective observational study | Nonalcoholic fatty liver disease (NAFLD) adult patients. A total of 1067 NAFLD adult patients; 834 patients with polypharmacy; patients with NAFLD and polypharmacy have lower QoL than those with NAFLD and nonpolypharmacy. Number of medications had a significant negative impact on PCS (physical component summary), MCS (mental component summary), and all SF-36 domains except mental health, role physical limitation and role emotional limitation domains. |
| Patel et al.: Multimorbidity and polypharmacy in diabetic patients with NAFLD. Implications for disease severity and management. (2017) [26] | Identifying characteristics that may impact liver disease severity or clinical management of patients with diabetes and NAFLD/Observational study | Adult patients with diabetes 2 and with NAFLD. A total of 95 patients; 10% took <5 regular medications; 59% polypharmacy (5–9 medications); 31% hyperpolypharmacy (≥10 medications). Older patients and patients with a history of IHD or osteoarthritis were taking more medicines (p = 0.01, p <0.01, and p = 0.05, respectively). Significant relationship between number of medications taken and number of co-morbidities. |
| Hudson et al.: Comorbidities and medications of patients with chronic hepatitis C under specialist care in the UK. (2017) [27] | Using patient data to describe the demographics currently under specialist hepatology care who are likely to be eligible for direct-acting antiviral (DAA) treatment over the next 5 years, and investigate the prevalence of comorbidities, adverse lifestyle factors, and use of medications with potential DDIs/Retrospective analysis. Data from National HCV Research UK Biobank | Adult chronic hepatitis C. A total of 6278 patients (70.5% white; median age, 52 years) from 59 UK specialist centres were included; 59.1% of patients had acquired HCV through injecting drug use (IDU). The most common medications with drug-drug interaction (DDI) potential were psychotropic agents (antidepressants, opioids, and hypnotics) (38.6%), antidiabetics (9.3%), immunosuppressants (6.1%), statins (4.9%), and antiretrovirals (4.9%). This study concurs that patients with CHC in the UK have high levels of non-HCV comorbidity and polypharmacy. |
| Hayward et al.: Changing Prevalence of Medication Use in People with Cirrhosis: A Retrospective Cohort Study Using Pharmaceutical Benefits Scheme Data. (2023) [28] | To characterise the prescriptions dispensed to people with cirrhosis and explore changes in the use of medication groups over time./Observational study/Data from a multi-site, prospective, observational study | Patients with diagnosed cirrhosis; 522 patients (mean age 60 years, 70% male, 34% decompensated at recruitment), 89,615 prescriptions during the follow-up period; median of 136 prescriptions and a median of 16 unique medicines per patient (total =9306 medicines). The most commonly used medicines were proton pump inhibitors (dispensed at least once to 73% of patients), opioids (68%) and antibiotics (89%). Polypharmacy: 59–69% of participants in each time period dispensed five or more unique medicines. Prescription medication use increased over time (p < 0.001) independently of age, comorbidity burden and liver disease aetiology. |
| Farooq et al.: Polypharmacy in chronic liver disease patients: Implications for disease severity, drug-drug interaction, and quality of life. (2023) [29] | To evaluate polypharmacy in patients with chronic liver disease and to identify potential drug-drug interactions associated with it/Cross-sectional study | Patients with chronic liver disease from various age groups. Number of prescribed drugs significantly correlated (p = 0.018) with the severity of liver disease in Child-Pugh categories B and C. Moderate polypharmacy reduced quality of life (p < 0.05), Drug-drug interactions were found in 108 out of the 118 examined prescriptions, Total of 586 interactions in the admission list and 405 interactions in the discharge list. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkultecka-Dębek, M.; Bułaś, L.; Skowron, A.; Drozd, M. Prevalence of Polypharmacy Among Patients with Chronic Liver Disease—A Narrative Literature Review. J. Clin. Med. 2025, 14, 6263. https://doi.org/10.3390/jcm14176263
Szkultecka-Dębek M, Bułaś L, Skowron A, Drozd M. Prevalence of Polypharmacy Among Patients with Chronic Liver Disease—A Narrative Literature Review. Journal of Clinical Medicine. 2025; 14(17):6263. https://doi.org/10.3390/jcm14176263
Chicago/Turabian StyleSzkultecka-Dębek, Monika, Lucyna Bułaś, Agnieszka Skowron, and Mariola Drozd. 2025. "Prevalence of Polypharmacy Among Patients with Chronic Liver Disease—A Narrative Literature Review" Journal of Clinical Medicine 14, no. 17: 6263. https://doi.org/10.3390/jcm14176263
APA StyleSzkultecka-Dębek, M., Bułaś, L., Skowron, A., & Drozd, M. (2025). Prevalence of Polypharmacy Among Patients with Chronic Liver Disease—A Narrative Literature Review. Journal of Clinical Medicine, 14(17), 6263. https://doi.org/10.3390/jcm14176263







