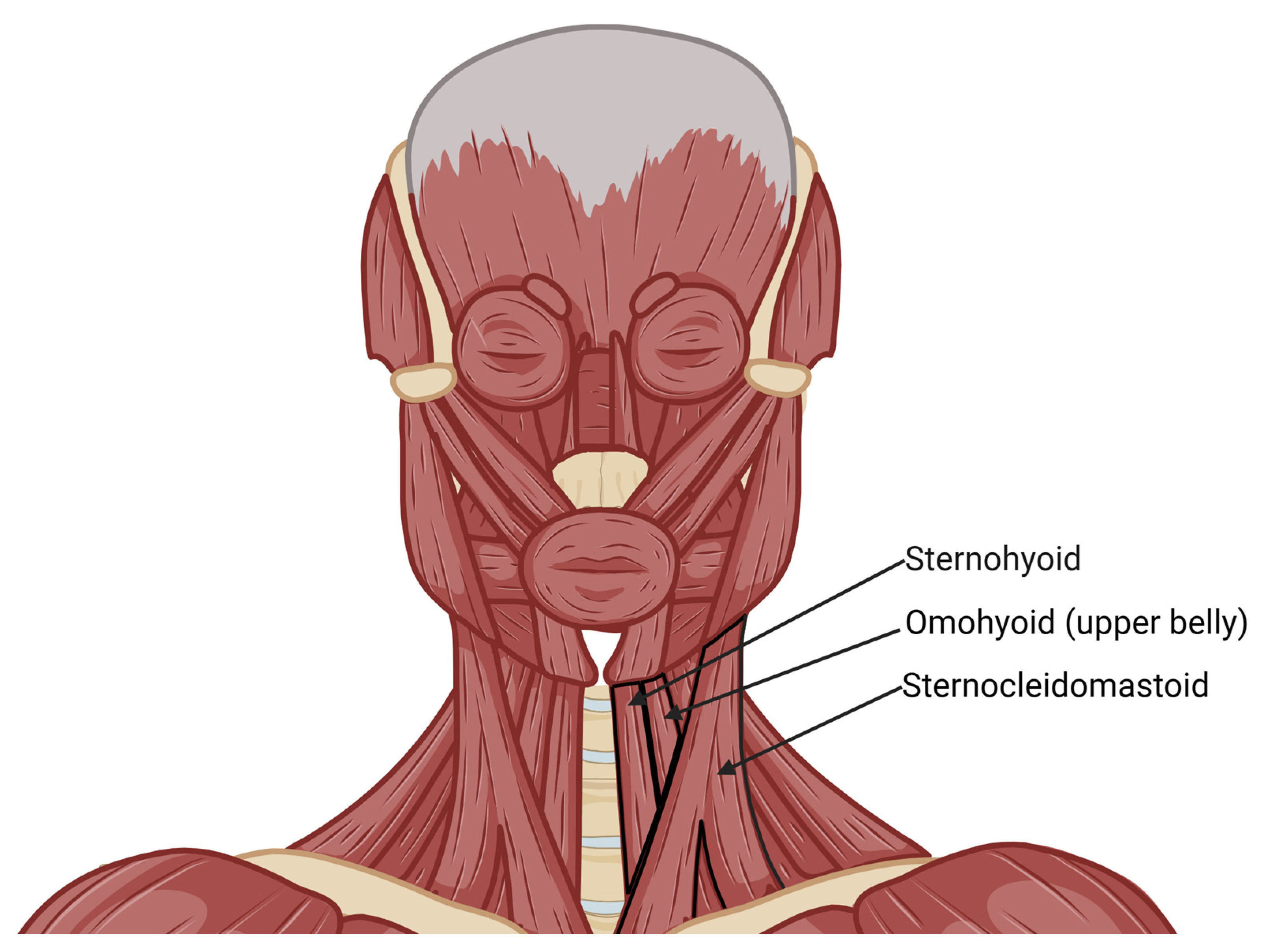
Anatomical Variations in the Superior Thyroid Artery: A Systematic Review and Implications for Free Flap Surgery
Abstract
1. Introduction
- What are the prevalence and types of anatomical variations in the origin, branching patterns, and perfusion territory of the STA?
- How do these variations relate to the SLN and to relevant surgical landmarks?
- What are the implications of these variations for surgical planning, including the selection of recipient vessels in free flap reconstruction and the prevention of iatrogenic nerve injury?
2. Materials and Methods
2.1. Study Protocol and Registration
2.2. Search Strategy
2.3. Selection Criteria
- It had to concern the anatomy of the superior thyroid artery or the usage of the superior thyroid artery in free flap surgery.
- It had to be a cadaveric, radiological, or surgical study.
- It had to be written in English.
- It had to be an original work presented as a complete, peer-reviewed article.
- It had to clearly state its objectives, methods, and results.
2.4. Study Appraisal
2.5. Data Extraction
2.6. Outcome Analysis
3. Results
3.1. Origin of STA
3.1.1. Influence of Sex and Laterality
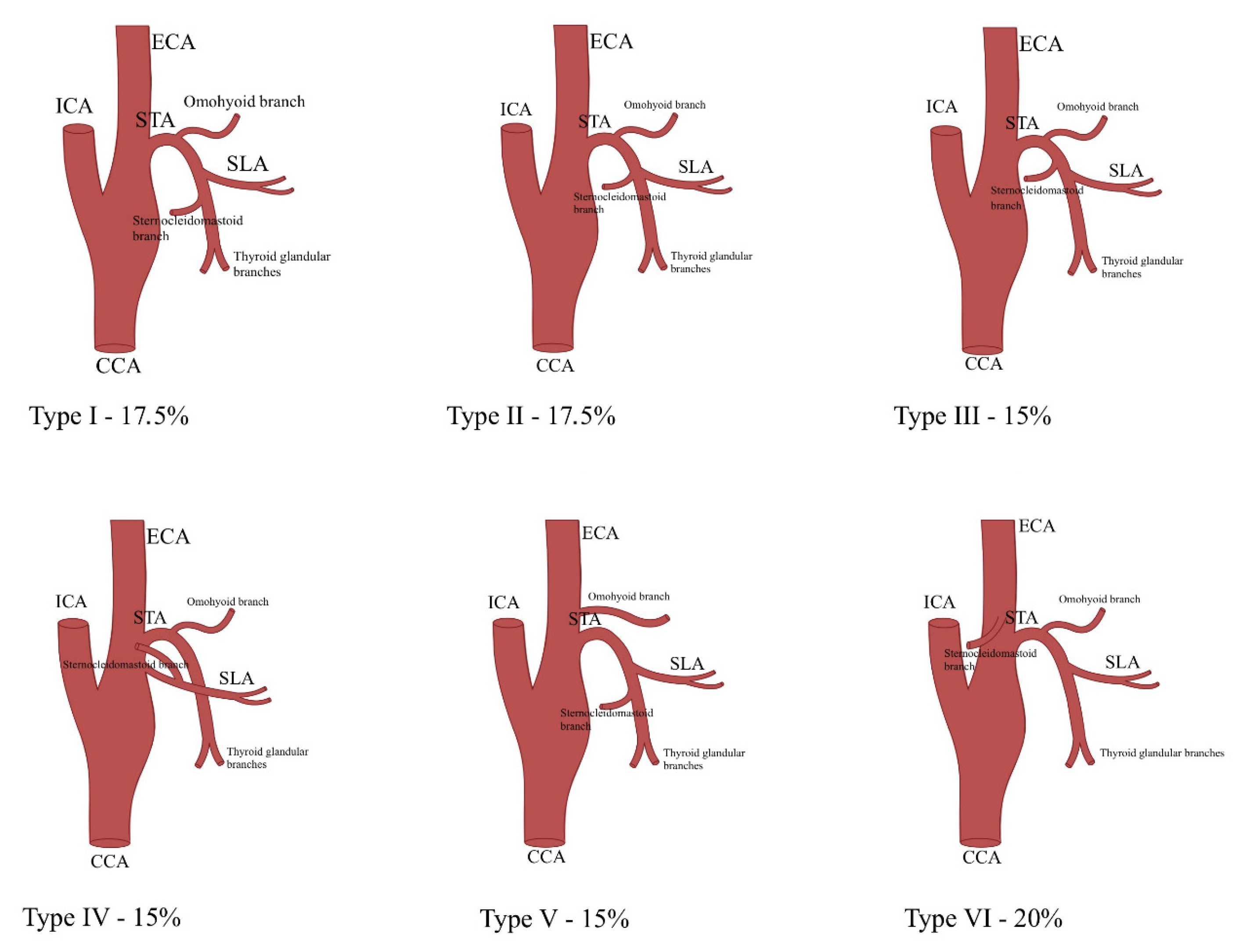
3.1.2. Common Variations and Additional Arterial Connections
3.2. Relationship with Anatomical Landmarks
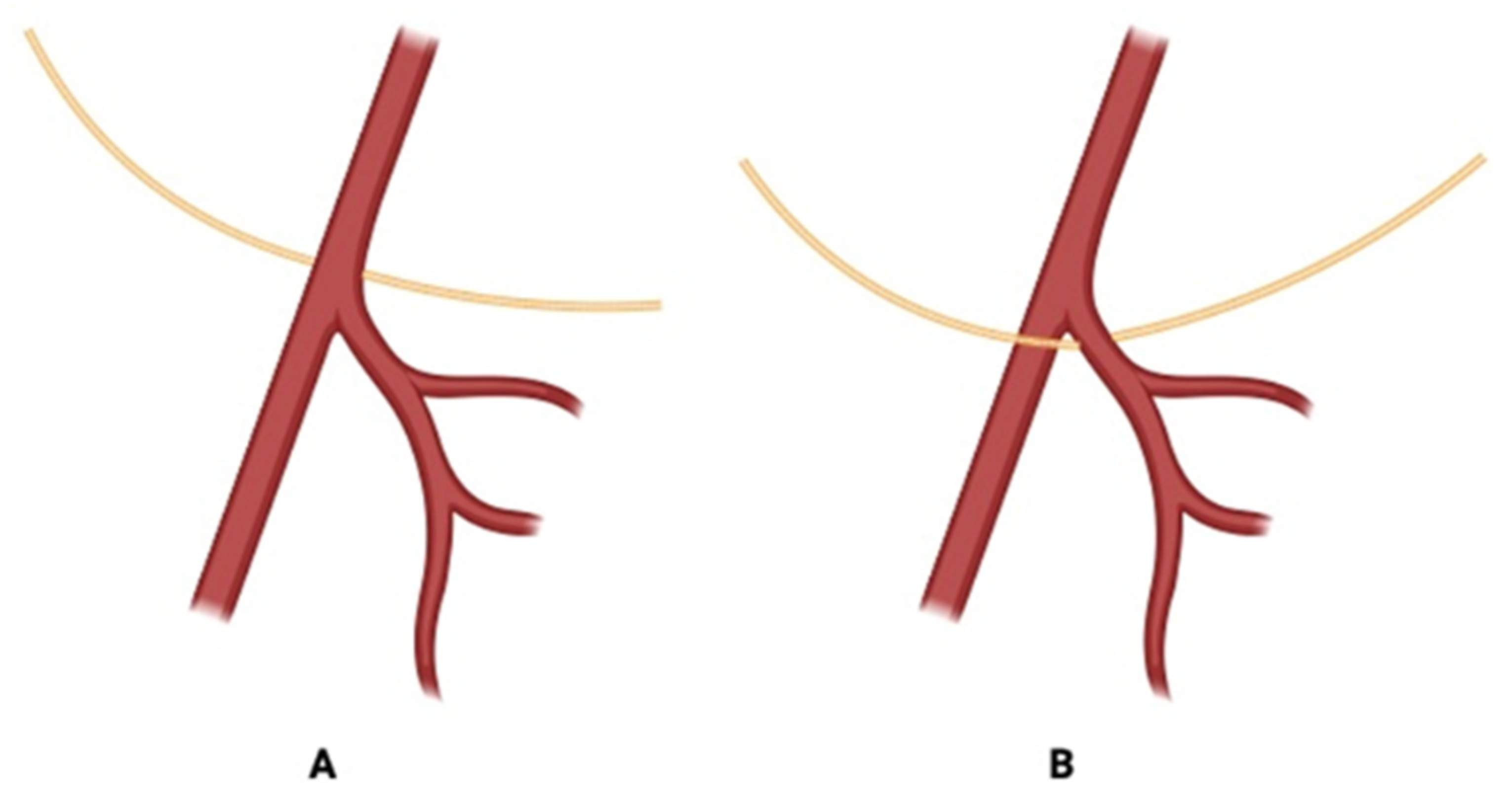
3.3. Correlation with Carotid Bifurcation Levels
3.4. Superior Thyroid Artery Perfusion Territory
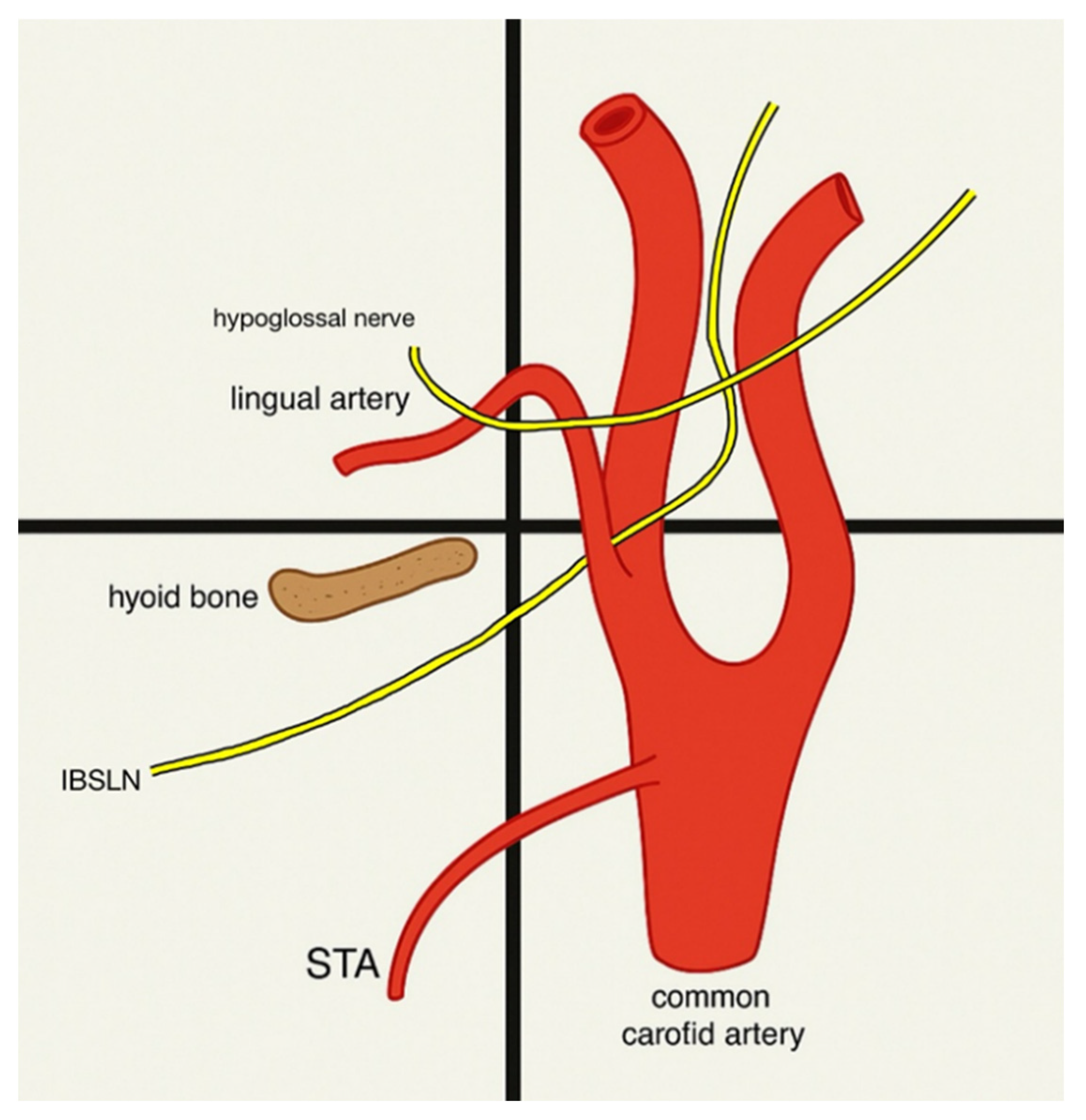
3.5. The Relationship Between the Superior Thyroid Artery and the Superior Laryngeal Nerve
3.6. Landmarks to Use During Surgeries
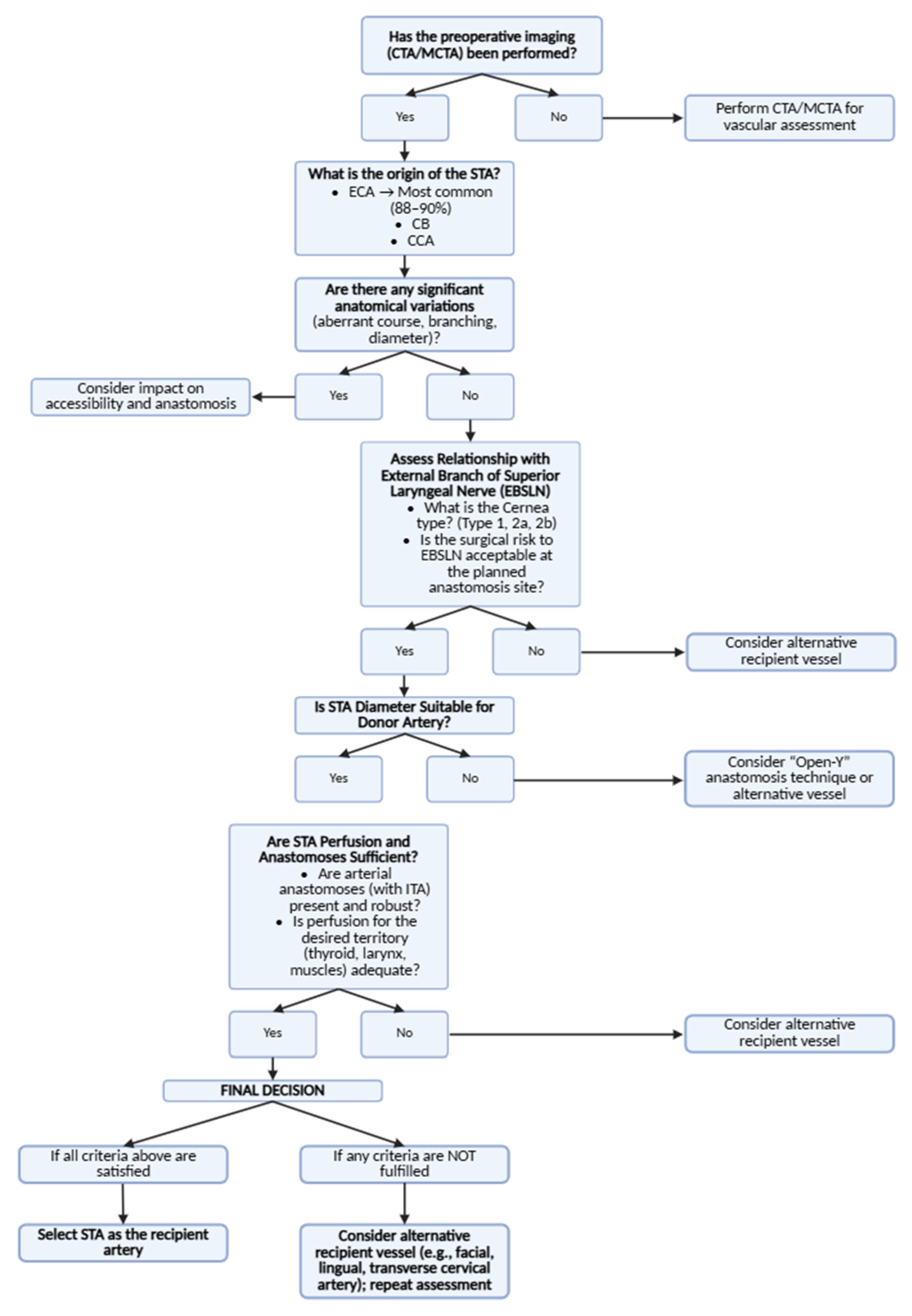
3.7. The Role of the Superior Thyroid Artery in Free Flap Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STA | superior thyroid artery |
| SLN | superior laryngeal nerve |
| ECA | external carotid artery |
| CB | carotid bifurcation |
| CCA | common carotid artery |
| EBSLN | external branch of superior laryngeal nerve |
| SLA | superior laryngeal artery |
| ITA | inferior thyroid artery |
| SCM | sternocleidomastoid muscle |
| OMO | omohyoid muscle |
| THB | tip of the greater horn of the hyoid bone |
| FA | facial artery |
| LA | lingual artery |
| TCA | transverse cervical artery |
| FFF | free fibula flap |
| STAP | superior thyroid artery perforator |
| CTA | computed tomography angiography |
References
- Abdalla, M.; Mohammed, N.; Abdallah, R.; Ahmed, M.K.; Ismaiel, M.; Abdelrahim, M.; Salih, A.; Yousif, E.; Abdalla, A.A.; Abdelrahim, M.A. Anatomical Variations of the Bifurcation Levels of the Common Carotid Artery and Superior Thyroid Artery. Cureus 2024, 16, e71120. [Google Scholar] [CrossRef]
- Al-Azzawi, A.; Takahashi, T. Anatomical variations of the thyroid gland: An experimental cadaveric study. Ann. Med. Surg. 2021, 70, 102823. [Google Scholar] [CrossRef]
- Cobiella, R.; Quinones, S.; Aragones, P.; León, X.; Abramovic, A.; Vazquez, T.; Sanudo, J.R.; Maranillo, E.; Olewnik, L.; de Blas, C.S.; et al. Anatomic mapping of the collateral branches of the external carotid artery with regard to daily clinical practice. Ann. Anat.—Anat. Anz. 2021, 238, 151789. [Google Scholar] [CrossRef]
- Estrela, F.; Leão, H.Z.; Jotz, G.P. Anatomic relation between the external branch of the superior laryngeal nerve and the thyroid gland. Braz. J. Otorhinolaryngol. 2011, 77, 249–258. [Google Scholar] [CrossRef]
- Haller, J.M.; Iwanik, M.; Shen, F.H. Clinically relevant anatomy of high anterior cervical approach. Spine 2011, 36, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.S.; Song, W.C.; Kim, S.H.; Choi, S.W.; Han, S.H.; Paik, D.J.; Kim, H.J.; Koh, K.S. Branching patterns of the arterial branches supplying the middle vascular pedicle of the sternocleidomastoid muscle: A topographic anatomical study with surgical applications for the use of pedicles osteomuscular flaps. Surg. Radiol. Anat. 2005, 28, 7–12. [Google Scholar] [CrossRef]
- Lo, A.; Oehley, M.; Bartlett, A.; Adams, D.; Blyth, P.; Al-Ali, S. Anatomical Variations of the Common Carotid Artery Bifurcation. ANZ J. Surg. 2006, 76, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-T.; Sun, S.-Q.; Huang, J.; Zhong, Y.; Xu, J.; Gan, S.-W.; Guo, L.; Mo, T.-T. An applied anatomical study on the external laryngeal nerve loop and the superior thyroid artery in the neck surgical region. Anat. Sci. Int. 2014, 90, 209–215. [Google Scholar] [CrossRef]
- Manjappa, T.; Pandit, R. Anatomical Variations in the Origin of Superior Thyroid Artery and its Relation with External Laryngeal Nerve and their Clinical Importance-A Cadaveric Study. J. Univers. Coll. Med. Sci. 2021, 9, 33–37. [Google Scholar] [CrossRef]
- Ozgur, Z.; Govsa, F.; Ozgur, T. Anatomic evaluation of the carotid artery bifurcation in cadavers: Implications for open and endovascular therapy. Surg. Radiol. Anat. 2008, 30, 475–480. [Google Scholar] [CrossRef]
- Ozgur, Z.; Govsa, F.; Celik, S.; Ozgur, T. Clinically relevant variations of the superior thyroid artery: An anatomic guide for surgical neck dissection. Surg. Radiol. Anat. 2009, 31, 151–159. [Google Scholar] [CrossRef]
- Patel, J.P.; Dave, R.V.; Shah, R.K.; Kanani, S.D.; Nirvan, A.B. A study of superior thyroid artery in 50 cadavers. Int. J. Biol. Med. Res. 2013, 4, 2875–2878. [Google Scholar]
- Poyraz, M.; Calguner, E. Bilateral Investigation of the Anatomical Relationships of the External Branch of the Superior Laryngeal Nerve and Superior Thyroid Artery, and also the Recurrent laryngeal Nerve and Inferior thyroid Artery. Okajimas Folia Anat. Jpn. 2001, 78, 65–74. [Google Scholar] [CrossRef]
- Al-Rafiah, A.; El-Haggagy, A.A.; Aal, I.A.; Zaki, A.I. Anatomical study of the carotid bifurcation and origin variations of the ascending pharyngeal and superior thyroid arteries. Folia Morphol. 2011, 70, 47–55. [Google Scholar]
- Shyamala, B.Y.; Akhilandeswari, B. Cadaveric study on variations in the source and level of origin of superior thyroid artery. J. Anat. Soc. India 2021, 70, 251–254. [Google Scholar] [CrossRef]
- Yalcin, B.; Develi, S.; Tubbs, R.S.; Poyrazoglu, Y. A detailed study of the relationship between the external laryngeal nerve and superior thyroid artery, including its glandular branches. Clin. Anat. 2012, 26, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, B.; Develi, S.; Tubbs, R.S.; Poyrazoglu, Y.; Yazar, F. Blood supply of the terminal part of the external branch of the superior laryngeal nerve. Surg. Today 2014, 45, 1160–1165. [Google Scholar] [CrossRef]
- Anand, A.; Metgudmath, R.B.; Belaldavar, B.P. Topographic Evaluation of Superior Thyroid Artery-A Terrain to be Well Versed for Surgeon’s Knife. Indian J. Otolaryngol. Head Neck Surg. 2021, 74 (Suppl. S3), 5994–6000. [Google Scholar] [CrossRef] [PubMed]
- Lučev, N.; Bobinac, D.; Marić, I.; Drešćik, I. Variations of the great arteries in the carotid triangle. Otolaryngol. Neck Surg. 2000, 122, 590–591. [Google Scholar] [CrossRef]
- EBSCOhost. Morphological Considerations on the Origin of the Superior Thyroid Artery. Available online: https://openurl.ebsco.com/EPDB%3Agcd%3A8%3A26281032/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A160189085&crl=c&link_origin=scholar.google.com (accessed on 10 November 2024).
- Lemaire, V.; Jacquemin, G.; Nelissen, X.; Heymans, O. Tip of the greater horn of the hyoid bone: A landmark for cervical surgery. Surg. Radiol. Anat. 2004, 27, 33–36. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, K.-T.; Jeong, B.-O.; Seo, E.-M.; Suk, K.-S.; Lee, J.-H.; Lee, G.-K. The safety zone of percutaneous cervical approach: A dynamic computed tomographic study. Spine 2007, 32, E569–E574. [Google Scholar] [CrossRef]
- Kierner, A.C.; Aigner, M.; Zelenka, I.; Riedl, G.; Burian, M. The Blood Supply of the Sternocleidomastoid Muscle and Its Clinical Implications. Arch. Surg. 1999, 134, 144–147. [Google Scholar] [CrossRef]
- Kierner, A.C.; Aigner, M.; Burian, M. The external branch of the superior laryngeal nerve: Its topographical anatomy as related to surgery of the neck. Arch. Otolaryngol. Neck Surg. 1998, 124, 301–303. [Google Scholar] [CrossRef]
- Kapre, M.; Mangalgiri, A.S.; Mahore, D. Study of Thyro-Lingual Trunk and its Clinical Relevance. Indian J. Otolaryngol. Head Neck Surg. 2011, 65, 102–104. [Google Scholar] [CrossRef]
- Gupta, P.; Bhalla, A.S.; Thulkar, S.; Kumar, A.; Mohanti, B.K.; Thakar, A.; Sharma, A. Variations in superior thyroid artery: A selective angiographic study. Indian J. Radiol. Imaging 2014, 24, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Devadas, D.; Pillay, M.; Sukumaran, T.T. Variations in the origin of superior laryngeal artery. Anat. Cell Biol. 2016, 49, 254–258. [Google Scholar] [CrossRef]
- Dessie, M.A.; Gadeau, A.-P. Variations of the origin of superior thyroid artery and its relationship with the external branch of superior laryngeal nerve. PLoS ONE 2018, 13, e0197075. [Google Scholar] [CrossRef]
- Wang, R.C.; Puig, C.M.; Brown, D.J. Strap muscle neurovascular supply. Laryngoscope 1998, 108, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Devaraja, K.; Punja, R.; Kalthur, S.G.; Pujary, K. Unmapped landmarks around branches of the Superior Laryngeal Nerve: An exploratory cadaveric study. J. Taibah Univ. Med. Sci. 2021, 16, 328–335. [Google Scholar] [CrossRef]
- Esen, K.; Ozgur, A.; Balci, Y.; Tok, S.; Kara, E. Variations in the origins of the thyroid arteries on CT angiography. Jpn. J. Radiol. 2017, 36, 96–102. [Google Scholar] [CrossRef]
- Sharma, N.; Pandit, R.; Neupane, B.; Sah, R.P.; Bhattarai, L.; Yadav, P.K. Right External Carotid Artery Originated Right Superior Thyroid Artery in Cadavers of a Medical College in Western Nepal: A Descriptive Cross-sectional Study. J. Nepal. Med. Assoc. 2021, 59, 906–909. [Google Scholar] [CrossRef]
- Salmeron, J.; Gannon, P.J.; Blackwell, K.E.; Shaari, C.M.; Urken, M.L. Tracheal Transplantation: Superior and Inferior Thyroid Artery Perfusion Territory. Laryngoscope 1998, 108, 849–853. [Google Scholar] [CrossRef]
- EBSCOhost. The Morphology and Topography of the Superior Laryngeal Artery. Available online: https://web-1p-1ebscohost-1com-15kyuz1800070.han.bg.umw.edu.pl/ehost/pdfviewer/pdfviewer?vid=110&sid=1fe85999-6f48-42f0-bfa7-62e9b243b014%40redis (accessed on 11 November 2024).
- Rabson, J.A.; Hurwitz, D.J.; Futrell, J.W. The cutaneous blood supply of the neck: Relevance to incision planning and surgical reconstruction. Br. J. Plast. Surg. 1985, 38, 208–219. [Google Scholar] [CrossRef]
- Ongeti, K.W.; Ogeng’O, J.A. Variant origin of the superior thyroid artery in a Kenyan population. Clin. Anat. 2011, 25, 198–202. [Google Scholar] [CrossRef]
- Park, S.-A.; Lee, J.-H.; Nam, Y.-S.; An, X.; Han, S.-H.; Ha, K.-Y. Topographical anatomy of the anterior cervical approach for c2-3 level. Eur. Spine J. 2013, 22, 1497–1503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmad, R.; Saraf, A.; Kishore, K.; Kalsotra, P. Relation of Superior Laryngeal Nerve and Superior Thyroid Artery with Superior Pole of Thyroid During Thyroid Surgery. Indian J. Otolaryngol. Head. Neck Surg. 2020, 74, 2095–2098. [Google Scholar] [CrossRef] [PubMed]
- Burger, F.; Fritsch, H.; Zwierzina, M.; Prommegger, R.; Konschake, M. Postoperative Hypoparathyroidism in Thyroid Surgery: Anatomic-Surgical Mapping of the Parathyroids and Implications for Thyroid Surgery. Sci. Rep. 2019, 9, 15700. [Google Scholar] [CrossRef] [PubMed]
- Jitpun, E.; Wattanasen, Y.; Tirakotai, W. Do Asians have Higher Carotid Bifurcation? A Computed Tomographic Angiogram Study of the Common Carotid Artery Bifurcation and External Carotid Artery Branching Patterns. Asian J. Neurosurg. 2019, 14, 1082–1088. [Google Scholar] [CrossRef]
- Griepp, D.W.; Sajan, A.; DiRaimo, R.; Starikov, L.; Márquez, S.; Paunovic, J. Quantitative Prediction of the Location of Carotid Bifurcation and Neurovascular Structures in the Carotid Region: A Cross-Sectional Cadaveric Study. Comput. Math. Methods Med. 2021, 2021, 9214104. [Google Scholar] [CrossRef]
- Magoma, G.; Saidi, H.; Kaisha, W. Relation of the external laryngeal nerve to superior thyroid artery in an African population. Anat. J. Afr. 2012, 1, 28–30. [Google Scholar]
- Monfared, A.; Gorti, G.; Kim, D. Microsurgical anatomy of the laryngeal nerves as related to thyroid surgery. Laryngoscope 2002, 112, 386–392. [Google Scholar] [CrossRef]
- Herrera-Núñez, M.; Menchaca-Gutiérrez, J.L.; Pinales-Razo, R.; Elizondo-Riojas, G.; Quiroga-Garza, A.; Fernandez-Rodarte, B.A.; Elizondo-Omaña, R.E.; Guzmán-López, S. Origin variations of the superior thyroid, lingual, and facial arteries: A computed tomography angiography study. Surg. Radiol. Anat. 2020, 42, 1085–1093. [Google Scholar] [CrossRef]
- Ortega, C.; Maranillo, E.; McHanwell, S.; Sañudo, J.; Vázquez-Osorio, T. External laryngeal nerve landmarks revisited. Head. Neck 2018, 40, 1926–1933. [Google Scholar] [CrossRef]
- Sasikumar, N.; S, V.; Raghunath, G.; Karunakaran, B.; S, N.; Ks, P.D.; M, K.; G, S.N.; Gurusamy, K.; Francis, Y.M. Morphometric Study and Branching Patterns of External Carotid Artery Using Computed Tomography Angiography Among the South Indian Population: A Retrospective Study. Cureus 2023, 15, e35624. [Google Scholar] [CrossRef]
- Sreedharan, R.; Krishna, L.; Shetty, A. Origin of superior thyroid artery: Under the surgeon’s knife. J. Vasc. Bras. 2018, 17, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Nagasao, T.; Sakamoto, Y.; Shimizu, Y.; Imanishi, N.; Kishi, K. An anatomical study on the availability of contralateral recipient vessels in hemi-mandibular reconstruction with vascularised free fibula transfer. J. Plast. Surg. Hand Surg. 2017, 51, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Tan, O.; Kantarci, M.; Parmaksizoglu, D.; Uyanik, U.; Durur, I. Determination of the recipient vessels in the head and neck using multislice spiral computed tomography angiography before free flap surgery: A preliminary study. J. Craniofacial Surg. 2007, 18, 1284–1289. [Google Scholar] [CrossRef]
- Chung, J.-H.; Kim, K.-J.; Jung, K.-Y.; Baek, S.-K.; Park, S.-H.; Yoon, E.-S. Recipient vessel selection for head and neck reconstruction: A 30-year experience in a single institution. Arch. Craniofacial Surg. 2020, 21, 269–275. [Google Scholar] [CrossRef]
- Lai, C.; Shen, C.; Chang, Y.; Liu, S.; Lu, C.; Tsai, Y.; Chen, I.; Feng, C.; Wu, C. Recipient vessel selection for multiple free flap transfers in head and neck reconstruction at different periods. Microsurgery 2021, 41, 438–447. [Google Scholar] [CrossRef]
- Chia, H.-L.; Wong, C.-H.; Tan, B.-K.; Tan, K.-C.; Ong, Y.-S. An algorithm for recipient vessel selection in microsurgical head and neck reconstruction. J. Reconstr. Microsurg. 2010, 27, 047–056. [Google Scholar] [CrossRef]
- Chen, H.-C.; Chang, H.-S. The Sternocleidomastoid Flap for Oral Cavity Reconstruction: Extended Indications and Technical Modifications. J. Oral. Maxillofac. Surg. 2015, 73, 2429–2439. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.L.F.; Rozen, W.M.M.; Ross, R.M.; Findlay, M.W.B.; Ashton, M.W.F.; Behan, F.C.F. The superior thyroid artery perforator flap: Anatomical study and clinical series. Plast. Reconstr. Surg. 2012, 129, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


| Title | Abstract | Introduction | Methodology | Results | Discussion | Conclusions | Other Information | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Background/Rationale | Objective | Study Design and Fundamentals | Setting | Sample Size | Subject | Reference Standard | Outcomes and/or Parameters | Measurement and Assessment | Modality | Technique | Bias | Statistical Approach | Ethics | Subjects | Main Results | Descriptive Anatomy | Confounders | Additional Analyses | Key Findings | Interpretation and Comparison | Implication | Limitation | Acknowledgement | Conflict of Interest | Funding | ||||
| Abdalla 2024 [1] | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + |
| Al-Azzawi 2021 [2] | + | + | + | + | + | - | + | + | + | + | + | + | + | - | + | - | - | + | - | + | - | + | + | + | + | + | + | - | + |
| Cobiella 2021 [3] | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | - | + | + | + | + | + | + | - | - | + | + | - | - |
| Estrela 2011 [4] | + | + | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | - | + | + | - | - | - |
| Haller 2011 [5] | + | + | + | + | + | - | - | + | + | - | + | + | + | - | + | - | + | + | + | + | - | + | + | - | - | + | - | - | - |
| Hu 2005 [6] | + | + | + | + | - | - | - | + | + | - | + | + | + | - | - | - | + | + | + | - | - | - | + | - | + | + | + | - | - |
| Lo 2006 [7] | + | + | + | + | - | + | + | + | + | - | + | + | + | - | + | - | + | + | - | + | + | + | + | + | - | + | + | - | - |
| Lu 2014 [8] | + | + | + | + | + | - | - | + | + | + | - | + | + | - | - | + | + | + | + | - | - | + | + | + | - | + | + | - | + |
| Manjappa 2021 [9] | + | + | + | - | + | + | - | + | + | - | + | + | + | - | - | + | - | + | + | - | - | + | + | - | - | + | - | - | - |
| Ozgur 2009 [10] | + | + | + | + | - | + | + | + | + | + | + | + | + | - | + | + | + | + | + | - | - | + | + | - | - | + | - | - | - |
| Ozgur 2008 [11] | + | + | + | + | - | + | + | + | + | + | + | + | - | - | + | - | + | + | + | - | - | + | + | - | - | + | - | - | - |
| Patel 2013 [12] | + | + | - | - | - | + | - | + | + | - | + | - | + | - | - | - | - | + | + | - | - | + | + | - | - | + | + | - | - |
| Poyraz 2001 [13] | + | + | + | + | - | - | - | + | + | + | + | - | + | - | + | - | + | + | + | - | + | + | + | - | - | + | - | + | - |
| Al-Rafiah 2011 [14] | + | + | + | + | + | + | - | + | + | + | + | - | + | + | + | - | + | + | + | - | + | + | + | + | - | + | + | - | - |
| Shyamala 2021 [15] | + | + | + | - | + | + | - | + | + | + | + | + | + | - | - | - | + | + | + | + | - | + | + | - | - | + | - | - | + |
| Yalcin 2012 [16] | + | + | + | + | + | - | - | + | + | + | - | + | + | - | - | - | - | + | + | + | - | + | + | - | - | + | - | - | - |
| Yalcin 2014 [17] | + | + | + | + | + | - | - | + | + | + | + | + | + | - | - | - | - | + | + | + | - | + | + | + | - | + | + | - | - |
| Anand 2021 [18] | + | + | + | + | + | - | + | + | - | + | + | + | + | - | + | - | - | + | + | - | - | + | + | + | - | + | - | - | - |
| Lucev 2000 [19] | + | + | + | + | + | - | + | + | - | + | + | - | - | - | - | - | + | + | - | - | - | + | + | - | - | + | - | - | - |
| Bunea 2022 [20] | + | + | + | + | + | + | - | - | + | + | + | + | + | - | - | - | + | + | + | + | - | + | + | - | - | + | - | - | - |
| Lemaire 2004 [21] | + | + | + | + | + | - | + | + | - | + | + | + | + | + | + | - | - | + | + | + | - | + | + | + | + | + | + | - | - |
| Lee 2007 [22] | + | + | + | + | + | - | + | + | - | + | + | + | + | - | + | + | + | + | + | - | - | + | + | + | - | + | - | - | - |
| Kierner 1999 [23] | + | + | + | + | + | + | + | + | - | + | - | + | + | + | - | - | + | + | + | - | - | + | + | + | - | + | + | - | - |
| Kierner 1998 [24] | + | + | + | + | + | + | + | + | - | + | - | - | + | - | - | - | + | + | + | - | + | + | + | + | - | + | - | - | - |
| Kapre 2011 [25] | + | + | + | + | + | + | + | + | - | + | - | - | + | - | - | - | - | + | + | - | - | + | + | + | - | + | - | - | - |
| Gupta 2014 [26] | + | + | + | + | + | + | + | + | - | + | + | + | + | - | - | + | + | + | + | - | + | + | + | + | - | + | - | - | + |
| Devadas 2016 [27] | + | + | + | + | + | - | + | + | + | - | - | - | + | - | - | - | + | + | + | - | - | + | + | + | - | - | - | - | - |
| Dessie 2018 [28] | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | - | - | + | + | + | - | + | + | - | - |
| Wang 1998 [29] | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | + | - | - |
| Devaraja 2021 [30] | + | + | + | + | + | + | + | + | - | + | + | + | + | - | - | + | + | + | + | - | - | + | + | + | + | + | + | - | - |
| Esen 2017 [31] | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | - | + | + | - | + | + | + | + | + | + | - | + | - |
| Sharma 2021 [32] | + | + | + | + | + | + | + | + | + | + | - | - | + | - | + | + | + | + | + | - | - | + | + | + | + | + | - | + | - |
| Salmeron 1998 [33] | + | + | + | + | + | + | + | + | + | + | + | + | + | - | - | - | + | + | + | + | - | + | + | + | + | + | - | - | + |
| Rusu 2007 [34] | + | + | + | + | + | - | + | + | - | - | - | + | + | - | - | - | + | + | + | + | + | + | + | + | - | - | - | - | - |
| Rabson 1985 [35] | + | + | + | + | + | - | + | + | - | + | + | + | + | - | - | - | + | + | + | - | - | + | + | + | - | - | - | - | - |
| Ongeti 2011 [36] | + | + | + | + | + | + | + | + | + | + | - | + | + | - | + | - | - | + | + | - | - | + | + | + | + | + | - | - | - |
| Park 2013 [37] | + | + | + | + | + | - | + | + | + | + | + | + | + | - | + | + | - | + | + | - | - | + | + | + | + | + | - | + | - |
| Ahmad 2020 [38] | + | + | + | + | + | + | + | + | + | + | + | - | + | - | + | - | + | + | + | + | + | + | - | + | - | + | + | - | + |
| Burger 2019 [39] | + | + | + | + | + | + | - | + | + | + | + | + | - | - | - | + | + | + | + | - | + | + | + | + | + | + | + | + | - |
| Jitpun 2019 [40] | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | - | + | + | + | + | + | + | - | + | - |
| Griepp 2021 [41] | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | - | - | + | + | + | + | + | + | + | - |
| Magoma 2012 [42] | + | + | + | - | + | + | + | + | + | + | + | + | + | - | + | - | + | + | + | - | - | + | + | + | + | + | - | - | - |
| Monfared 2002 [43] | + | + | + | + | + | + | - | + | + | - | + | + | + | - | - | - | + | + | + | - | - | + | + | + | + | + | + | - | - |
| Herrera Nunez 2020 [44] | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | - | + | + | + | - | + | + | - | + | + | + | + | - |
| Ortega 2018 [45] | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | - | + | + | + | - | + | + | - | - | + |
| Sasikumar 2023 [46] | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | - | - | + | + | - | - | + | + | + | + |
| Sreedharan 2018 [47] | + | + | + | + | + | - | + | + | - | + | + | + | + | - | + | + | + | + | + | - | + | + | - | + | + | - | + | + | + |
| Kasai 2017 [48] | + | + | + | + | + | + | - | + | + | + | + | + | + | - | + | + | + | + | + | - | - | + | + | + | + | + | - | + | + |
| Tan 2007 [49] | + | + | + | + | + | + | + | + | - | + | + | + | + | - | - | - | + | + | + | - | - | + | + | - | - | - | - | - | - |
| Chung 2020 [50] | + | + | + | + | + | + | + | + | + | + | + | - | + | - | + | + | + | + | - | - | - | + | + | + | - | + | - | - | - |
| Lai 2021 [51] | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | + | - |
| Chia 2010 [52] | + | + | + | + | + | + | - | + | - | + | - | - | + | - | - | - | + | + | + | + | + | - | + | + | - | + | - | - | - |
| Chen 2015 [53] | + | + | + | + | + | + | + | + | - | - | - | + | + | - | + | - | + | + | + | - | + | - | + | + | - | + | + | - | - |
| Wilson 2012 [54] | + | + | + | + | + | + | + | + | - | + | + | - | + | - | - | - | + | + | + | - | + | + | + | + | + | + | + | + | + |
| Study | ECA (%) [95% Cl] | CB (%) [95% Cl] | CCA (%) [95% Cl] |
|---|---|---|---|
| Sreedharan et al. [47] (n = 60) | 53 (88.33%) [77.82–94.23] | 5 (8.33%) [3.61–18.07] | 2 (3.33%) [0.92–11.36] |
| Sharma et al. [32] (n = 30) | 27 (90%) [74.38–96.54] | 2 (6.67%) [1.85–21.32] | 1 (3.33%) [0.59–16.67] |
| IN TOTAL | 80 (88.89%) [80.74–93.85] | 7 (7.78%) [3.82–15.19] | 3 (3.33%) [1.14–9.35] |
| Author | Nitash Sharma | Maria Christina Bunea | Mario Herrera-Núñez | B.Y. Shyamala [15] | ||||
|---|---|---|---|---|---|---|---|---|
| Gender | Male | Female | Male | Female | Male | Female | Male | Female |
| External Carotid Artery | 90.90% | 87.50% | 88.33% | 86.49% | 59% | 50% | 51% | 37% |
| Carotid Bifurcation | X | X | 0% | 4.05% | 29% | 9% | 44% | 58% |
| Common Carotid Artery | X | X | 11.67% | 8.11% | 12% | 16% | 5% | 5% |
| Parathyroid Glands | Mean Prevalence of STA of ITA Anastomoses | Supply by the Same Vessel on Both Sides |
|---|---|---|
| Superior | 24.9% | 39% |
| Inferior | 6.8% | 66% |
| Author | Number of Subjects | Area of SCM Supplied by STA |
|---|---|---|
| Kierner | 31 | Middle part |
| Hu | 26 | Lower half |
| Author | Number of Subjects | 1 (95% Cl) | 2a (95% Cl) | 2b (95% Cl) | No Specific Pattern (95%Cl) |
|---|---|---|---|---|---|
| Dessie | 43 | 57% (43.33–71.62) | 40.7% (26.37–54.42) | 2.3% (0.41–12.06) | - |
| Ahmad | 50 | 53.2% (40.40–67.03) | 17.7% (9.77–30.80) | 22.5% (12.75–35.24) | 6.4% (0.00–32.44) |
| Devaraja | 8 | 25% (7.15–59.07) | 58.3% (30.57–86.32) | 16.6% (2.24–47.09) | - |
| IN TOTAL | 101 | 52.6% (43.79–62.89) | 30.7% (22.54–40.26) | 13.4% (7.68–20.78) | 3.2% (1.02–8.37) |
| Structure | Cervical Level | Frequency (%) | Additional Notes |
|---|---|---|---|
| STA | C3–C4 | 86.7% | Within “safety zone” |
| STA | C4–C5 | 26.7% | Partially safe |
| STA | C5–C6, C6–C7 | 0% | Unsafe levels |
| STA | C3 | 44.4% | Common STA origin level |
| STA | C3–C4 | 22.2% | — |
| ISLN | C3 | 52.8% | Most common level |
| ISLN | C2 | 19.4% | — |
| ISLN | C3–C4 | 11.1% | — |
| ESLN | C3 | 33.3% | Most common ESLN level |
| ESLN | C2–C3 | 13.9% | — |
| ESLN | C3–C4 | 22.2% | — |
| ELN | — | 100% | Deep in ascending pharyngeal vein |
| ELN | — | 89% | Passes medial to STA origin |
| ELN | — | 80% | Passes through inferior pharyngeal constrictor |
| ELN | — | 47% | Crosses carotid axis at STA origin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandra, K.; Stokłosa, J.; Patkowska, A.; Rudko, W.; Mazurek, M.; Domagała, Z. Anatomical Variations in the Superior Thyroid Artery: A Systematic Review and Implications for Free Flap Surgery. J. Clin. Med. 2025, 14, 6250. https://doi.org/10.3390/jcm14176250
Aleksandra K, Stokłosa J, Patkowska A, Rudko W, Mazurek M, Domagała Z. Anatomical Variations in the Superior Thyroid Artery: A Systematic Review and Implications for Free Flap Surgery. Journal of Clinical Medicine. 2025; 14(17):6250. https://doi.org/10.3390/jcm14176250
Chicago/Turabian StyleAleksandra, Królikowska, Julia Stokłosa, Alicja Patkowska, Wiktoria Rudko, Mateusz Mazurek, and Zygmunt Domagała. 2025. "Anatomical Variations in the Superior Thyroid Artery: A Systematic Review and Implications for Free Flap Surgery" Journal of Clinical Medicine 14, no. 17: 6250. https://doi.org/10.3390/jcm14176250
APA StyleAleksandra, K., Stokłosa, J., Patkowska, A., Rudko, W., Mazurek, M., & Domagała, Z. (2025). Anatomical Variations in the Superior Thyroid Artery: A Systematic Review and Implications for Free Flap Surgery. Journal of Clinical Medicine, 14(17), 6250. https://doi.org/10.3390/jcm14176250