Frequency and Characteristics of Craniomaxillofacial Tumors: A Five-Year Retrospective Institutional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Sources and Eligibility
2.3. Case Definitions, Coding, and Unit of Analysis
- Oral/oropharyngeal (C00–C14; SCC-predominant sites);
- Salivary glands (C07–C08);
- Cutaneous head/neck (C43.3–C43.4, C44.3–C44.4; in situ D03.3–D03.4, D04.3–D04.4);
- Sinonasal/middle ear (C30–C31; D14.1 benign middle ear);
- Eye/adnexa (C69);
- Craniofacial bone/soft tissue/peripheral nerve (head/neck) (C41.0, C49.0, C47.0; selected benign D16.4/D17.0/D18.x);
- Regional nodes/lymphoid presentations (C77.0; C82/C83 head–neck).Note: ICD-10 does not encode morphology; therefore, SCC cannot be confirmed at the record level. Odontogenic tumors cannot be isolated cleanly and are reported within the craniofacial bone/soft-tissue/nerve class.
2.4. Variables and Measures
2.5. Outcomes
2.6. Data Management and Quality Assurance
2.7. Handling of Missing Data
2.8. Statistical Analysis
2.9. Reporting Standards and Ethics
3. Results
3.1. Frequency of CMF Tumor Diagnoses (2012–2016)
3.1.1. Outpatient and Emergency Service Volumes
3.1.2. Malignant Share Within the CMF Case Mix (Primary Analysis)
3.1.3. Department-Wide Context (Table 2 and Table 3; Not CMF Prevalence)
3.1.4. Tumor Distribution by ICD-10 Site Category
3.1.5. Trends in Emergency, Urgent Care, and Ambulatory Streams
3.2. Department Admissions (All Causes), Context-Only
3.2.1. Sex-Based Distribution
3.2.2. Age-Based Distribution
3.2.3. Urban vs. Rural Distribution
3.3. Most Common Surgical Procedures for Tumor Treatment (2012–2016)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMF | craniomaxillofacial |
| ED | emergency department |
| EHR | electronic health record |
| ICD-10 | International Classification of Diseases, 10th Revision |
| WHO | World Health Organization |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Schache, A.G.; Powell, N.G.; Cuschieri, K.S.; Robinson, M.; Leary, S.; Mehanna, M.; Rapozo, D.; Long, A.; Cubie, H.; Junor, E.; et al. HPV-Related Oropharynx Cancer in the United Kingdom: An Evolution in the Understanding of Disease Etiology. Cancer Res. 2016, 76, 6598–6606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Shah, J.P. Head and Neck cancers—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Living with oral cancer: Epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol. 2010, 46, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Kanatas, A.; Walshaw, E.G.; Wu, J.; Fabbroni, G.; Chengot, P. Prognostic factors in oral cancer surgery-results from a UK tertiary centre. Eur. J. Surg. Oncol. 2023, 49, 755–759. [Google Scholar] [CrossRef]
- Kowalski, L.P.; Carvalho, A.L. Natural history of untreated head and neck cancer. Eur. J. Cancer 2000, 36, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.N.; Lowe, D.; Yueh, B.; Weymuller, E.A., Jr. The physical function and social-emotional function subscales of the University of Washington Quality of Life Questionnaire. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 352–357. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 5 August 2025).
- Sheikh, M.; Virani, S.; Robbins, H.A.; Foretova, L.; Holcatova, I.; Janout, V.; Lissowska, J.; Navratilova, M.; Mukeriya, A.; Ognjanovic, M.; et al. Survival and prognostic factors of early-stage non-small cell lung cancer in Central and Eastern Europe: A prospective cohort study. Cancer Med. 2023, 12, 10563–10574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pehalova, L.; Krejci, D.; Snajdrova, L.; Dusek, L. Cancer incidence trends in the Czech Republic. Cancer Epidemiol. 2021, 74, 101975. [Google Scholar] [CrossRef] [PubMed]
- Valean, S.; Chira, R.; Dumitrascu, D. Epidemiological trends in digestive cancers in Romania, 1955–2012, compared to alcohol consumption. Correlation or coincidence? Clujul Med. 2018, 91, 376–386. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer–systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E. Oral cancer prevention and control—The approach of the World Health Organization. Oral Oncol. 2009, 45, 454–460. [Google Scholar] [CrossRef] [PubMed]
- WHO. International Classification of Diseases for Oncology (ICD-O), 3rd ed.; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- WHO. Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Carvalho, A.L.; Nishimoto, I.N.; Califano, J.A.; Kowalski, L.P. Trends in incidence and prognosis for head and neck cancer in the United States: A site-specific analysis of the SEER database. Int. J. Cancer 2005, 114, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 5, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Ariyawardana, A. Malignant transformation of oral leukoplakia: A systematic review of observational studies. J. Oral Pathol. Med. 2016, 45, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef] [PubMed]
- de Camargo Cancela, M.; de Souza, D.L.; Curado, M.P. International incidence of oropharyngeal cancer: A population-based study. Oral Oncol. 2012, 48, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Fossum, G.H.; Lie, A.K.; Jebsen, P.; Sandlie, L.E.; Mork, J. Human papillomavirus in oropharyngeal squamous cell carcinoma in South-Eastern Norway: Prevalence, genotype, and survival. Eur. Arch. Otorhinolaryngol. 2017, 274, 4003–4010. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, J.N.; Li, C.Z.; Guo, C.B. Elective neck dissection versus observation in the management of early tongue carcinoma with clinically node-negative neck: A retrospective study of 229 cases. J. Craniomaxillofac. Surg. 2014, 42, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Caudell, J.J.; Gillison, M.L.; Maghami, E.; Spencer, S.; Pfister, D.G.; Adkins, D.; Birkeland, A.C.; Brizel, D.M.; Busse, P.M.; Cmelak, A.J.; et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 1.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 224–234. [Google Scholar] [CrossRef] [PubMed]


| Year | CMF Tumor Diagnoses | Non-CMF Tumor Diagnoses (Other Body Sites) | Total |
|---|---|---|---|
| 2012 | 1698 | 930 | 2628 |
| 2013 | 2337 | 515 | 2852 |
| 2014 | 1304 | 1497 | 2801 |
| 2015 | 905 | 957 | 1862 |
| 2016 | 1725 | 91 | 1816 |
| TOTAL | 7969 | 3990 | 11,959 |
| YEAR | Total Recorded Conditions | Malignant Tumor Diagnoses—CMF | Malignant Tumor Diagnoses—NON-CMF | Total Malignant Tumor Diagnoses | Benign Tumor Diagnoses |
|---|---|---|---|---|---|
| 2012 | 13,368 | 1606 | 552 | 2158 | 470 |
| 2013 | 12,411 | 1813 | 412 | 2225 | 627 |
| 2014 | 12,772 | 1055 | 1030 | 2085 | 716 |
| 2015 | 8895 | 671 | 612 | 1283 | 579 |
| 2016 | 9120 | 580 | 76 | 656 | 1160 |
| Year | Benign (N) | Benign (%) | Malignant (N) | Malignant (%) |
|---|---|---|---|---|
| 2012 | 470 | 3.52 | 2158 | 16.14 |
| 2013 | 627 | 5.05 | 2225 | 17.93 |
| 2014 | 716 | 5.61 | 2085 | 16.32 |
| 2015 | 579 | 6.51 | 1283 | 14.42 |
| 2016 | 1160 | 12.72 | 656 | 7.19 |
| ICD-10 | ICD-10 DIAGNOSIS | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|
| C00 | Malignant neoplasm of lip | 1 | 7 | 7 | 18 | 23 |
| C01 | Malignant neoplasm of base of tongue | 1 | 1 | 4 | 107 | 16 |
| C02 | Malignant neoplasm of other/unspecified parts of tongue | 40 | 1 | 2 | 5 | 97 |
| C03 | Malignant neoplasm of gum | 41 | 72 | 51 | 114 | 4 |
| C04 | Malignant neoplasm of floor of mouth | 157 | 53 | 47 | 17 | 70 |
| C05 | Malignant neoplasm of palate | 15 | 155 | 190 | 46 | 10 |
| C06 | Malignant neoplasm of other/unspecified parts of mouth | 103 | 8 | 10 | 29 | 29 |
| C07 | Malignant neoplasm of parotid gland | 7 | 133 | 196 | 10 | 30 |
| C08 | Malignant neoplasm of other/unspecified major salivary glands | 21 | 14 | 19 | 9 | 9 |
| C09 | Malignant neoplasm of tonsil | 34 | 41 | 49 | 10 | 6 |
| C10 | Malignant neoplasm of oropharynx | 28 | 44 | 43 | 1 | 6 |
| C13 | Malignant neoplasm of hypopharynx | 7 | 21 | 20 | 2 | 1 |
| C14 | Other and ill-defined sites in the lip, oral cavity, and pharynx | 22 | 5 | 19 | 27 | 13 |
| C30.0/C30.1 | Malignant neoplasm of nasal cavity and middle ear | 39 | 70 | 1 | 33 | 10 |
| C31 | Malignant neoplasm of paranasal sinuses | 2 | 13 | 41 | 2 | 3 |
| C41.0 | Malignant neoplasm of bones of skull and face | 1 | 3 | 6 | 5 | 2 |
| C41.0/C41.9 | Malignant neoplasm of bone (skull/face and unspecified) * | 1 | 213 | 5 | 34 | 83 |
| C43.3–C43.4 | Malignant melanoma of skin of face/scalp/neck | 160 | 9 | 2 | 1 | 10 |
| C44.3–C44.4 | Other malignant neoplasms of skin of face/scalp/neck | 2 | 59 | 19 | 18 | 53 |
| C47.0 | Malignant neoplasm of peripheral nerves of head/face/neck * | 7 | 11 | 54 | 2 | 1 |
| C49.0 | Malignant neoplasm of connective/soft tissue of head/face/neck * | 1 | 2 | 1 | 1 | 16 |
| C69 | Malignant neoplasm of eye and adnexa | 1 | 4 | 52 | 20 | 40 |
| C77.0 | Secondary malignant neoplasm of lymph nodes of head/face/neck † | 2 | 3 | 45 | 1 | 9 |
| C82 (HN) | Follicular lymphoma (head/neck presentation) † | 2 | 1 | 2 | 11 | 1 |
| C83 (HN) | Diffuse non-Hodgkin lymphoma (head/neck presentation) † | 7 | 1 | 5 | 1 | 4 |
| D00.0 | Carcinoma in situ of oral cavity | 3 | 12 | 5 | 1 | 17 |
| D02.0 | Carcinoma in situ of middle ear * | 2 | 8 | 10 | 4 | 1 |
| D03.3–D03.4 | Melanoma in situ of face/scalp/neck | 106 | 110 | 10 | 4 | 1 |
| D04.3–D04.4 | Carcinoma in situ of skin of face/scalp/neck | 785 | 708 | 137 | 107 | 5 |
| D16.4 | Benign neoplasm of bones of skull and face | 1 | 2 | 191 | 131 | 376 |
| D17.0 | Benign lipomatous neoplasm of skin/subcutis of head/face/neck | 1 | 61 | 2 | 1 | 25 |
| D18.X | Hemangioma and lymphangioma (head/neck sites) | 4 | 445 | 1 | 1 | 16 |
| D23.3–D23.4 | Benign neoplasm of skin of face/scalp/neck | 71 | 6 | 1 | 65 | 185 |
| D10.X | Benign neoplasm of mouth and pharynx * | 11 | 2 | 3 | 1 | 396 |
| D14.1 | Benign neoplasm of middle ear * | 1 | 6 | 11 | 14 | 2 |
| TOTAL (INCLUDED ROWS) | 1687 | 2304 | 1261 | 853 | 1570 |
| Year | Outpatient Total | Outpatient Urgent | ED Total | ED Urgent |
|---|---|---|---|---|
| 2012 | 12,565 | 329 | 4399 | 2908 |
| 2013 | 10,422 | 402 | 4205 | 2775 |
| 2014 | 10,071 | 314 | 4209 | 2692 |
| 2015 | 9089 | 239 | 4004 | 2762 |
| 2016 | 9795 | 343 | 3972 | 2609 |
| TOTAL | 51,942 | 1627 | 20,789 | 13,746 |
| Month | Total (Urban + Rural) | Urban Total | Urban Adults | Urban Children | Rural Total | Rural Adults | Rural Children |
|---|---|---|---|---|---|---|---|
| January | 815 | 491 | 457 | 34 | 324 | 290 | 34 |
| February | 779 | 515 | 484 | 31 | 264 | 236 | 28 |
| March | 944 | 591 | 563 | 28 | 353 | 316 | 37 |
| April | 692 | 437 | 407 | 30 | 255 | 231 | 24 |
| May | 799 | 507 | 483 | 24 | 292 | 256 | 36 |
| June | 820 | 576 | 539 | 37 | 244 | 212 | 32 |
| July | 801 | 519 | 480 | 39 | 282 | 252 | 30 |
| August | 641 | 387 | 356 | 31 | 254 | 230 | 24 |
| September | 718 | 445 | 423 | 22 | 273 | 248 | 25 |
| October | 805 | 490 | 459 | 31 | 315 | 281 | 34 |
| November | 726 | 469 | 444 | 25 | 257 | 235 | 22 |
| December | 506 | 321 | 301 | 20 | 185 | 168 | 17 |
| Total | 9046 | 5748 | 5396 | 352 | 3298 | 2955 | 343 |
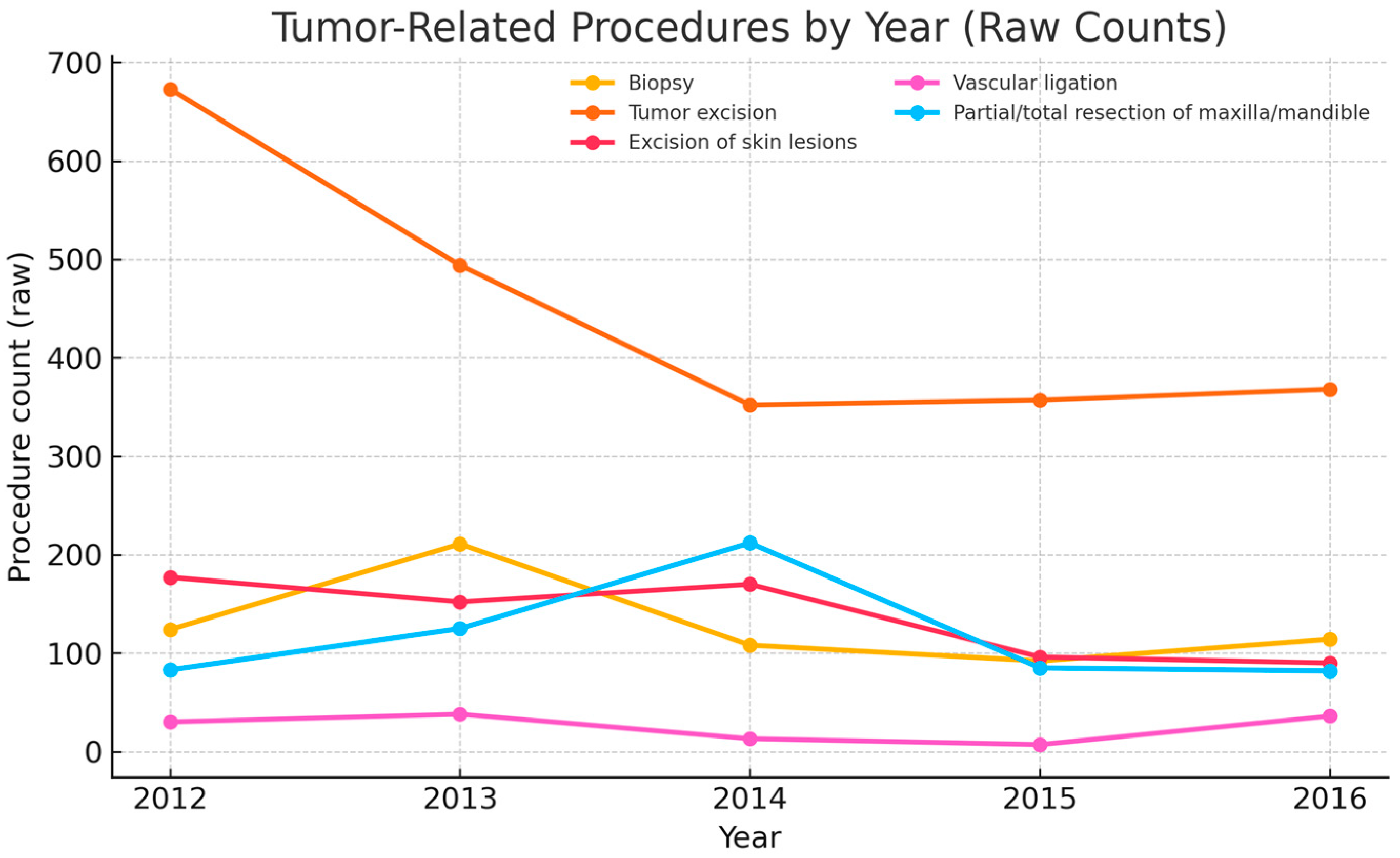
| Procedure | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|
| Biopsy | 124 | 211 | 108 | 92 | 114 |
| Tumor excision | 673 | 494 | 352 | 357 | 368 |
| Excision of skin lesions | 177 | 152 | 170 | 96 | 90 |
| Vascular ligation | 30 | 38 | 13 | 7 | 36 |
| Partial/total resection of maxilla/mandible | 83 | 125 | 212 | 85 | 82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, G.-D.; Veja, I.; Niculescu, S.T.; Mazilescu, C.-A.; Hoinoiu, T.; Buda, V.O.; Oancea, R. Frequency and Characteristics of Craniomaxillofacial Tumors: A Five-Year Retrospective Institutional Study. J. Clin. Med. 2025, 14, 6256. https://doi.org/10.3390/jcm14176256
Constantin G-D, Veja I, Niculescu ST, Mazilescu C-A, Hoinoiu T, Buda VO, Oancea R. Frequency and Characteristics of Craniomaxillofacial Tumors: A Five-Year Retrospective Institutional Study. Journal of Clinical Medicine. 2025; 14(17):6256. https://doi.org/10.3390/jcm14176256
Chicago/Turabian StyleConstantin, George-Dumitru, Ioana Veja, Serban Talpos Niculescu, Crisanta-Alina Mazilescu, Teodora Hoinoiu, Valentina Oana Buda, and Roxana Oancea. 2025. "Frequency and Characteristics of Craniomaxillofacial Tumors: A Five-Year Retrospective Institutional Study" Journal of Clinical Medicine 14, no. 17: 6256. https://doi.org/10.3390/jcm14176256
APA StyleConstantin, G.-D., Veja, I., Niculescu, S. T., Mazilescu, C.-A., Hoinoiu, T., Buda, V. O., & Oancea, R. (2025). Frequency and Characteristics of Craniomaxillofacial Tumors: A Five-Year Retrospective Institutional Study. Journal of Clinical Medicine, 14(17), 6256. https://doi.org/10.3390/jcm14176256









