Outcomes of Vitrectomy for Macular Pathologies Associated with Idiopathic Vasoproliferative Retinal Tumor
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Techniques
2.3. Statistical Analyses
3. Results
3.1. Preoperative Demographics and Surgical Procedures
3.2. Postoperative Outcomes After Vitrectomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rennie, I.G. Retinal vasoproliferative tumours. Eye 2010, 24, 468–471. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A.; Barrett, J.; De Potter, P. Vasoproliferative tumors of the ocular fundus. Classification and clinical manifestations in 103 patients. Arch. Ophthalmol. 1995, 113, 615–623. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Al-Dahmash, S.; Rojanaporn, D.; Shukla, S.Y.; Reilly, B.; Shields, J.A. Retinal vasoproliferative tumors: Comparative clinical features of primary vs secondary tumors in 334 cases. JAMA Ophthalmol. 2013, 131, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Baines, P.S.; Hiscott, P.S.; McLeod, D. Posterior non-vascularized proliferative extraretinopathy and peripheral nodular retinal telangiectasis. Trans. Ophthalmol. Soc. UK 1982, 102 Pt 4, 487–491. [Google Scholar]
- Laqua, H.; Wessing, A. Peripheral retinal telangiectasis in adults simulating a vascular tumor or melanoma. Ophthalmology 1983, 90, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Decker, W.L.; Sanborn, G.E.; Augsburger, J.J.; Goldberg, R.E. Presumed acquired retinal hemangiomas. Ophthalmology 1983, 90, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Pichi, F.; Neri, P.; Agarwal, A.; Invernizzi, A.; Choudhry, N.; Amer, R.; Lembo, A.; Nucci, P.; Thompson, I.; Sen, H.N.; et al. Vasoproliferative tumors in intermediate uveitis. Retina 2020, 40, 1765–1773. [Google Scholar] [CrossRef]
- Shields, J.A.; Reichstein, D.; Mashayekhi, A.; Shields, C.L. Retinal vasoproliferative tumors in ocular conditions of childhood. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2012, 16, 6–9. [Google Scholar] [CrossRef]
- Li, H.K.; Shields, J.A.; Shields, C.L.; Maguire, J.I.; Garg, S.J. Retinal vasoproliferative tumour as the initial manifestation of retinitis pigmentosa. Clin. Exp. Ophthalmol. 2008, 36, 895–897. [Google Scholar] [CrossRef]
- Ledesma-Gil, G.; Moreno Andrade, A.B.; Shields, C.L. Secondary vasoproliferative tumor in adult-onset Coats disease. Can. J. Ophthalmol. 2021, 57, 69–70. [Google Scholar] [CrossRef]
- Bottazzi, L.; L’Abbate, G.; Fossataro, C.; Savastano, M.C.; Sacconi, R.; Rizzo, S.; Bandello, F.; Querques, G. Management of Vasoproliferative Tumor in a Patient With Coats’ Disease: Multiple Therapeutic Approach in Current Era. Ophthalmic Surg. Lasers Imaging Retina. 2025, 56, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Kitei, P.M.; Say, E.A.; Shields, C.L.; Regillo, C.D.; Shields, J.A. Management of retinal vasoproliferative tumor associated with ROP by plaque brachytherapy. J. Pediatr. Ophthalmol. Strabismus. 2011, 48, e10–e12. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Pellegrini, M.; Kaliki, S.; Mashayekhi, A.; Shields, C.L. Retinal vasoproliferative tumors in 6 patients with neurofibromatosis type 1. JAMA Ophthalmol. 2014, 132, 190–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Au, A.K.; Shields, C.L.; Kalina, R.E.; Wells, C.G.; Shieldsm, J.A. Bilateral vasoproliferative retinal tumors in a patient with autosomal dominant aniridia. Retin. Cases Brief. Rep. 2007, 1, 249–250. [Google Scholar] [CrossRef]
- Rundle, P.; Shields, J.A.; Shields, C.L.; Singh, A.D.; Peairs, R. Vasoproliferative tumour of the ocular fundus associated with Waardenburg’s syndrome. Eye 2000, 14 Pt 1, 105–106. [Google Scholar] [CrossRef]
- Chen, C.Y.; Engelbert, M.; Freund, K.B.; Shields, C.L.; Barile, G. Choroidal neovascularization and chorioretinal anastomoses following laser treatment of a secondary vasoproliferative tumor. JAMA Ophthalmol. 2014, 132, 1488–1490. [Google Scholar] [CrossRef]
- Manjandavida, F.P.; Shields, C.L.; Kaliki, S.; Shields, J.A. Cryotherapy-induced release of epiretinal membrane associated with retinal vasoproliferative tumor: Analysis of 16 cases. Retina 2014, 34, 1644–1650. [Google Scholar] [CrossRef]
- Makdoumi, K.; Crafoord, S. Vasoproliferative retinal tumours in a Swedish population. Acta Ophthalmol. 2011, 89, 91–94. [Google Scholar] [CrossRef]
- Chu, T.W.; Tsai, S.H.; Chen, L.J. Retinal vasoproliferative tumor regression after intravitreal aflibercept. Taiwan J. Ophthalmol. 2022, 13, 249–252. [Google Scholar] [CrossRef]
- Nickerson, S.J.; Al-Dahmash, S.A.; Shields, C.L.; Shields, J.A. Retinal vasoproliferative tumor with total retinal detachment managed with plaque radiotherapy. Oman J. Ophthalmol. 2012, 5, 53–54. [Google Scholar] [CrossRef]
- Cohen, V.M.; Shields, C.L.; Demirci, H.; Shields, J.A. Iodine I 125 plaque radiotherapy for vasoproliferative tumors of the retina in 30 eyes. Arch. Ophthalmol. 2008, 126, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Poole Perry, L.J.; Jakobiec, F.A.; Zakka, F.R.; Reichel, E.; Herwig, M.C.; Perry, A.; Brat, D.J.; Grossniklaus, H.E. Reactive retinal astrocytic tumors (so-called vasoproliferative tumors): Histopathologic, immunohistochemical, and genetic studies of four cases. Am. J. Ophthalmol. 2013, 155, 593–608.e1. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L. Reactive retinal astrocytic tumors (so-called vasoproliferative tumors): Histopathologic, immunohistochemical, and genetic studies of four cases. Am. J. Ophthalmol. 2013, 156, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Chen, S.J. Clinical characters and treatments of retinal vasoproliferative tumors. Taiwan J. Ophthalmol. 2016, 6, 85–88. [Google Scholar] [CrossRef]
- Castro-Navarro, V.; Saktanasate, J.; Say, E.A.; Chiang, A.; Shields, C.L. Role of pars plana vitrectomy and membrane peel in vitreomacular traction associated with retinal vasoproliferative tumors. Oman J. Ophthalmol. 2016, 9, 167–169. [Google Scholar] [CrossRef]
- Jong, J.L.Z.; Jawaheer, L.; Spiteri-Cornish, K.; Chawla, A. Surgical outcomes of pars plana vitrectomy for intraocular complications related to vasoproliferative tumor of the retina. Retina 2023, 43, 1980–1987. [Google Scholar] [CrossRef]
- Diafas, A.; Toumanidou, V.; Kassos, I.; Samouilidou, M.; Dastiridou, A.; Ziakas, N.; Androudi, S. Macular sequelae in vasoproliferative tumors: Results of surgical approach. Int. Ophthalmol. 2021, 41, 3515–3522. [Google Scholar] [CrossRef]
- Zhang, W.; Qiang, Z.; Song, H.; Li, X.; Dan, H.; Ge, K.; Li, P.; Huang, Z.; Wang, D.; Chen, F.; et al. Management of Vasoproliferative Tumors of the Retina with Macular Complications by Pars Plana Vitrectomy Combined with Episcleral Cryotherapy. J. Ophthalmol. 2021, 13, 6667755. [Google Scholar] [CrossRef]
- Garcia-Arumi, J.; Distefano, L.N.; Fonollosa, A.; Quijano, C.; Corcostegui, B. Management of Vision-Threatening Complications of Vasoproliferative Tumors of the Retina. Ophthalmic Res. 2015, 54, 34–40. [Google Scholar] [CrossRef]
- Grondin, C.; Lorenzi, U.; Beral, L.; Morane, S.; Guillaumie, T.; David, T. Successful closure of a large full-thickness macular hole secondary to an idiopathic retinal vasoproliferative tumor using the inverted internal limiting membrane flap technique. Retin. Cases Brief. Rep. 2022, 16, 490–493. [Google Scholar] [CrossRef]
- Hashimoto, I.; Takase, H.; Kase, S.; Iwasaki, Y.; Kobayashi, D.; Ohno-Matsui, K. Clinicopathological analysis of secondary retinal vasoproliferative tumor/reactive retinal astrocytic tumor successfully treated by endoresection. Retin. Cases Brief. Rep. 2024, 18, 106–111. [Google Scholar] [CrossRef]
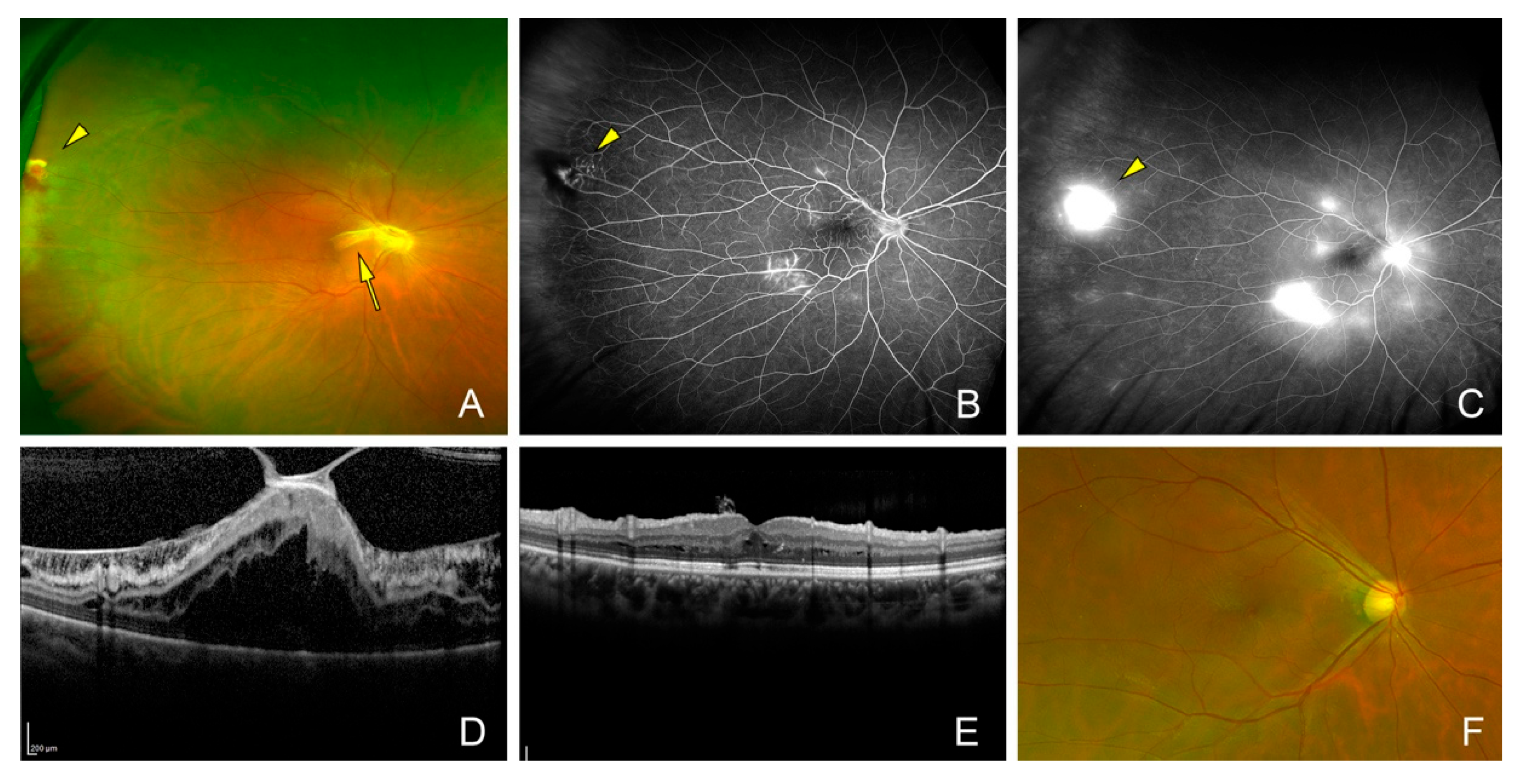

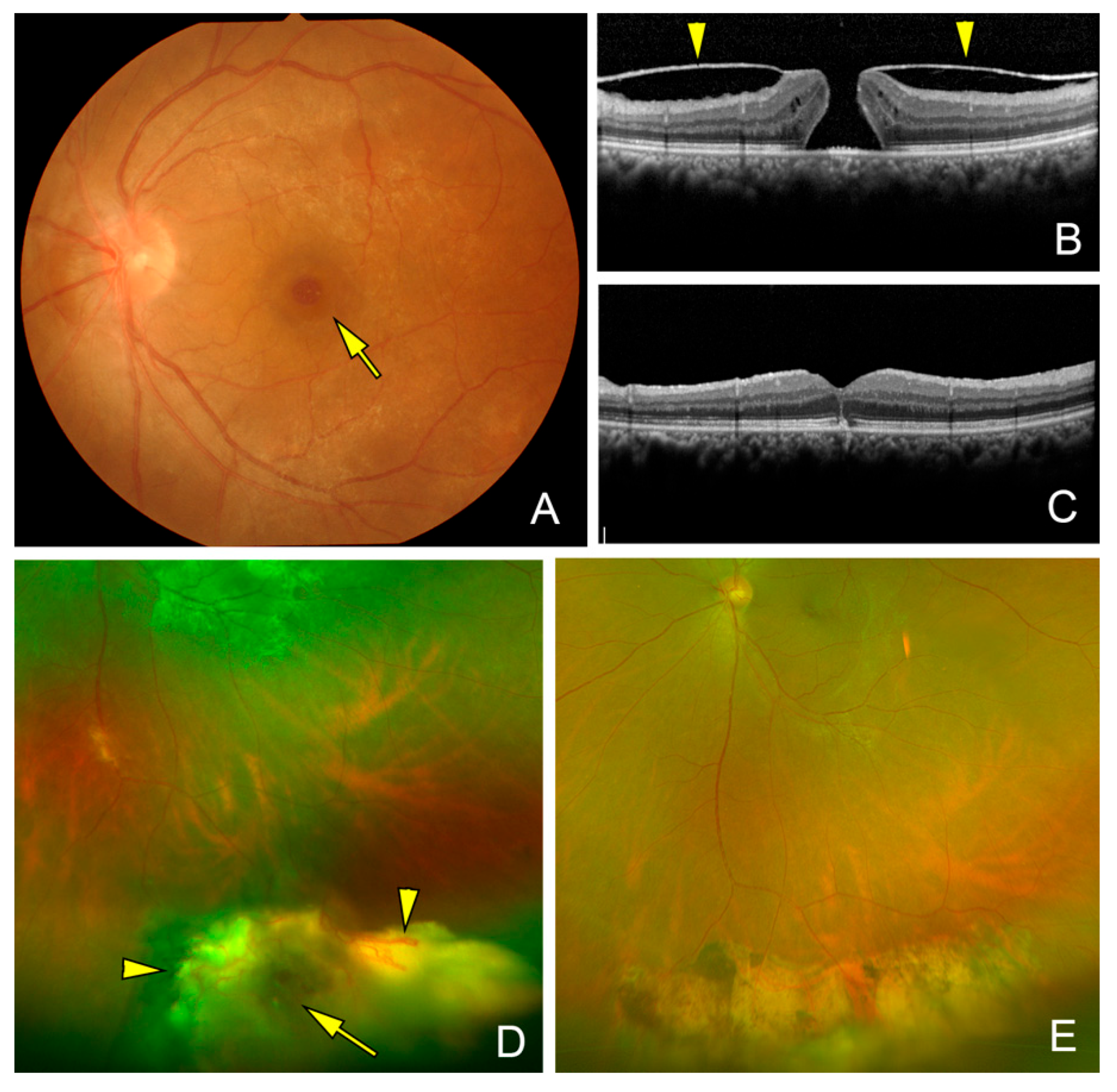
| Case | Side | Age | Sex | Symptom | Location of Tumor | Size of Tumor (DD) | Extent of Exudation (DD) | PVD | MH | ERM | VH | Exudative RD | Tractional RD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | L | 22 | F | decreased VA | temporal-inferior | 1 | 2 | − | − | + | − | − | + |
| 2 | R | 51 | F | metamorphopsia | temporal-inferior | 2 | 8 | − | − | + | − | − | − |
| 3 | L | 52 | F | metamorphopsia | temporal-inferior | 3 | 7 | − | + | + | − | − | − |
| 4 | R | 53 | F | decreased VA | temporal-inferior | 2 | 4 | − | + | + | − | − | − |
| 5 | R | 35 | F | blurry vision | temporal-inferior | 2 | 4 | − | − | + | − | − | + |
| 6 | L | 72 | F | metamorphopsia | temporal-inferior | 6 | 8 | + | − | + | + | + | − |
| 7 | R | 36 | F | metamorphopsia | temporal-superior | 3 | 7 | − | − | + | − | − | − |
| 8 | R | 72 | M | decreased VA | temporal-inferior | 1 | 3 | − | − | + | − | + | − |
| 8 | L | 72 | M | decreased VA | temporal-inferior | 3 | 6 | − | − | + | − | + | − |
| 9 | L | 68 | F | decreased VA | temporal-inferior | 2 | 7 | − | − | + | − | + | − |
| 10 | R | 70 | M | decreased VA | temporal-inferior | 3 | 5 | + | − | + | + | − | + |
| 11 | R | 35 | M | metamorphopsia | temporal-superior | 1 | 2 | − | − | + | + | − | + |
| 12 | R | 48 | M | metamorphopsia | temporal-inferior | 2 | 4 | + | − | + | − | − | − |
| Case | Side | Follow Up Duration (Month) | Baseline BCVA | Refractive Error (D) | Treatment | BCVA-1M | BCVA-3M | BCVA-6M | BCVA-Final |
|---|---|---|---|---|---|---|---|---|---|
| 1 | L | 12 | 0.1 | −4.25 | PC | 0.3 | 0.4 | 0.8 | 0.8 |
| 2 | R | 14 | 0.4 | −0.75 | PC | 1.0 | 1.0 | 1.2 | 1.2 |
| 3 | L | 27 | 0.3 | 0.5 | PC, cryo | 0.5 | 0.5 | 0.6 | 0.7 |
| 4 | R | 12 | 0.8 | 2.25 | PC, dia | 1.0 | 1.0 | 0.8 | 1.0 |
| 5 | R | 14 | 0.6 | −9 | PC | 1.2 | 1.2 | 1.2 | 1.2 |
| 6 | L | 21 | 0.8 | −3 | PC, cryo | 0.7 | 0.5 | 0.7 | 1.0 |
| 7 | R | 84 | 0.9 | −2.75 | PC, cryo, dia | 1.0 | 1.2 | 1.2 | 1.2 |
| 8 | R | 12 | 0.8 | 0 | PC | 0.9 | 1.0 | 1.0 | 1.0 |
| 8 | L | 12 | 1.0 | 0.25 | PC | 1.0 | 1.0 | 1.0 | 1.0 |
| 9 | L | 121 | 0.01 | 1.5 | PC, cryo | 1.0 | 0.9 | 1.0 | 1.0 |
| 10 | R | 12 | 0.8 | 2.25 | PC | 1.2 | 1.2 | 1.2 | 1.2 |
| 11 | R | 12 | 0.02 | −2.5 | PC | 0.1 | 0.2 | 0.3 | 0.2 |
| 12 | R | 20 | 0.5 | 0.5 | PC | 1.0 | 1.0 | 1.2 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinozaki, M.; Ohara, H.; Torikai, T.; Koto, T.; Inoue, M. Outcomes of Vitrectomy for Macular Pathologies Associated with Idiopathic Vasoproliferative Retinal Tumor. J. Clin. Med. 2025, 14, 6221. https://doi.org/10.3390/jcm14176221
Shinozaki M, Ohara H, Torikai T, Koto T, Inoue M. Outcomes of Vitrectomy for Macular Pathologies Associated with Idiopathic Vasoproliferative Retinal Tumor. Journal of Clinical Medicine. 2025; 14(17):6221. https://doi.org/10.3390/jcm14176221
Chicago/Turabian StyleShinozaki, Masatoshi, Hiromi Ohara, Tomohiko Torikai, Takashi Koto, and Makoto Inoue. 2025. "Outcomes of Vitrectomy for Macular Pathologies Associated with Idiopathic Vasoproliferative Retinal Tumor" Journal of Clinical Medicine 14, no. 17: 6221. https://doi.org/10.3390/jcm14176221
APA StyleShinozaki, M., Ohara, H., Torikai, T., Koto, T., & Inoue, M. (2025). Outcomes of Vitrectomy for Macular Pathologies Associated with Idiopathic Vasoproliferative Retinal Tumor. Journal of Clinical Medicine, 14(17), 6221. https://doi.org/10.3390/jcm14176221






