Redox Status in Patients Suffering from Multiple Chemical Sensitivity (MCS): A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sample Collection
2.3. Oxidative Stress Studies
2.3.1. Lipid Peroxidation (LPO) in Plasma
2.3.2. Plasma Total Antioxidant Activity (TAA)
2.3.3. Amount of ATP in Plasma
2.3.4. Antioxidant Enzyme Activities in Erythrocytes
2.3.5. Glutathione Cycle in Erythrocytes
2.4. Statistical Analysis
3. Results
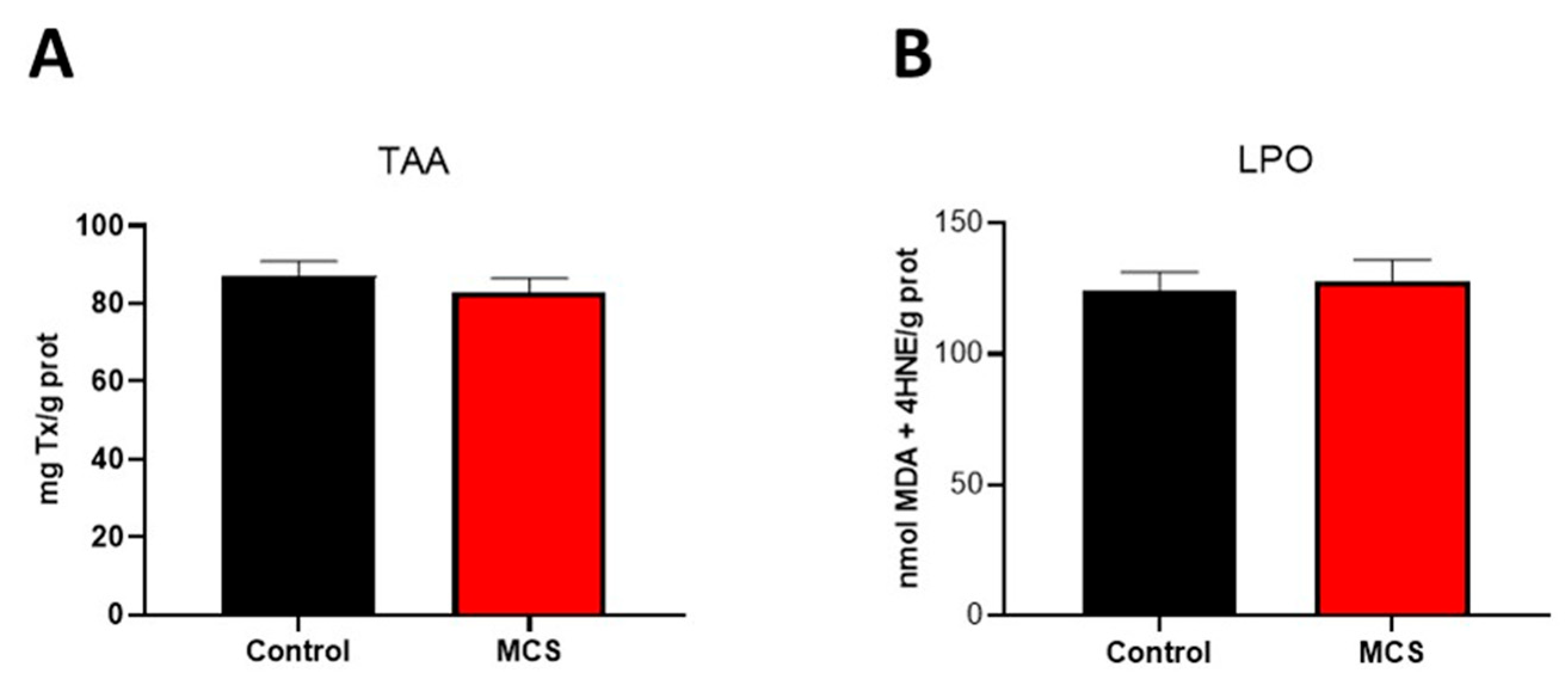
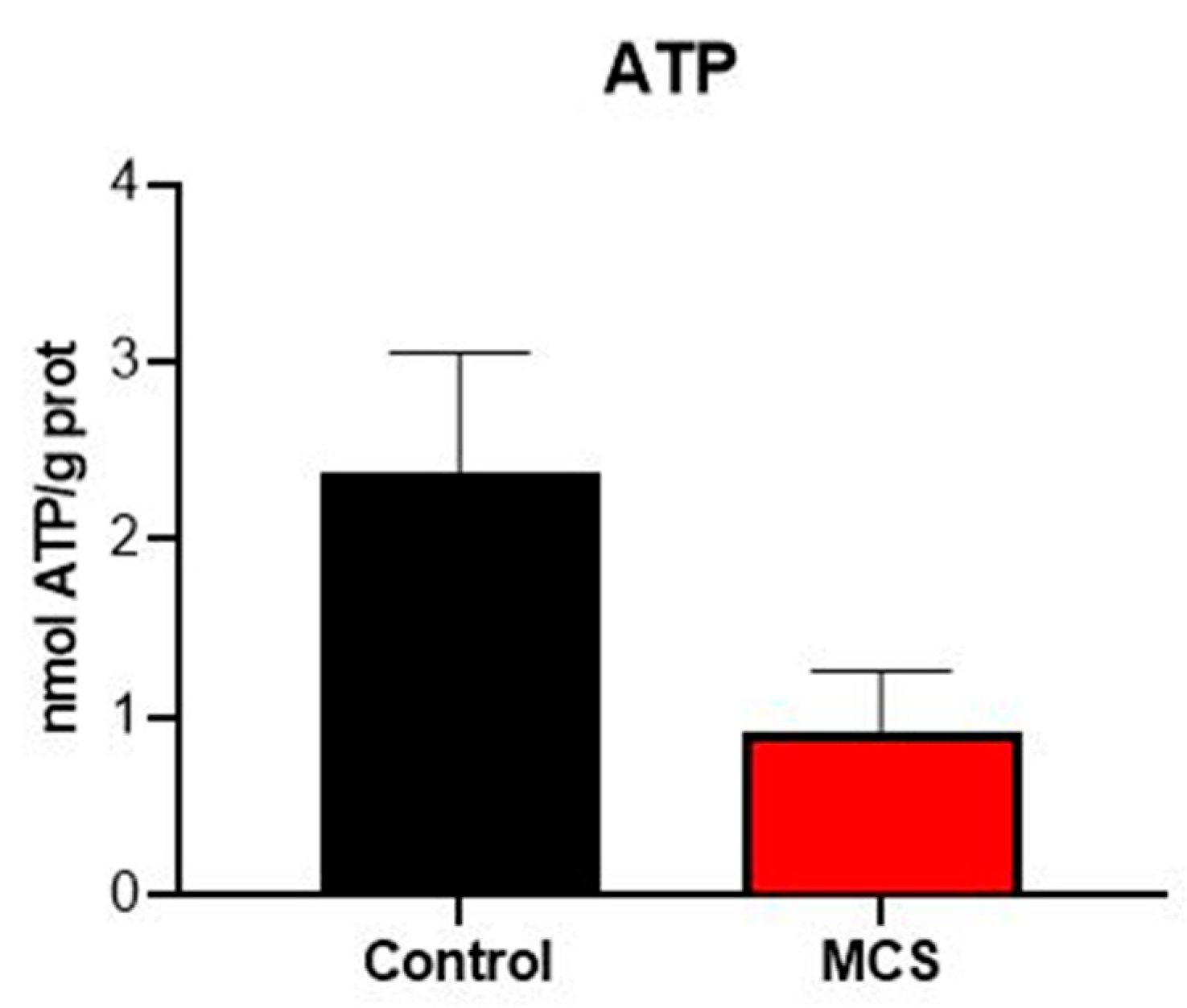
3.1. Markers of Plasma Oxidative Stress
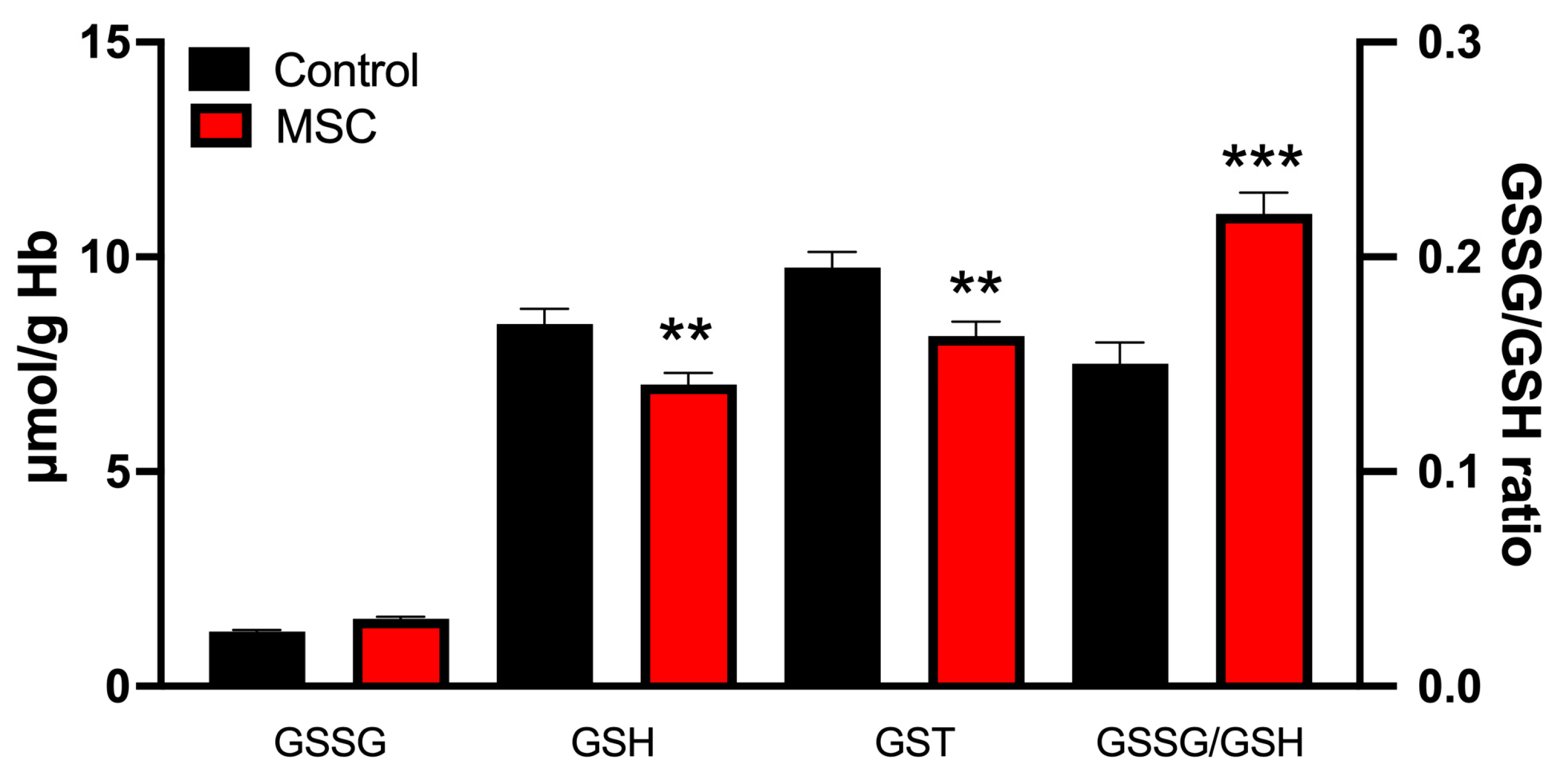
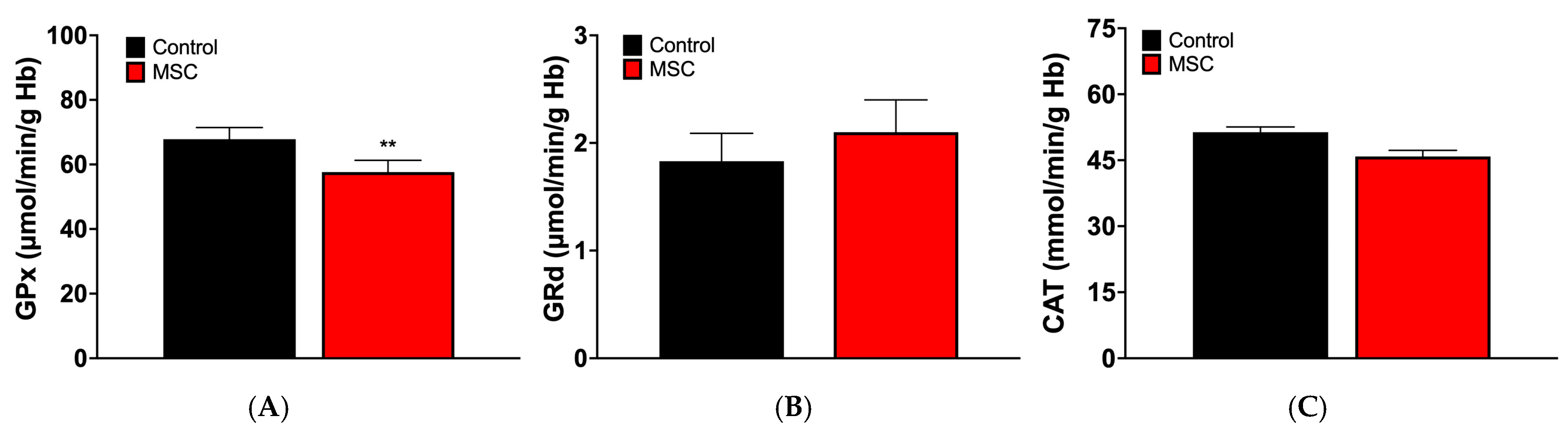
3.2. Markers of Oxidative Stress in Erythrocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCS | Multiple chemical sensitivity |
| TAA | Total antioxidant activity |
| LPO | Lipid peroxidation |
| ATP | Adenosine triphosphate |
| GSSG | Disulfide glutathione |
| GSH | Glutathione |
| GST | Total glutathione (oxidized plus GSH) |
| GPx | Glutathione peroxidase |
| GRd | Glutathione reductase |
| CAT | Catalase |
References
- Zucco, G.M.; Doty, R.L. Multiple Chemical Sensitivity. Brain Sci. 2021, 12, 46. [Google Scholar] [CrossRef]
- Labarge, A.S.; McCaffrey, R.J. Multiple Chemical Sensitivity: A Review of the Theoretical and Research Literature. Neuropsychol. Rev. 2000, 10, 183–211. [Google Scholar] [CrossRef]
- Sears, M.; Eng, M.; Lee, T.M. The Medical Perspective on Environmental Sensitivities. Available online: https://www.researchgate.net/publication/343699036_The_Medical_Perspective_on_Environmental_Sensitivities (accessed on 10 April 2025).
- Cullen, M.R. The Worker with Multiple Chemical Sensitivities: An Overview. Occup. Med. 1987, 2, 655–661. [Google Scholar]
- Lacour, M.; Zunder, T.; Schmidtke, K.; Vaith, P.; Scheidt, C. Multiple Chemical Sensitivity Syndrome (MCS)—Suggestions for an Extension of the U.S. MCS-Case Definition. Int. J. Hyg. Environ. Health 2005, 208, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Magill, M.K.; Suruda, A. Multiple Chemical Sensitivity Syndrome. Am. Fam. Physician 1998, 58, 721–728. [Google Scholar]
- Bornschein, S.; Hausteiner, C.; Römmelt, H.; Nowak, D.; Förstl, H.; Zilker, T. Double-Blind Placebo-Controlled Provocation Study in Patients with Subjective Multiple Chemical Sensitivity (MCS) and Matched Control Subjects. Clin. Toxicol. 2008, 46, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Nogué Xarau, S.; Alarcón Romay, M.; Martínez Martínez, J.-M.; Delclós Clanchet, J.; Rovira Prat, E.; Fernández Solà, J. Multiple chemical sensitivity: Epidemiological, clinical and prognostic differences between occupational and non-occupational cases. Med. Clin. 2010, 135, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bailer, J.; Witthöft, M.; Rist, F. Psychological Predictors of Short- and Medium Term Outcome in Individuals with Idiopathic Environmental Intolerance (IEI) and Individuals with Somatoform Disorders. J. Toxicol. Environ. Health A 2008, 71, 766–775. [Google Scholar] [CrossRef]
- Meggs, W.J. Mechanisms of Allergy and Chemical Sensitivity. Toxicol. Ind. Health 1999, 15, 331–338. [Google Scholar] [CrossRef]
- Antelman, S.M. Time-Dependent Sensitization in Animals: A Possible Model of Multiple Chemical Sensitivity in Humans. Toxicol. Ind. Health 1994, 10, 335–342. [Google Scholar] [CrossRef]
- Belpomme, D.; Campagnac, C.; Irigaray, P. Reliable Disease Biomarkers Characterizing and Identifying Electrohypersensitivity and Multiple Chemical Sensitivity as Two Etiopathogenic Aspects of a Unique Pathological Disorder. Rev. Environ. Health 2015, 30, 251–271. [Google Scholar] [CrossRef]
- Hirvonen, M.R.; Ruotsalainen, M.; Roponen, M.; Hyvärinen, A.; Husman, T.; Kosma, V.M.; Komulainen, H.; Savolainen, K.; Nevalainen, A. Nitric Oxide and Proinflammatory Cytokines in Nasal Lavage Fluid Associated with Symptoms and Exposure to Moldy Building Microbes. Am. J. Respir. Crit. Care Med. 1999, 160, 1943–1946. [Google Scholar] [CrossRef]
- Jacques, L. Multiple Chemical Sensitivity: A Clinical Perspective. Brain Sci. 2024, 14, 1261. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of Aldehydic Lipid Peroxidation Products: Malonaldehyde and 4-Hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Jaskot, R.H.; Charlet, E.G.; Grose, E.C.; Grady, M.A.; Roycroft, J.H. An Automated Analysis of Glutathione Peroxidase, S-Transferase, and Reductase Activity in Animal Tissue. J. Anal. Toxicol. 1983, 7, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Hissin, P.J.; Hilf, R. A Fluorometric Method for Determination of Oxidized and Reduced Glutathione in Tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Molot, J.; Sears, M.; Anisman, H. Multiple Chemical Sensitivity: It’s Time to Catch up to the Science. Neurosci. Biobehav. Rev. 2023, 151, 105227. [Google Scholar] [CrossRef]
- Damiani, G.; Alessandrini, M.; Caccamo, D.; Cormano, A.; Guzzi, G.; Mazzatenta, A.; Micarelli, A.; Migliore, A.; Piroli, A.; Bianca, M.; et al. Italian Expert Consensus on Clinical and Therapeutic Management of Multiple Chemical Sensitivity (MCS). Int. J. Environ. Res. Public Health 2021, 18, 11294. [Google Scholar] [CrossRef]
- De Luca, C.; Gugliandolo, A.; Calabrò, C.; Currò, M.; Ientile, R.; Raskovic, D.; Korkina, L.; Caccamo, D. Role of Polymorphisms of Inducible Nitric Oxide Synthase and Endothelial Nitric Oxide Synthase in Idiopathic Environmental Intolerances. Mediat. Inflamm. 2015, 2015, 245308. [Google Scholar] [CrossRef]
- Cannata, A.; De Luca, C.; Andolina, G.; Caccamo, D.; Currò, M.; Ferlazzo, N.; Ientile, R.; Alibrandi, A.; Korkina, L. Influence of the SOD2 A16V Gene Polymorphism on Alterations of Redox Markers and Erythrocyte Membrane Fatty Acid Profiles in Patients with Multiple Chemical Sensitivity. Biomed. Rep. 2021, 15, 101. [Google Scholar] [CrossRef]
- Pall, M.L.; Satterlee, J.D. Elevated Nitric Oxide/Peroxynitrite Mechanism for the Common Etiology of Multiple Chemical Sensitivity, Chronic Fatigue Syndrome, and Posttraumatic Stress Disorder. Ann. N. Y. Acad. Sci. 2001, 933, 323–329. [Google Scholar] [CrossRef]
- Huang, J.; Philbert, M.A. Cellular Responses of Cultured Cerebellar Astrocytes to Ethacrynic Acid-Induced Perturbation of Subcellular Glutathione Homeostasis. Brain Res. 1996, 711, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.V.; Holt, R.; Wieder, L.; Terhune, D.B. Responsiveness to Direct Verbal Suggestions and Dissociation Independently Predict Symptoms Associated with Environmental Factors. Available online: https://www.researchgate.net/publication/364157386_Responsiveness_to_Direct_Verbal_Suggestions_and_Dissociation_Independently_Predict_Symptoms_Associated_with_Environmental_Factors (accessed on 10 April 2025).
- Rodriguez Izquierdo, D. Preliminary Study of the Physiopathology of Multiple Chemical Sensitivity. Bachelor’s Thesis, Universidad de Oviedo, Oviedo, Spain, 2023. [Google Scholar]
- Giuliani, A.L.; Sarti, A.C.; Di Virgilio, F. Ectonucleotidases in Acute and Chronic Inflammation. Front. Pharmacol. 2020, 11, 619458. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Emberson, J.; Collins, R. Strategic Need for Large Prospective Studies in Different Populations. JAMA 2020, 323, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Hausteiner-Wiehle, C.; Henningsen, P. Irritable Bowel Syndrome: Relations with Functional, Mental, and somatoform disorders. World J. Gastroenterol. 2014, 20, 6024–6030. [Google Scholar] [CrossRef]
| MCS | Control | p-Value | |
|---|---|---|---|
| Age (years) | 54.8 ± 7.9 | 51.4 ± 8.8 | |
| Number of subjects (women) | 40 (88.9%) | 40 (88.9%) | |
| Weight (kg) | 62.8 ± 12.5 | 63.7 ± 10.4 | |
| Symptoms | Allergies and Intolerances 19 (42.2%); | Allergies and Intolerances 5 (11.1%) | 0.0008 |
| Chronic Widespread Pain Conditions 9 (20%) | Chronic Widespread Pain Conditions 4 (8.9%) | 0.1338 | |
| Thyroid Disorders 4 (8.9%) | Thyroid Disorders 2 (4.4%) | 0.3980 | |
| Migraines 4 (8.9%) | Migraines 1 (2.2%) | 0.1674 | |
| Gastrointestinal Disorders (Crohn’s disease, gastritis, hiatus hernia, Irritable Bowel Syndrome) 7 (15.6%) | Gastrointestinal Disorders (gastritis, hiatus hernia) 2 (4.4%) | 0.0789 | |
| Cardiovascular Disorders (: hyper or hypotension, arrhythmia, atrial fibrillation, sinus tachycardia, extrasystoles) 5 (11.1%) | Cardiovascular Disorders (hyper or hypotension, extrasystoles) 2 (4.4%) | 0.2377 | |
| Skeletal and Connective Tissue Disorders 9 (20%) | Skeletal and Connective Tissue Disorders 3 (6.7%) | 0.0628 | |
| Treatments | Vitamins and Supplements 27 (60%) | Vitamins and Supplements 14 (31.1%) | 0.0059 |
| Cardiovascular Issues (amlodipine, losartan, bisoprolol) 9 (20%) | Cardiovascular Issues (amlodipine, losartan, bisoprolol) 7 (15.6%) | 0.5814 | |
| Neurological Medications (fluoxetine, trankimazin, mirtazapine, sertraline) 14 (31.1%) | Neurological Medications (fluoxetine, trankimazin, sertraline) 2 (4.4%) | 0.0009 | |
| Anti-inflammatory/Analgesics 2 (4.4%) | Anti-inflammatory/Analgesics 2 (4.4%) | >0.9999 | |
| Thyroid Medications 8 (17.8%) | Thyroid Medications 2 (4.4%) | 0.0442 | |
| Geographical distribution | Galicia 10 (22.2%), Murcia 10 (22.2%), Asturias 5 (11.1%), Madrid 4 (8.9%), Toledo 4 (8.9%), Ciudad Real 3 (6.7%), Granada 3 (6.7%), Cádiz 2 (4.4%), Cantabria 1 (2.2%), Córdoba 1 (2.2%), Salamanca 1 (2.2%), Ibiza 1 (2.2%) | Galicia 10 (22.2%), Murcia 10 (22.2%), Asturias 5 (11.1%), Madrid 4 (8.9%), Toledo 4 (8.9%), Ciudad Real 3 (6.7%), Granada 3 (6.7%), Cádiz 2 (4.4%), Cantabria 1 (2.2%), Córdoba 1 (2.2%), Salamanca 1 (2.2%), Ibiza 1 (2.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranda-Martínez, P.; Menéndez-Coto, N.; Coto-Montes, A.; Martín-Estebané, M.; Escames, G.; Acuña-Castroviejo, D. Redox Status in Patients Suffering from Multiple Chemical Sensitivity (MCS): A Pilot Study. J. Clin. Med. 2025, 14, 6185. https://doi.org/10.3390/jcm14176185
Aranda-Martínez P, Menéndez-Coto N, Coto-Montes A, Martín-Estebané M, Escames G, Acuña-Castroviejo D. Redox Status in Patients Suffering from Multiple Chemical Sensitivity (MCS): A Pilot Study. Journal of Clinical Medicine. 2025; 14(17):6185. https://doi.org/10.3390/jcm14176185
Chicago/Turabian StyleAranda-Martínez, Paula, Nerea Menéndez-Coto, Ana Coto-Montes, María Martín-Estebané, Germaine Escames, and Darío Acuña-Castroviejo. 2025. "Redox Status in Patients Suffering from Multiple Chemical Sensitivity (MCS): A Pilot Study" Journal of Clinical Medicine 14, no. 17: 6185. https://doi.org/10.3390/jcm14176185
APA StyleAranda-Martínez, P., Menéndez-Coto, N., Coto-Montes, A., Martín-Estebané, M., Escames, G., & Acuña-Castroviejo, D. (2025). Redox Status in Patients Suffering from Multiple Chemical Sensitivity (MCS): A Pilot Study. Journal of Clinical Medicine, 14(17), 6185. https://doi.org/10.3390/jcm14176185







