Association of Aspiration Pneumonia-Related Factors with the Incidence of Healthcare-Associated Pneumonia in Elderly with Dementia
Abstract
1. Introduction
2. Method
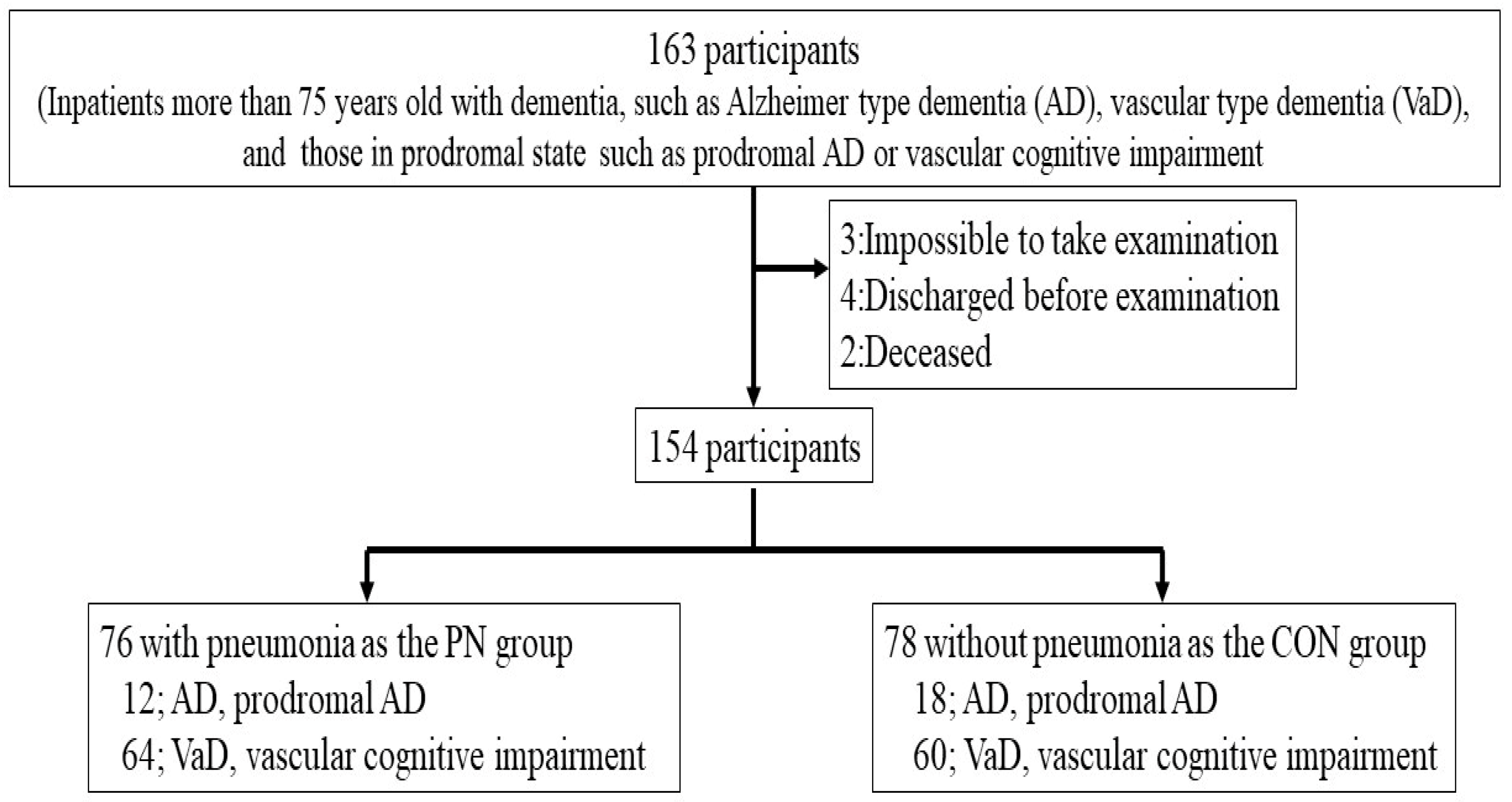
2.1. Study Design and Subjects
2.2. Diagnosis of Pneumonia and Dementia
2.3. Evaluation of Dysphagic Risk in Aspiration Pneumonia-Related Factors
2.4. Other Factors Affecting the Incidence of Aspiration Pneumonia
2.4.1. Comprehensive Geriatric Assessment
2.4.2. Aspiration Pneumonia-Related Factors for Lifestyle Habits, Comorbidities, and Medications
2.4.3. Cerebral Infarction
2.5. Statistics
3. Result
3.1. Patient’s Characteristics
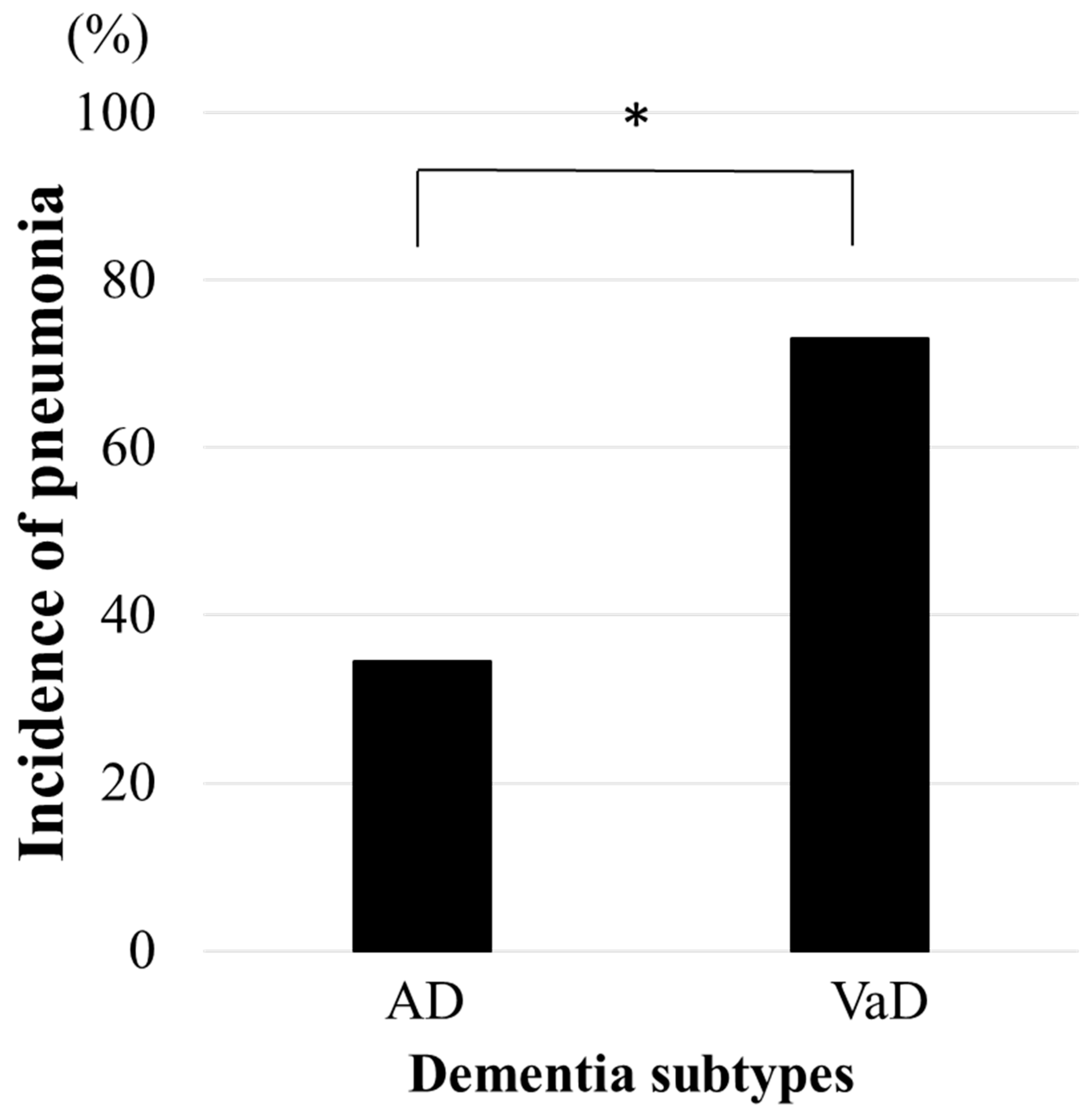
3.2. HCAP by Dementia Subtype and Stage
3.3. Association Between HCAP and Aspiration Pneumonia-Related Factors by Dementia Subtypes
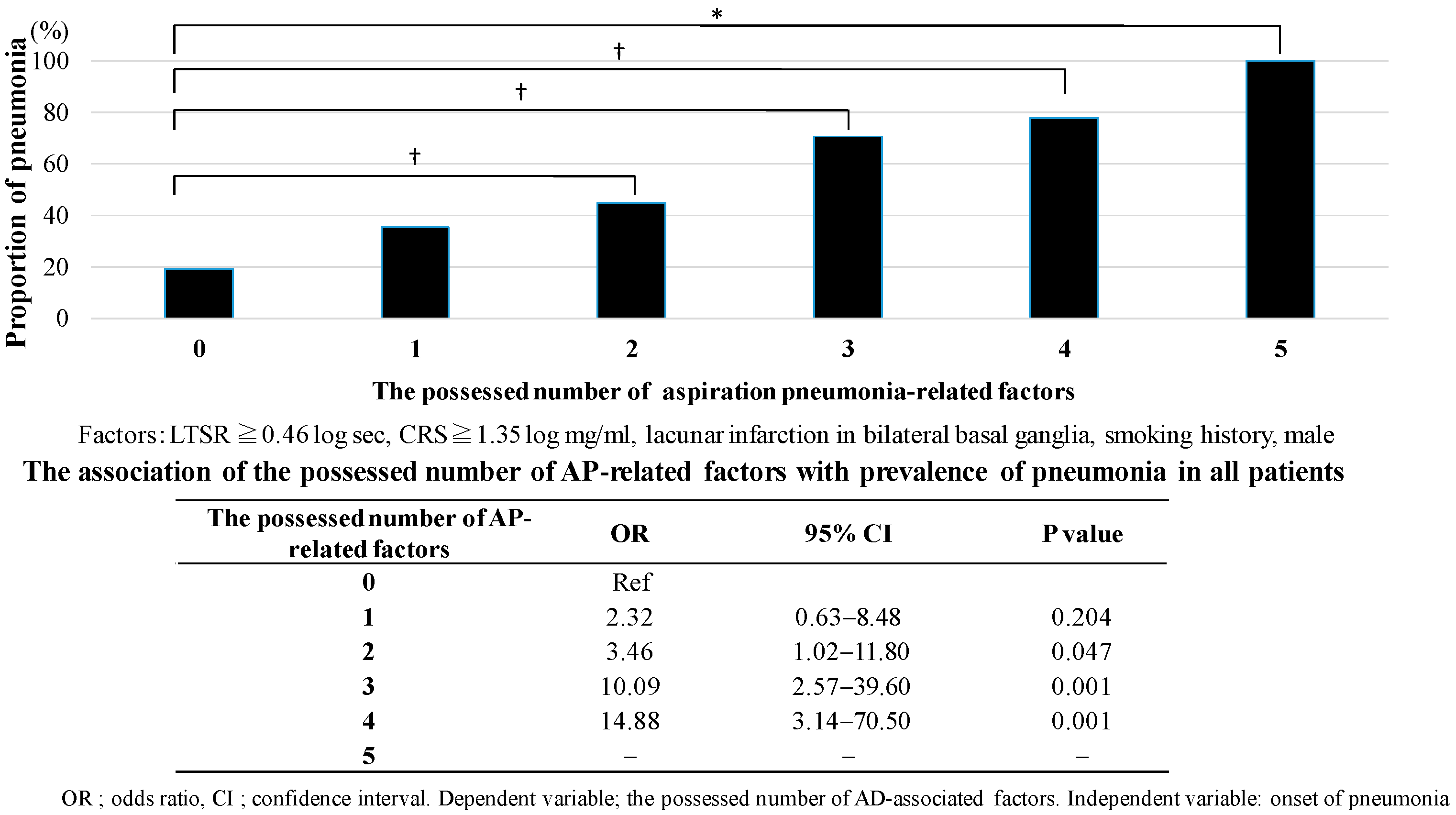
3.4. The Number of Aspiration Pneumonia-Related Factors in HCAP by Dementia Subtype
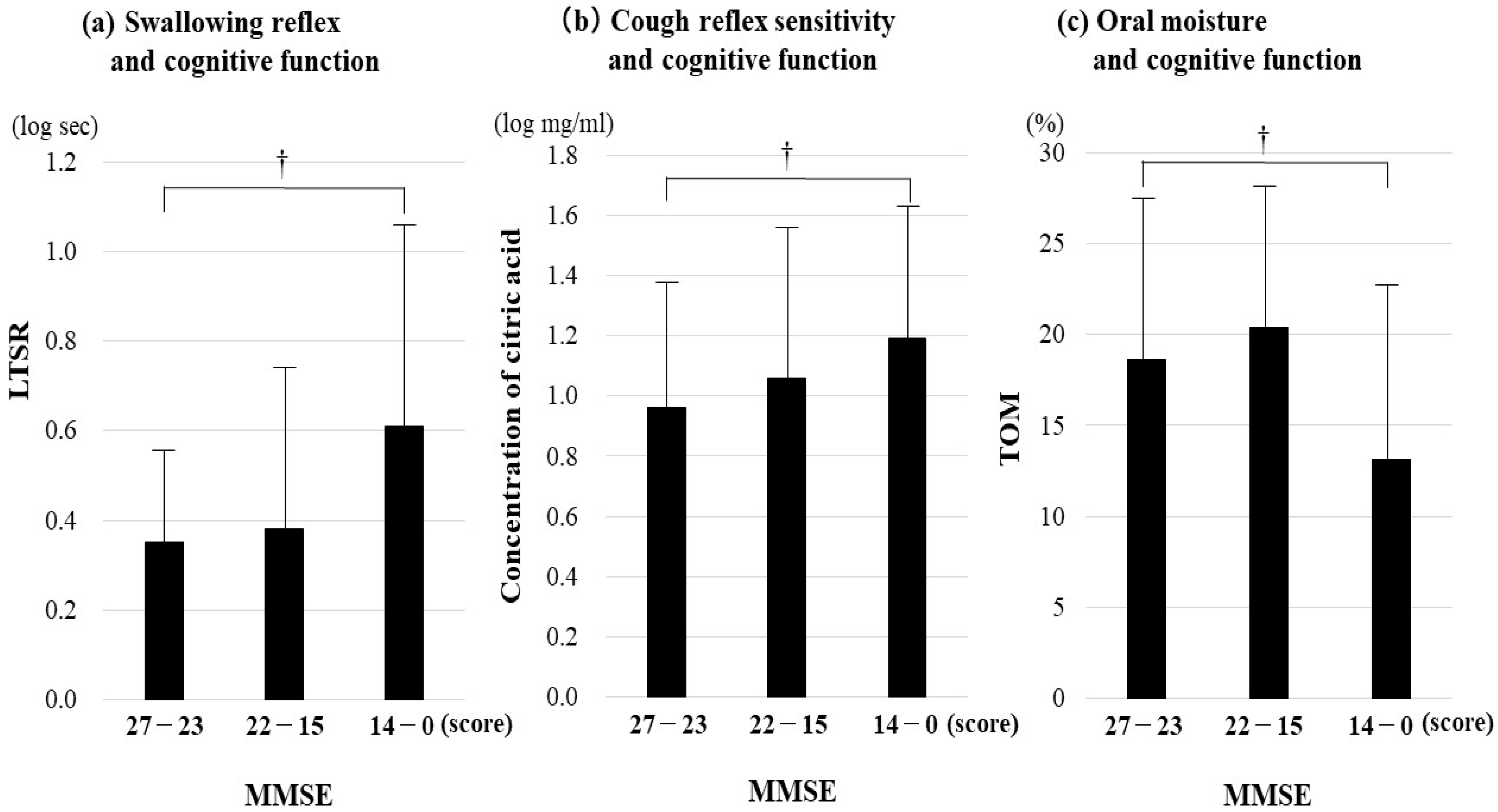
3.5. Relationship Between Aspiration Pneumonia-Related Factors and Cognitive Function by Dementia Subtype
3.6. Association of HCAP with Cerebral Infarction
4. Discussion
4.1. Association Between HCAP and the Aspiration Pneumonia-Related Factor by Dementia Subtypes
4.2. The Number of Aspiration Pneumonia-Related Factors in HCAP by Dementia Subtype
4.3. Relationship Between Aspiration Pneumonia-Related Factors and Cognitive Function by Dementia Subtype
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teramoto, S.; Fukuchi, Y.; Sasaki, H.; Sato, K.; Sekizawa, K.; Matsuse, T. High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: A multicenter, prospective study in Japan. J. Am. Geriatr. Soc. 2008, 56, 577–579. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Teno, J.M.; Kiely, D.K.; Shaffer, M.L.; Jones, R.N.; Prigerson, H.G.; Volicer, L.; Givens, J.L.; Hamel, M.B. The clinical course of advanced dementia. N. Engl. J. Med. 2009, 361, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Data Base by The Ministry of Health, Labor and Welfare. Available online: https://www.mhlw.go.jp/content/001279920.pdf (accessed on 8 May 2025).
- Ebihara, T. Comprehensive Approaches to aspiration pneumonia and dysphagia in the elderly on the disease time-axis. J. Clin. Med. 2022, 10, 5323. [Google Scholar] [CrossRef]
- Takeshita, T.; Tomioka, M.; Shimazaki, Y.; Matsuyama, M.; Koyano, K.; Matsuda, K.; Yamashita, Y. Microfloral characterization of the tongue coating and associated risk for pneumonia-related health problems in institutionalized older adults. J. Am. Geriatr. Soc. 2010, 58, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Langmore, S.E.; Terpenning, M.S.; Schork, A.; Chen, Y.; Murray, J.T.; Lopatin, D.; Loesche, W.J. Predictors of aspiration pneumonia: How important is dysphagia? Dysphagia 1998, 13, 69–81. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kubo, H.; Yanai, M. Impairment of the swallowing reflex in exacerbations of COPD. Thorax 2007, 62, 1017. [Google Scholar] [CrossRef]
- Marumo, K.; Homma, S.; Fukuchi, Y. Post-gastrectomy aspiration pneumonia. Chest 1995, 107, 453–456. [Google Scholar] [CrossRef]
- Herzig, S.J.; LaSalvia, M.T.; Naidus, E.; Rothberg, M.B.; Zhou, W.; Gurwitz, J.H.; Marcantonio, E.R. Antipsychotics and the Risk of Aspiration Pneumonia in Individuals Hospitalized for Nonpsychiatric Conditions: A Cohort Study. J. Am. Geriatr. Soc. 2017, 65, 2580–2586. [Google Scholar] [CrossRef] [PubMed]
- Kose, E.; Hirai, T.; Seki, T. Assessment of aspiration pneumonia using the Anticholinergic Risk Scale. Geriatr. Gerontol. Int. 2018, 18, 1230–1235. [Google Scholar] [CrossRef]
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical MANUAL of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2003. [Google Scholar]
- Hirata, Y.; Matsuda, H.; Nemoto, K.; Ohnishi, T.; Hirao, K.; Yamashita, F.; Asada, T.; Iwabuchi, S.; Samejima, H. Voxel-based morphometry to discriminate early Alzheimer’s disease from controls. Neurosci. Lett. 2005, 382, 269–274. [Google Scholar] [CrossRef]
- Minoshima, S.; Foster, N.L.; Kuhl, D.E. Posterior cingulate cortex in Alzheimer’s disease. Lancet 1994, 344, 895. [Google Scholar] [CrossRef]
- Román, G.C.; Tatemichi, T.K.; Erkinjuntti, T.; Cummings, J.L.; Masdeu, J.C.; Garcia, J.H.; Amaducci, L.; Orgogozo, J.M.; Brun, A.; Moody, D.M.; et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993, 43, 250–260. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Reisberg, B. Functional assessment staging (FAST). Psychopharmacol. Bull. 1988, 24, 653–659. [Google Scholar] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Kunieda, K.; Ohno, T.; Fujishima, I.; Hojo, K.; Morita, T. Reliability and validity of a tool to measure the severity of dysphagia: The Food Intake LEVEL Scale. J. Pain Symptom Manag. 2013, 46, 201–206. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, M.; Kleinman, J.T.; Ky, P.K.; Palmer, J.B.; Hillis, A.E. Supratentorial regions of acute ischemia associated with clinically important swallowing disorders: A pilot study. Stroke 2008, 39, 3022–3028. [Google Scholar] [CrossRef]
- Steinhagen, V.; Grossmann, A.; Benecke, R.; Walter, U. Swallowing disturbance pattern relates to brain lesion location in acute stroke patients. Stroke 2009, 40, 1903–1906. [Google Scholar] [CrossRef] [PubMed]
- Lowell, S.Y.; Poletto, C.J.; Knorr-Chung, B.R.; Reynolds, R.C.; Simonyan, K.; Ludlow, C.L. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage 2008, 42, 285–295. [Google Scholar] [CrossRef]
- Ebihara, S.; Ebihara, T.; Yamasaki, M.; Asada, M.; Yamanda, S.; Niu, K.; Sasaki, H.; Arai, H. Contribution of gastric acid in elderly nursing home patients with cough reflex hypersensitivity. J. Am. Geriatr. Soc. 2007, 55, 1686–1688. [Google Scholar] [CrossRef] [PubMed]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Nakajoh, K.; Satoh-Nakagawa, T.; Suzuki, T.; Ohrui, T.; Arai, H.; Sasaki, H. Risk factors of aspiration pneumonia in Alzheimer’s disease patients. Gerontology 2001, 47, 271–276. [Google Scholar] [CrossRef] [PubMed]
- DeMyer, W. Neuroanatomy; Williams & Wilkins: Ambler, PA, USA, 1998; ISBN 9780683300758. [Google Scholar]
| All Participants | AD Participants | VaD Participants | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PN | CON | p Value | PN | CON | p Value | PN | CON | p Value | |
| N | 76 | 78 | — | 12 | 18 | — | 64 | 60 | — |
| Sex (male n, (%)) | 47 (61.8) | 27 (34.6) | 0.001 | 6 (50.0) | 5 (27.8) | 0.266 | 41 (64.1) | 22 (36.7) | 0.002 |
| Age mean ± SD (years old) | 87.4 ± 5.3 | 88.3 ± 4.9 | 0.225 | 87.0 ± 5.1 | 86.6 ± 3.7 | 0.810 | 87.5 ± 5.4 | 88.8 ± 5.1 | 0.185 |
| Smoking history n, (%) | 30 (39.5) | 18 (23.1) | 0.028 | 7 (58.3) | 3 (16.7) | 0.045 | 23 (35.9) | 15 (25.0) | 0.187 |
| Cognition | |||||||||
| MMSE score mean ± SD (score) | 17.1 ± 8.8 | 15.2 ± 8.5 | 0.072 | 15.0 ± 8.2 | 14.1 ± 9.7 | 1.000 | 17.5 ± 8.9 | 15.6 ± 8.2 | 0.053 |
| FAST medial value(quantile), (level) | 6b (4–6e) | 6c (4–6e) | 0.829 | 6a (4–6c) | 6c (4–7a) | 0.632 | 6b (4–7a) | 6b (4–6e) | 0.580 |
| Physical function | |||||||||
| PS score medial value(quantile), (level) | 2 (1–3) | 2 (1–3) | 0.537 | 2 (2–3) | 2 (1–3) | 0.602 | 2 (1–3) | 2 (1–3) | 0.672 |
| Barthel Index mean ± SD (score) | 48.0 ± 36.3 | 46.3 ± 35.5 | 0.962 | 44.2 ± 42.1 | 51.7 ± 38.3 | 0.662 | 48.8 ± 35.5 | 44.8 ± 34.7 | 0.680 |
| Eating ability | |||||||||
| FILS medial value(quantile), (level) | 6 (2–8) | 7 (5–10) | 0.004 | 7 (5–7) | 5 (4–9) | 0.787 | 4 (2–8) | 7 (5–10) | 0.002 |
| Comorbidities contributes to onset of AP | |||||||||
| Postoperative state of gastrointestinal tract, n (%) | 7 (9.2) | 2 (2.6) | 0.096 | 2 (16.7) | 1 (5.6) | 0.548 | 5 (7.8) | 1 (1.7) | 0.209 |
| COPD, n (%) | 10 (13.2) | 6 (7.7) | 0.266 | 1 (8.3) | 2 (11.1) | 1.000 | 9 (14.1) | 4 (6.7) | 0.179 |
| Medications which affect the development of pneumonia | |||||||||
| Neuroleptics, n (%) | 4 (5.3) | 1 (1.3) | 0.207 | 1 (8.3) | 0 | 0.400 | 3 (4.7) | 1 (1.7) | 0.620 |
| Anticholinergic agent, n (%) | 4 (5.2) | 5 (6.4) | 1.000 | 0 | 2 (11.1) | 0.503 | 4 (6.3) | 3 (5.0) | 1.000 |
| ACE-Inhibitor, n (%) | 6 (7.9) | 7 (9.0) | 0.810 | 0 | 3 (16.7) | 0.255 | 6 (9.4) | 4 (6.7) | 0.745 |
| Dopamine-releasing hormone agent, n (%) | 2 (2.6) | 0 | 0.242 | 0 | 0 | — | 2 (3.1) | 0 | 0.496 |
| PDE-III Inhibitor, n (%) | 4 (5.3) | 4 (5.1) | 1.000 | 1 (8.3) | 3 (16.7) | 0.632 | 3 (4.7) | 1 (1.7) | 0.620 |
| All Participants | AD Participants | VaD Participants | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PN | CON | p Value | Odds Ratio [95% CI] | p Value | PN | CON | p Value | PN | CON | p Value | Odds Ratio [95% CI] | p Value | |
| LTSR mean ± SD, log sec. | 0.52 ± 0.41 | 0.38 ± 0.33 | 0.010 | 3.98 [1.38–11.50] | 0.011 | 0.37 ± 0.29 | 0.35 ± 0.40 | 0.573 | 0.55 ± 0.42 | 0.39 ± 0.31 | 0.008 | 4.71 [1.35–6.49] | 0.015 |
| CRS mean ± SD, log mg/mL | 1.13 ± 0.49 | 1.02 ± 0.47 | 0.097 | 1.55 [0.74–3.25] | 0.244 | 1.07 ± 0.45 | 0.98 ± 0.63 | 0.669 | 1.14 ± 0.50 | 1.03 ± 0.41 | 0.084 | 1.78 [0.75–4.24] | 0.192 |
| TOM mean ± SD, % | 18.1 ± 9.6 | 16.6 ± 9.3 | 0.252 | 1.02 [0.98–1.06] | 0.251 | 21.7 ± 9.5 | 15.1 ± 10.5 | 0.053 | 17.4 ± 9.5 | 17.1 ± 8.9 | 0.669 | 1.01 [0.97–1.05] | 0.661 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, T.; Karita, K.; Kozaki, K.; Ebihara, T. Association of Aspiration Pneumonia-Related Factors with the Incidence of Healthcare-Associated Pneumonia in Elderly with Dementia. J. Clin. Med. 2025, 14, 6186. https://doi.org/10.3390/jcm14176186
Miyamoto T, Karita K, Kozaki K, Ebihara T. Association of Aspiration Pneumonia-Related Factors with the Incidence of Healthcare-Associated Pneumonia in Elderly with Dementia. Journal of Clinical Medicine. 2025; 14(17):6186. https://doi.org/10.3390/jcm14176186
Chicago/Turabian StyleMiyamoto, Takahide, Kanae Karita, Koichi Kozaki, and Takae Ebihara. 2025. "Association of Aspiration Pneumonia-Related Factors with the Incidence of Healthcare-Associated Pneumonia in Elderly with Dementia" Journal of Clinical Medicine 14, no. 17: 6186. https://doi.org/10.3390/jcm14176186
APA StyleMiyamoto, T., Karita, K., Kozaki, K., & Ebihara, T. (2025). Association of Aspiration Pneumonia-Related Factors with the Incidence of Healthcare-Associated Pneumonia in Elderly with Dementia. Journal of Clinical Medicine, 14(17), 6186. https://doi.org/10.3390/jcm14176186





