Current Advancement of Respiratory Stability Time-Guided Heart Failure Management
Abstract
1. Heart Failure Pandemic
2. The Prognostic Significance of HF Re-Hospitalization
3. How to Prevent HF Re-Hospitalization
4. CardioMEMS
5. Thoracic Impedance Monitoring
6. Remote Dielectric Sensing
7. Telemonitoring Programs
| Technology | Physiological Parameter | Method | Invasiveness | Predictive Value | Representative Study Outcome | Strengths/Weaknesses |
|---|---|---|---|---|---|---|
| CardioMEMS | Pulmonary artery pressure | Implantable sensor (pulmonary artery) | Invasive (trans-catheter) | High (in selected high-risk patients) | CHAMPION and MONITOR-HF: HF hospitalization ↓ [21,22] | Strength: strong outcome data. Weakness: requires implantation. Sensitivity high, but limited generalizability. |
| OptiVol | Intrathoracic impedance (fluid accumulation) | Pacemaker-based impedance measurement | Minimally invasive | Limited (low specificity) | SENSE-HF: poor predictive accuracy [24] | Strength: available in pacemaker patients. Weakness: high false positive. Sensitivity low. |
| Remote dielectric sensing | Lung fluid content | Electromagnetic signal through thorax | Non-invasive | Moderate to high (validated in trials) | ReDS-SAFE HF: early readmission risk ↓ [29] | Strength: quantitative. Weakness: device cost/logistics. Specificity moderate, confounded by lung disease. |
| Telemonitoring | Symptoms, weight, blood pressure, heart rate | Patient-reported or home-based devices | Non-invasive | Variable (depends on adherence and setting) | TIM-HF2: mortality and HF hospitalization ↓ [33] | Strength: wide accessibility. Weakness: reliance on adherence, subjective data. Sensitivity low. |
8. What Is Respiratory Stability Time?
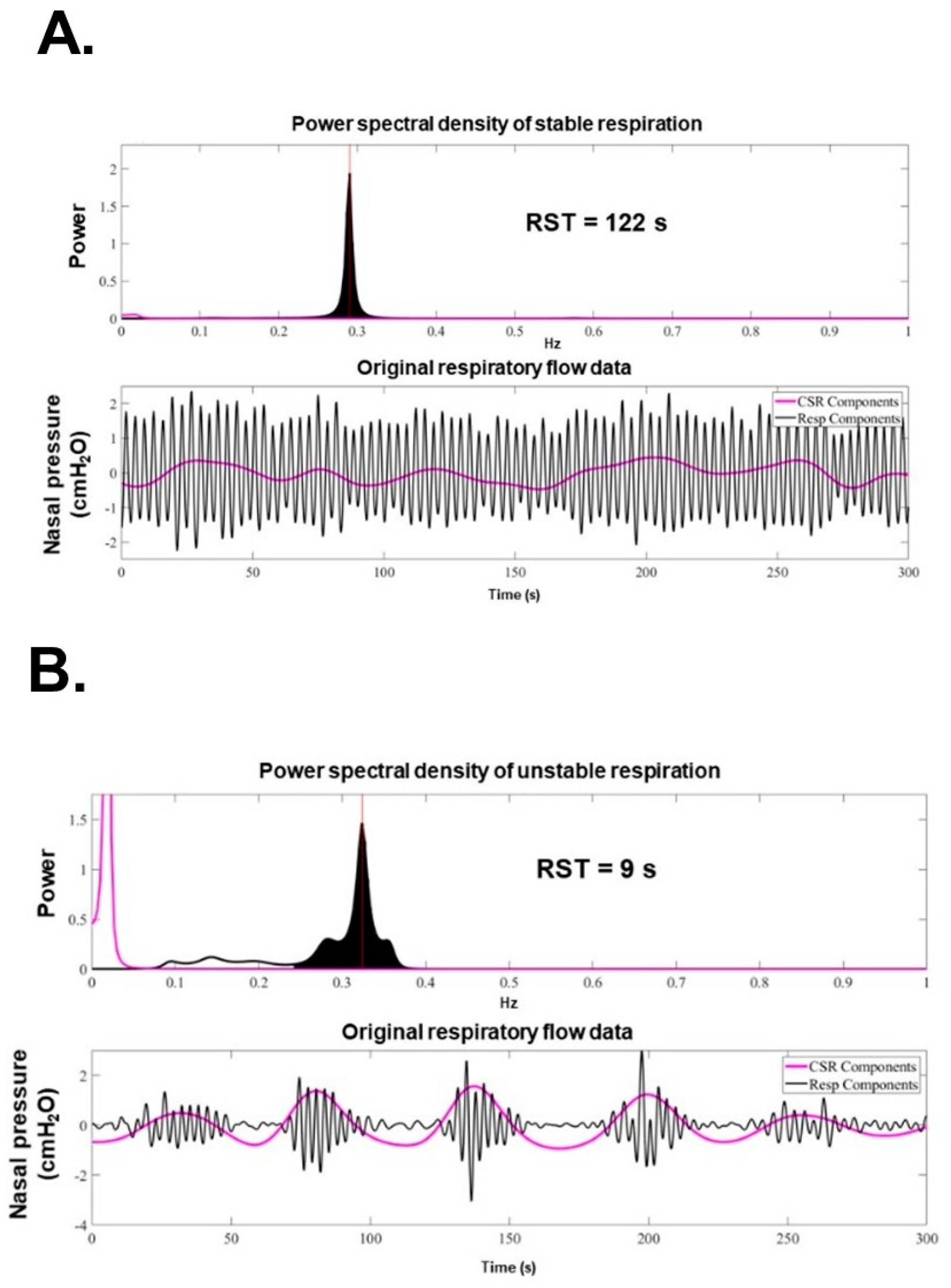
9. How to Calculate RST
10. Theoretical Physiological Relationship Between RST and HF
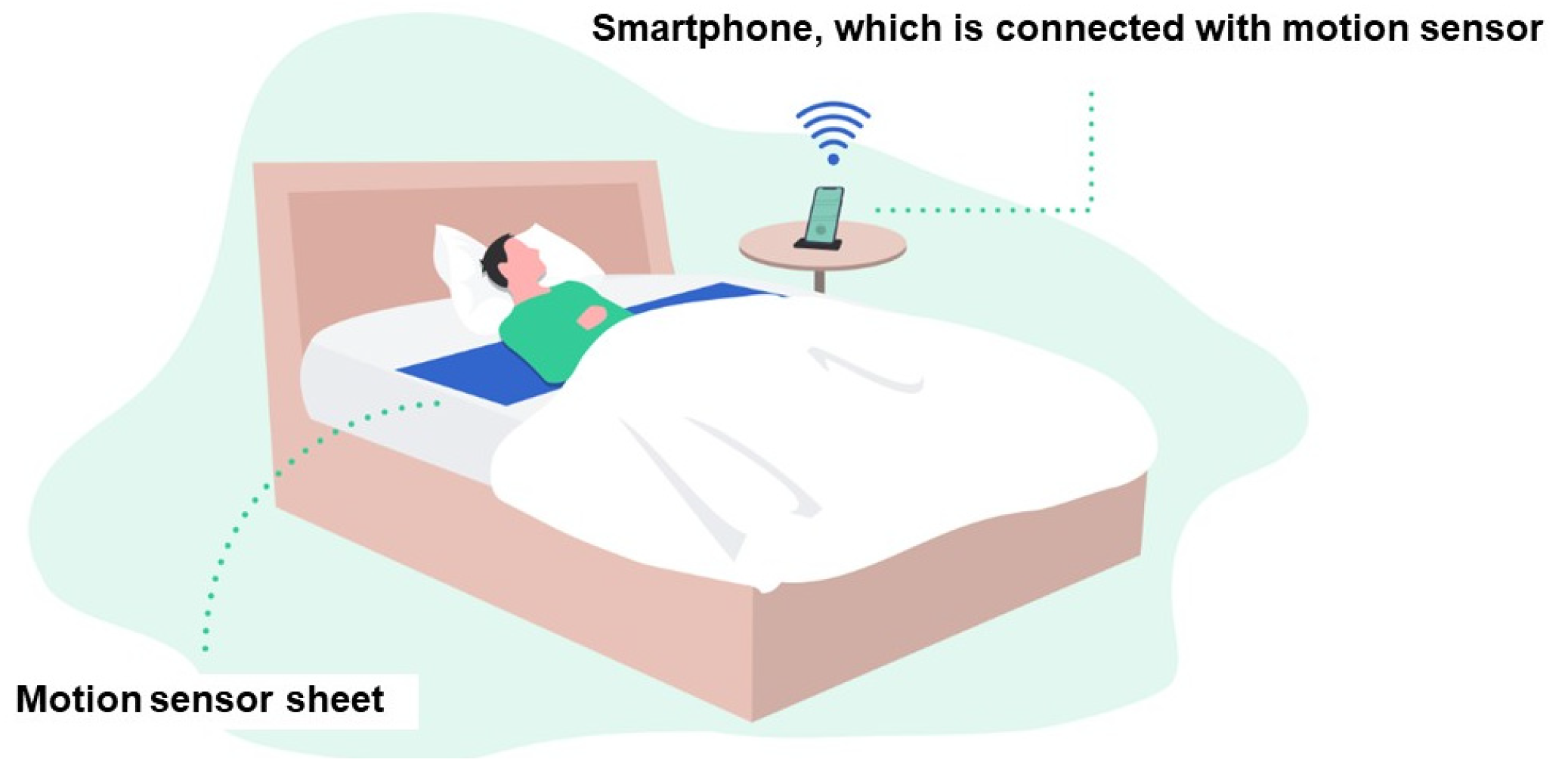
11. How to Measure Respiratory Pattern
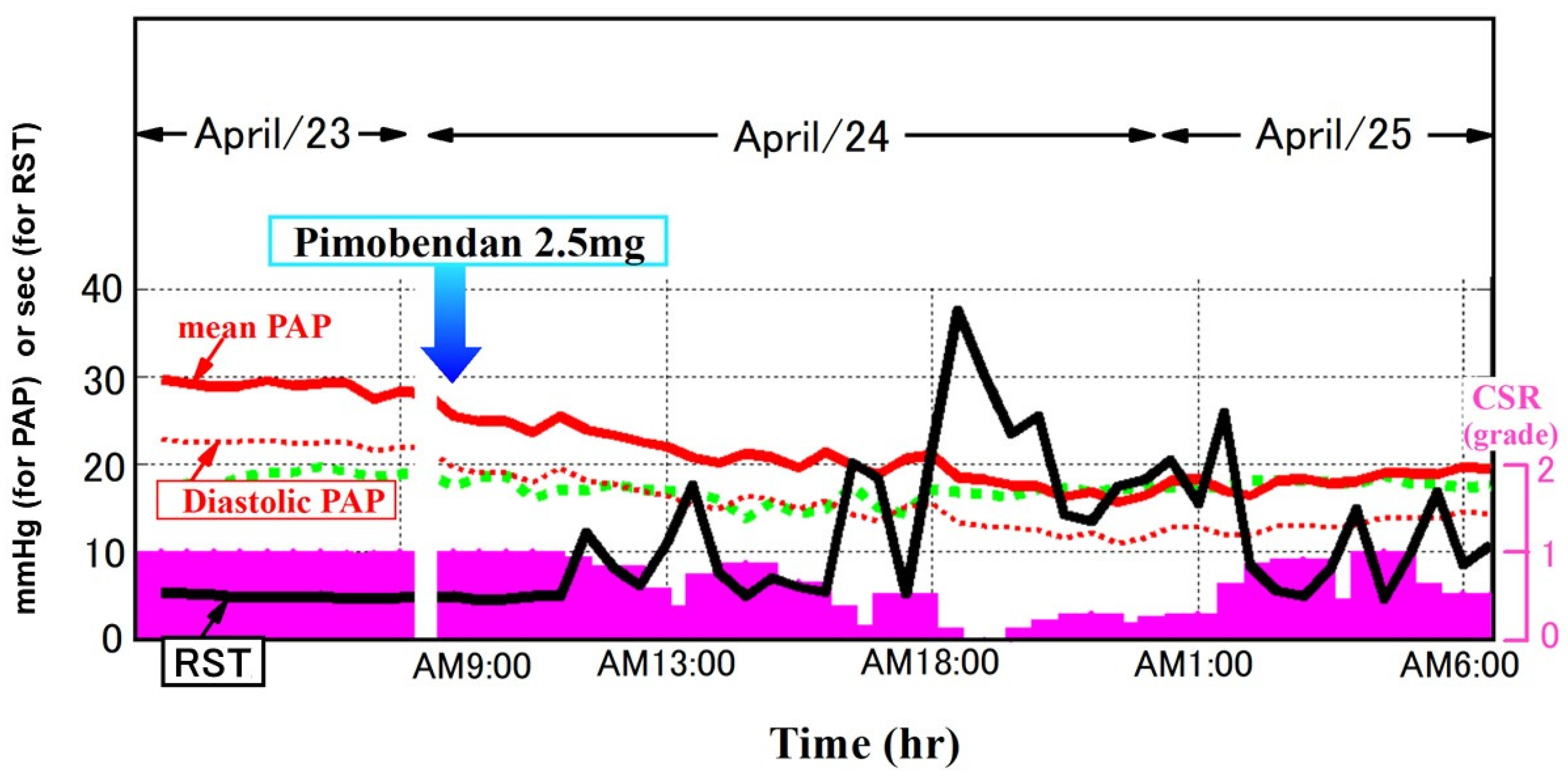
12. RST as a Marker of Prognosis and Congestion
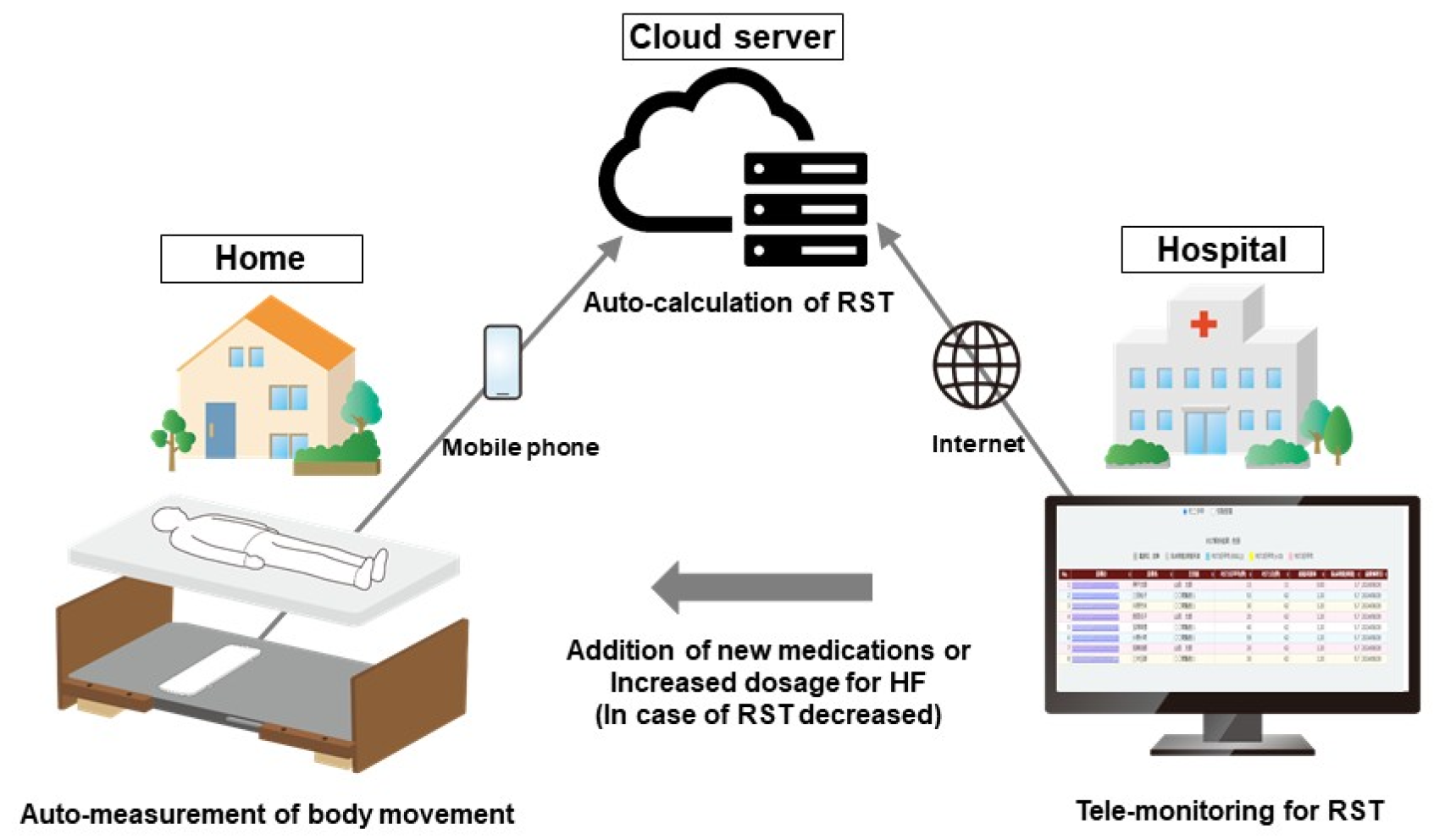
13. Remote Monitoring of RST and Its Potential Utility in Predicting Worsening HF
14. Ongoing Prospective Study Using RST-Guided HF Management
15. How to Improve RST Levels
16. Future Concerns
17. Conclusions
Funding
Conflicts of Interest
References
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Blecker, S.; Herrin, J.; Li, L.; Yu, H.; Grady, J.N.; Horwitz, L.I. Trends in Hospital Readmission of Medicare-Covered Patients With Heart Failure. J. Am. Coll. Cardiol. 2019, 73, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Miura, M.; Nochioka, K.; Sakata, Y. Heart failure as a general pandemic in Asia. Eur. J. Heart Fail. 2015, 17, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Komuro, I.; Kaneko, H.; Morita, H.; Isobe, M.; Nakayama, H.; Minematsu, K.; Yamaguchi, T.; Yazaki, Y. Nationwide Actions Against Heart Failure Pandemic in Japan—What Should We Do From Academia? Circ. J. 2019, 83, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T.; Kohsaka, S.; Kato, T.; Kato, E.; Sato, K.; Teramoto, K.; Yaku, H.; Akiyama, E.; Ando, M.; Izumi, C.; et al. JCS/JHFS 2025 Guideline on Diagnosis and Treatment of Heart Failure. J. Card. Fail. 2025, 89, 1278–1444. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Bauersachs, J. Heart failure drug treatment: The fantastic four. Eur. Heart J. 2021, 42, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Prinzen, F.W.; Vernooy, K.; Auricchio, A. Cardiac resynchronization therapy: State-of-the-art of current applications, guidelines, ongoing trials, and areas of controversy. Circulation 2013, 128, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.T.; Khera, R.; Lau, G.; Qiu, F.; Wang, Y.; Austin, P.C.; Koh, M.; Lin, Z.; Lee, D.S.; Wijeysundera, H.C.; et al. Readmission and Mortality After Hospitalization for Myocardial Infarction and Heart Failure. J. Am. Coll. Cardiol. 2020, 75, 736–746. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C., Jr.; et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Vaduganathan, M.; Fonarow, G.C.; Bonow, R.O. Rehospitalization for heart failure: Problems and perspectives. J. Am. Coll. Cardiol. 2013, 61, 391–403. [Google Scholar] [CrossRef]
- Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Miller, A.B.; Blair, J.E.; Konstam, M.A.; Wedge, P.; Bahit, M.C.; Carson, P.; Haass, M.; Hauptman, P.J.; Metra, M.; et al. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: Results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am. Heart J. 2010, 159, 841–849.e1. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; Abraham, W.T.; Albert, N.M.; Stough, W.G.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Pieper, K.; Sun, J.L.; Yancy, C.W.; et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: Findings from OPTIMIZE-HF. Arch. Intern. Med. 2008, 168, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Bennett, T.D.; St John Sutton, M.; Cho, Y.K.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M., Jr.; Abraham, W.T.; Smart, F.W.; Stevenson, L.W.; et al. Transition from chronic compensated to acute decompensated heart failure: Pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008, 118, 1433–1441. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Brugts, J.J.; Radhoe, S.P.; Clephas, P.R.D.; Aydin, D.; van Gent, M.W.F.; Szymanski, M.K.; Rienstra, M.; van den Heuvel, M.H.; da Fonseca, C.A.; Linssen, G.C.M.; et al. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF): A randomised clinical trial. Lancet 2023, 401, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- van Veldhuisen, D.J.; Braunschweig, F.; Conraads, V.; Ford, I.; Cowie, M.R.; Jondeau, G.; Kautzner, J.; Aguilera, R.M.; Lunati, M.; Yu, C.M.; et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011, 124, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Conraads, V.M.; Tavazzi, L.; Santini, M.; Oliva, F.; Gerritse, B.; Yu, C.M.; Cowie, M.R. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: The SENSE-HF trial. Eur. Heart J. 2011, 32, 2266–2273. [Google Scholar] [CrossRef]
- Amir, O.; Rappaport, D.; Zafrir, B.; Abraham, W.T. A novel approach to monitoring pulmonary congestion in heart failure: Initial animal and clinical experiences using remote dielectric sensing technology. Congest. Heart Fail. 2013, 19, 149–155. [Google Scholar] [CrossRef]
- Amir, O.; Azzam, Z.S.; Gaspar, T.; Faranesh-Abboud, S.; Andria, N.; Burkhoff, D.; Abbo, A.; Abraham, W.T. Validation of remote dielectric sensing (ReDS) technology for quantification of lung fluid status: Comparison to high resolution chest computed tomography in patients with and without acute heart failure. Int. J. Cardiol. 2016, 221, 841–846. [Google Scholar] [CrossRef]
- Nomoto, Y.; Imamura, T.; Izumida, T.; Narang, N.; Kinugawa, K. Clinical Implications of Remote Dielectric Sensing-Guided Management. J. Clin. Med. 2024, 13, 2906. [Google Scholar] [CrossRef]
- Alvarez-Garcia, J.; Lala, A.; Rivas-Lasarte, M.; De Rueda, C.; Brunjes, D.; Lozano-Jimenez, S.; Garcia-Sebastian, C.; Mitter, S.; Remior, P.; Jimenez-Blanco Bravo, M.; et al. Remote Dielectric Sensing Before and After Discharge in Patients With ADHF: The ReDS-SAFE HF Trial. JACC Heart Fail. 2024, 12, 695–706. [Google Scholar] [CrossRef]
- Amir, O.; Ben-Gal, T.; Weinstein, J.M.; Schliamser, J.; Burkhoff, D.; Abbo, A.; Abraham, W.T. Evaluation of remote dielectric sensing (ReDS) technology-guided therapy for decreasing heart failure re-hospitalizations. Int. J. Cardiol. 2017, 240, 279–284. [Google Scholar] [CrossRef]
- Imamura, T.; Narang, N.; Kinugawa, K. Clinical implications of remote dielectric sensing system to estimate lung fluid levels. J. Cardiol. 2023, 81, 276–282. [Google Scholar] [CrossRef]
- Koehler, F.; Winkler, S.; Schieber, M.; Sechtem, U.; Stangl, K.; Bohm, M.; Boll, H.; Baumann, G.; Honold, M.; Koehler, K.; et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: The telemedical interventional monitoring in heart failure study. Circulation 2011, 123, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.I.; Mattera, J.A.; Curtis, J.P.; Spertus, J.A.; Herrin, J.; Lin, Z.; Phillips, C.O.; Hodshon, B.V.; Cooper, L.S.; Krumholz, H.M. Telemonitoring in patients with heart failure. N. Engl. J. Med. 2010, 363, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Bax, J.; Bruining, N.; Cleland, J.G.; Koehler, F.; Malik, M.; Pinto, F.; van der Velde, E.; Vardas, P. e-Health: A position statement of the European Society of Cardiology. Eur. Heart J. 2016, 37, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Asanoi, H.; Harada, D.; Oda, Y.; Ueno, H.; Takagawa, J.; Ishise, H.; Goso, Y.; Joho, S.; Inoue, H. Independent prognostic importance of respiratory instability and sympathetic nerve activity in patients with chronic heart failure. J. Cardiol. 2017, 70, 476–483. [Google Scholar] [CrossRef]
- Ueno, Y.; Imamura, T.; Oshima, A.; Onoda, H.; Ushijima, R.; Sobajima, M.; Fukuda, N.; Ueno, H.; Kinugawa, K. Clinical Implications of Changes in Respiratory Instability Following Transcatheter Aortic Valve Replacement. J. Clin. Med. 2022, 11, 280. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Katsumata, Y.; Sadahiro, T.; Azuma, K.; Akita, K.; Isobe, S.; Yashima, F.; Miyamoto, K.; Nishiyama, T.; Tamura, Y.; et al. Real-Time Analysis of the Heart Rate Variability During Incremental Exercise for the Detection of the Ventilatory Threshold. J. Am. Heart Assoc. 2018, 7, e006612. [Google Scholar] [CrossRef]
- Naughton, M.T.; Lorenzi-Filho, G. Sleep in heart failure. Prog. Cardiovasc. Dis. 2009, 51, 339–349. [Google Scholar] [CrossRef]
- Melenovsky, V.; Andersen, M.J.; Andress, K.; Reddy, Y.N.; Borlaug, B.A. Lung congestion in chronic heart failure: Haemodynamic, clinical, and prognostic implications. Eur. J. Heart Fail. 2015, 17, 1161–1171. [Google Scholar] [CrossRef]
- Sakoda, M.; Asanoi, H.; Ohtani, T.; Nakamoto, K.; Harada, D.; Noto, T.; Takagawa, J.; Wada, O.; Nakane, E.; Inoko, M.; et al. Early Detection of Worsening Heart Failure in Patients at Home Using a New Telemonitoring System of Respiratory Stability. Circ. J. 2022, 86, 1081–1091. [Google Scholar] [CrossRef]
- Tobushi, T.; Kadokami, T.; Takagawa, J.; Dohi, K.; Joho, S.; Wada, O.; Momomura, S.I.; Koyama, T.; Haruki, N.; Ando, S.I.; et al. Blood Oxygen, Sleep Disordered Breathing, and Respiratory Instability in Patients With Chronic Heart Failure—PROST Subanalysis. Circ. Rep. 2019, 1, 414–421. [Google Scholar] [CrossRef]
- Imamura, T.; Akazawa, Y.; Saito, S.; Sakata, Y.; Miyagawa, S.; Yamada, T.; Asanoi, H.; Kinugawa, K. Usefulness of Respiratory Stability Time-Guided Management to Prevent Readmission of Chronic Heart Failure Patients at Home: A Multicenter, Single-Arm, Open-Label Clinical Study (ITMETHOD-HF-III). J. Clin. Med. 2025, 14, 4653. [Google Scholar] [CrossRef]
- Volpe, M.; Rao, M.A.; Tritto, C.; Pisani, A.; Mele, A.F.; Enea, I.; Condorelli, M. Transition from asymptomatic left ventricular dysfunction to congestive heart failure. J. Card. Fail. 1995, 1, 409–419. [Google Scholar] [CrossRef]
- Kohnlein, T.; Welte, T. Treatment strategies for Cheyne-Stokes respiration. Eur. Respir. J. 2006, 27, 238. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, T. Current Advancement of Respiratory Stability Time-Guided Heart Failure Management. J. Clin. Med. 2025, 14, 6182. https://doi.org/10.3390/jcm14176182
Imamura T. Current Advancement of Respiratory Stability Time-Guided Heart Failure Management. Journal of Clinical Medicine. 2025; 14(17):6182. https://doi.org/10.3390/jcm14176182
Chicago/Turabian StyleImamura, Teruhiko. 2025. "Current Advancement of Respiratory Stability Time-Guided Heart Failure Management" Journal of Clinical Medicine 14, no. 17: 6182. https://doi.org/10.3390/jcm14176182
APA StyleImamura, T. (2025). Current Advancement of Respiratory Stability Time-Guided Heart Failure Management. Journal of Clinical Medicine, 14(17), 6182. https://doi.org/10.3390/jcm14176182





