The Use of Advanced Glycation End-Product Measurements to Predict Post-Operative Complications After Cardiac Surgery
Abstract
1. Introduction
- (A)
- Non-receptor-mediated interactions with lipoproteins or the extracellular matrix (ECM) can cause direct disruption of structural stability of tissues by forming cross-links [3]. This includes interactions with blood vessel walls which can increase vascular permeability and lead to the formation of reactive oxygen species (ROS) [4].
- (B)
- Receptor-mediated binding and activation of ‘receptors of advanced glycation end products’ (RAGE). This leads to oxidative and inflammatory cascades being triggered, stimulating the production of cytokines like tumour necrosis factor alpha (TNF-alpha) and various interleukins [5], thereby enabling the development of inflammatory disorders.
2. AGEs and Frailty
3. Materials and Methods
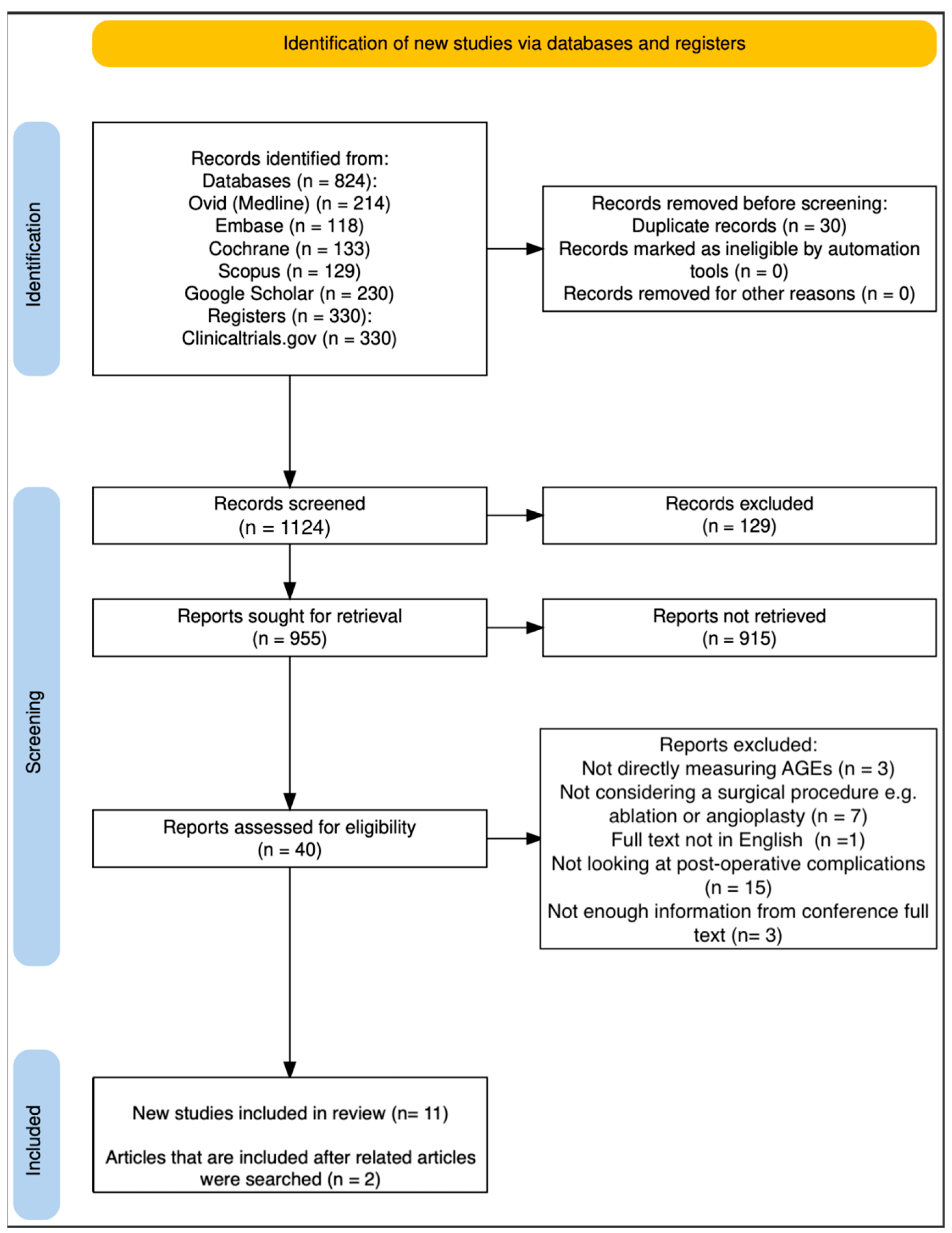
3.1. Search Strategy
3.2. Study Selection
3.3. Data Extraction
3.4. Quality Appraisal of Included Articles
4. Results
4.1. Main Study Characteristics Assessment
4.1.1. AGEs as Predictors of Post-Operative Complications in Cardiac Surgeries
4.1.2. AGEs as Predictors of Post-Operative Complications in General Surgery
4.1.3. AGEs as Predictors of Post-Operative Complications in Thoracic Surgeries
5. Discussion
5.1. Why Might AGEs Be Associated with Post-Operative Morbidity and Mortality?
5.1.1. Post-Operative Complication of Cardiovascular Disease
5.1.2. Post-Operative Complication of Infections
5.1.3. Post-Operative Complication of Kidney Disease
5.1.4. Post-Operative Complication of Transplant Graft Failure
5.2. How Should AGEs Be Measured?
5.3. Can Anything Be Conducted for Patients with High AGEs Levels?
5.4. Limitations
5.5. Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end products |
| AKI | Advanced kidney injury |
| CRP | C-reactive protein |
| ELISA | Enzyme-linked immunosorbent assay |
| sAF | Skin autofluorescence |
| sRAGE | Skin Receptor of Advanced glycation end products |
Appendix A
References
- Swarbrick, C.J.; Williams, K.; Evans, B.; Blake, H.A.; Poulton, T.; Nava, S.; Shah, A.; Martin, P.; Partridge, J.S.; Moppett, I.K. Postoperative outcomes in older patients living with frailty and multimorbidity in the UK: SNAP-3, a snapshot observational study. Br. J. Anaesth. 2025, 135, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Tarnowski, M. A Role for Advanced Glycation End Products in Molecular Ageing. Int. J. Mol. Sci. 2023, 24, 9881. [Google Scholar] [CrossRef] [PubMed]
- Basta, G. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, Y.; Shi, L.; Li, L.; Zhang, D.; Gong, Z.; Wu, Q. Activation and modulation of the AGEs-RAGE axis: Implications for inflammatory pathologies and therapeutic interventions—A review. Pharmacol. Res. 2024, 206, 107282. [Google Scholar] [CrossRef]
- Goh, S.Y.; Cooper, M.E. The Role of Advanced Glycation End Products in Progression and Complications of Diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef]
- Rojas, A.; Lindner, C.; Schneider, I.; González, I.; Morales, M.A. Contributions of the receptor for advanced glycation end products axis activation in gastric cancer. World J. Gastroenterol. 2023, 29, 997–1010. [Google Scholar] [CrossRef]
- Pan, S.; Guan, Y.; Ma, Y.; Cui, Q.; Tang, Z.; Li, J.; Zu, C.; Zhang, Y.; Zhu, L.; Jiang, J.; et al. Advanced glycation end products correlate with breast cancer metastasis by activating RAGE/TLR4 signaling. BMJ Open Diabetes Res. Care 2022, 10, e002697. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Nakaigawa, N.; Miyoshi, Y.; Fujinami, K.; Kubota, Y.; Uemura, H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate 2005, 64, 92–100. [Google Scholar] [CrossRef]
- Hegab, Z.; Gibbons, S.; Neyses, L.; Mamas, M.A. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 2012, 4, 90–102. [Google Scholar] [CrossRef]
- Atzeni, I.M.; van de Zande, S.C.; Westra, J.; Zwerver, J.; Smit, A.J.; Mulder, D.J. The AGE Reader: A non-invasive method to assess long-term tissue damage. Methods 2022, 203, 533–541. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Links, T.; Graaff, R.; Thorpe, S.R.; Baynes, J.W.; Hartog, J.; Gans, R.; Smit, A. Simple Noninvasive Measurement of Skin Autofluorescence. Ann. N. Y. Acad. Sci. 2005, 43, 290–298. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, H.; Zhu, L.; Wu, X.; Han, C. Accumulation of Advanced Glycation Endproducts and Subclinical Inflammation in Deep Tissues of Adult Patients With and Without Diabetes. Can. J. Diabetes 2018, 42, 525–532.e4. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty in Relation to the Accumulation of Deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef]
- Drenth, H.; Zuidema, S.U.; Krijnen, W.P.; Bautmans, I.; Smit, A.J.; Van Der Schans, C.; Hobbelen, H. Advanced Glycation End Products Are Associated With Physical Activity and Physical Functioning in the Older Population. J. Gerontol. Ser. A 2018, 73, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, S.; Smidt, N.; Swertz, M.A.; Bakker, S.J.; Dotinga, A.; Vonk, J.M.; Van Dijk, F.; van Zon, S.K.; Wijmenga, C.; Wolffenbuttel, B.H.; et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2015, 44, 1172–1180. [Google Scholar] [CrossRef]
- Yabuuchi, J.; Ueda, S.; Yamagishi, S.I.; Nohara, N.; Nagasawa, H.; Wakabayashi, K.; Matsui, T.; Yuichiro, H.; Kadoguchi, T.; Otsuka, T.; et al. Association of advanced glycation end products with sarcopenia and frailty in chronic kidney disease. Sci. Rep. 2020, 10, 17647. [Google Scholar] [CrossRef]
- Waqas, K.; Chen, J.; Rivadeneira, F.; Uitterlinden, A.G.; Voortman, T.; Zillikens, M.C. Skin Autofluorescence, a Noninvasive Biomarker of Advanced Glycation End-products, Is Associated With Frailty: The Rotterdam Study. J. Gerontol. Ser. A 2022, 77, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.A.; Brusselle, G.; Ghanbari, M.; Goedegebure, A.; Ikram, M.K.; Kavousi, M.; Kieboom, B.C.; Klaver, C.C.; de Knegt, R.J.; Luik, A.I.; et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur. J. Epidemiol. 2020, 35, 483–517. [Google Scholar] [CrossRef] [PubMed]
- Butcher, L.; Carnicero, J.A.; Pérès, K.; Bandinelli, S.; García-García, F.J.; Rodriguez-Artalejo, F.; Rodriguez-Mañas, L.; Erusalimsky, J.D. Frailty Influences the Relationship between the Soluble Receptor for Advanced Glycation-End Products and Mortality in Older Adults with Diabetes Mellitus. Gerontology 2024, 70, 585–594. [Google Scholar] [CrossRef]
- Erusalimsky, J.D.; Grillari, J.; Grune, T.; Jansen-Duerr, P.; Lippi, G.; Sinclair, A.J.; Tegnér, J.; Viña, J.; Durrance-Bagale, A.; Miñambres, R.; et al. In search of ‘Omics’-based biomarkers to predict risk of frailty and its consequences in older individuals: The FRAILOMIC initiative. Gerontology 2016, 62, 182–190. [Google Scholar] [CrossRef]
- Iida, H.; Takegami, Y.; Osawa, Y.; Funahashi, H.; Ozawa, Y.; Ido, H.; Asamoto, T.; Otaka, K.; Tanaka, S.; Nakashima, H.; et al. Association between advanced glycation end-products and fall risk in older adults: The Yakumo Study. Geriatr. Gerontol. Int. 2024, 24, 517–522. [Google Scholar] [CrossRef]
- Iida, H.; Seki, T.; Takegami, Y.; Osawa, Y.; Kato, D.; Takemoto, G.; Ando, K.; Ishizuka, S.; Hasegawa, Y.; Imagama, S. Association between locomotive syndrome and fall risk in the elderly individuals in Japan: The Yakumo study. J. Orthop. Sci. 2024, 29, 327–333. [Google Scholar] [CrossRef]
- Kuiper, L.M.; Picavet, H.S.J.; Rietman, M.L.; Dollé, M.E.; Verschuren, W.M. Advanced glycation end-products and metabolomics are independently associated with frailty: The longitudinal Doetinchem Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 80, glae272. [Google Scholar] [CrossRef]
- Verschuren, W.M.M.; Blokstra, A.; Picavet, H.S.J.; Smit, H.A. Cohort profile: The Doetinchem Cohort Study. Int. J. Epidemiol. 2008, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Ochi, M.; Shiraoka, A.; Matsumoto, S.; Fujishita, S.; Okada, Y.; Miura, S.; Ochi, H.; Igase, M.; Ohyagi, Y. Frailty and aging are associated with cognitive decline and dermal advanced glycation end-product accumulation in older Japanese men. Arch. Gerontol. Geriatr. Plus 2024, 1, 100071. [Google Scholar] [CrossRef]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef]
- Castellani, R.J.; Harris, P.L.; Sayre, L.M.; Fujii, J.; Taniguchi, N.; Vitek, M.P.; Founds, H.; Atwood, C.S.; Perry, G.; Smith, M.A. Active glycation in neurofibrillary pathology of Alzheimer disease: Nε-(Carboxymethyl) lysine and hexitol-lysine. Free Radic. Biol. Med. 2001, 31, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Simm, A.; Wagner, J.; Gursinsky, T.; Nass, N.; Friedrich, I.; Schinzel, R.; Czeslik, E.; Silber, R.E.; Scheubel, R.J. Advanced glycation endproducts: A biomarker for age as an outcome predictor after cardiac surgery? Exp. Gerontol. 2007, 42, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Creagh-Brown, B.C.; Quinlan, G.J.; Hector, L.R.; Evans, T.W.; Burke-Gaffney, A. Association between preoperative plasma sRAGE levels and recovery from cardiac surgery. Mediat. Inflamm. 2013, 2013, 496031. [Google Scholar] [CrossRef]
- Hofmann, B.; Gerull, K.A.; Bloch, K.; Riemer, M.; Erbs, C.; Fröhlich, A.; Richter, S.; Ehrhardt, M.; Zitterbart, C.; Bartel, F.F.; et al. It’s all in our skin—Skin autofluorescence—A promising outcome predictor in cardiac surgery: A single centre cohort study. PLoS ONE 2020, 15, e0234847. [Google Scholar] [CrossRef]
- Reichert, S.; Hofmann, B.; Kohnert, M.; Santos, A.N.; Friebe, L.; Grollmitz, J.; Schaller, H.-G.; Schulz, S. Advanced Glycation End Product (AGE) and Soluble Receptor of AGE (sRAGE) Levels in Relation to Periodontitis Severity and as Putative 3-Year Outcome Predictors in Patients Undergoing Coronary Artery Bypass Grafting (CABG). J. Clin. Med. 2022, 11, 4105. [Google Scholar] [CrossRef]
- Smoor, R.M.; van Dongen, E.P.; Verwijmeren, L.; Emmelot-Vonk, M.H.; Vernooij, L.M.; Cremer, O.L.; Noordzij, P.G. Advanced glycation end products for preoperative frailty screening in older cardiac surgery patients. J. Am. Geriatr. Soc. 2023, 71, 2520–2529. [Google Scholar] [CrossRef]
- Pol, H.W.D.; Sibma, E.; Zeebregts, C.J.; Pierik, E.G.; Meerwaldt, R. Increased skin autofluorescence after colorectal operation reflects surgical stress and postoperative outcome. Am. J. Surg. 2011, 202, 583–589. [Google Scholar] [CrossRef]
- Serpa Neto, A.; Campos, P.P.; Hemmes, S.N.; Bos, L.D.; Bluth, T.; Ferner, M.; Güldner, A.; Hollmann, M.W.; India, I.; Kiss, T.; et al. Kinetics of plasma biomarkers of inflammation and lung injury in surgical patients with or without postoperative pulmonary complications. Eur. J. Anaesthesiol. 2017, 34, 229–238. [Google Scholar] [CrossRef]
- Choi, S.; Yang, S.; Choi, G.; Kim, B.; Kang, H. Comparison of pressure- and volume-controlled ventilation during laparoscopic colectomy in patients with colorectal cancer. Sci. Rep. 2019, 9, 17007. [Google Scholar] [CrossRef] [PubMed]
- Krasnodębski, M.; Grąt, K.; Morawski, M.; Borkowski, J.; Krawczyk, P.; Zhylko, A.; Skalski, M.; Kalinowski, P.; Zieniewicz, K.; Grąt, M. Skin autofluorescence as a novel predictor of acute kidney injury after liver resection. World J. Surg. Oncol. 2021, 19, 276. [Google Scholar] [CrossRef]
- Morawski, M.; Grąt, M.; Krasnodębski, M.; Grąt, K.; Krawczyk, P.; Stypułkowski, J.; Borkowski, J.; Rykowski, P.; Zhylko, A.; Skalski, M.; et al. Prediction of Incisional Hernia Using Skin Autofluorescence in Patients after Subcostal Laparotomy. HPB 2022, 24, S154. [Google Scholar] [CrossRef]
- Calfee, C.S.; Budev, M.M.; Matthay, M.A.; Church, G.; Brady, S.; Uchida, T.; Ishizaka, A.; Lara, A.; Ranes, J.L.; decamp, M.M.; et al. Plasma Receptor for Advanced Glycation End-products Predicts Duration of ICU Stay and Mechanical Ventilation in Patients After Lung Transplantation. J. Heart Lung Transplant. 2007, 26, 675–680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, R.J.; Bellamy, S.L.; Lee, J.C.; Cantu, E.; Diamond, J.M.; Mangalmurti, N.; Kawut, S.M.; Ware, L.B.; Christie, J.D. Early plasma soluble receptor for advanced glycation end-product levels are associated with bronchiolitis obliterans syndrome. Am. J. Transpl. 2013, 13, 754–759. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakao, S.; Yamaguchi, K.; Iwamoto, H.; Kagimoto, A.; Mimae, T.; Tsutani, Y.; Miyata, Y.; Hamada, H.; Okada, M.; Hattori, N. Role of Soluble Receptor for Advanced Glycation End Products in Postoperative Fibrotic Lung Injury. Ann. Thorac. Surg. 2022, 113, 1617–1623. [Google Scholar] [CrossRef]
- Shohat, N.; Tarabichi, M.; Tan, T.L.; Goswami, K.; Kheir, M.; Malkani, A.L.; Shah, R.P.; Schwarzkopf, R.; Parvizi, J.A. 2019 John Insall Award: Fructosamine is a better glycaemic marker compared with glycated haemoglobin (HbA1C) in predicting adverse outcomes following total knee arthroplasty: A prospective multicentre study. Bone Jt. J. 2019, 101 (Suppl. S7), 3–9. [Google Scholar] [CrossRef]
- Chen, S.; Chen, G.; Jin, Y.; Zhu, S.; Jia, L.; Zhao, C.; Jin, C.; Xiang, M. Association between glycated albumin and adverse outcomes in patients with heart failure. J. Diabetes Investig. 2024, 15, 1457–1463. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Wang, Z.; Li, T.; Liu, C.; Kang, X.; Cui, X.; Yang, J.; Qu, H.; Duanmu, J.; et al. Extracellular RIPK3 acts as a damage-associated molecular pattern to exaggerate cardiac ischemia/reperfusion injury. Circulation 2024, 150, 1791–1811. [Google Scholar] [CrossRef]
- Lagier, D.; Velly, L.J.; Guinard, B.; Bruder, N.; Guidon, C.; Melo, M.F.; Alessi, M.C. Perioperative open-lung approach, regional ventilation, and lung injury in cardiac surgery: A PROVECS trial substudy. Anesthesiology 2020, 133, 1029. [Google Scholar] [CrossRef]
- Redondo, A.; Paradela-Dobarro, B.; Moscoso, I.; Moure-Álvarez, M.; Cebro-Márquez, M.; González-Juanatey, J.R.; García-Seara, J.; Álvarez, E. Galectin-3 and soluble RAGE as new biomarkers of post-infarction cardiac remodeling. J. Mol. Med. 2021, 99, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Böhm, A.; Tothova, L.; Urban, L.; Slezak, P.; Bacharova, L.; Musil, P.; Hatala, R. The relation between oxidative stress biomarkers and atrial fibrillation after pulmonary veins isolation. J. Electrocardiol. 2016, 49, 423–428. [Google Scholar] [CrossRef] [PubMed]
- González-Ferrero, T.; Bergonti, M.; López-Canoa, J.N.; Arias, F.G.; Eiras Penas, S.; Spera, F.; González-Maestro, A.; Minguito-Carazo, C.; Martínez-Sande, J.L.; González-Melchor, L.; et al. Atrial fibrillation ablation in patients with arrhythmia-induced cardiomyopathy: A prospective multicentre study. ESC Heart Fail. 2023, 10, 3055–3066. [Google Scholar] [CrossRef]
- Hartog, J.W.; de Vries, A.P.; Bakker, S.J.; Graaff, R.; van Son, W.J.; van der Heide, J.J.; Gans, R.O.; Wolffenbuttel, B.H.; de Jong, P.E.; Smit, A.J. Risk factors for chronic transplant dysfunction and cardiovascular disease are related to accumulation of advanced glycation end-products in renal transplant recipients. Nephrol. Dial. Transplant. 2006, 21, 2263–2269. [Google Scholar] [CrossRef]
- Kottmaier, M.; Mayer, S.; Bourier, F.; Reents, T.; Semmler, V.; Telishevska, M.; Kornmayer, M.; Brooks, S.; Lengauer, S.; Berger, F.; et al. P989 Association between circulating biomarkers of fibrosis and left atrial voltage in patients undergoing atrial fibrillation ablation. A pilot study. Eur. Heart J. 2018, 39 (Suppl. S1), ehy564-P989. [Google Scholar] [CrossRef]
- Perkins, G.D.; Gates, S.; Park, D.; Gao, F.; Knox, C.; Holloway, B.; McAuley, D.F.; Ryan, J.; Marzouk, J.; Cooke, M.W.; et al. The beta agonist lung injury trial prevention. A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2014, 189, 674–683. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M. Introduction to the GRADE tool for rating certainty in evidence and recommendations. Clin. Epidemiol. Glob. Health 2024, 25, 101484. [Google Scholar] [CrossRef]
- Tang, J.L.; Liu, J.L. Misleading funnel plot for detection of bias in meta-analysis. J. Clin. Epidemiol. 2000, 53, 477–484. [Google Scholar] [CrossRef]
- Nashef, S.A.; Roques, F.; Michel, P.; Gauducheau, E.; Lemeshow, S.; Salamon, R.; EuroSCORE Study Group. European system for cardiac operative risk evaluation (Euro SCORE). Eur. J. Cardio-Thorac. Surg. 1999, 16, 9–13. [Google Scholar] [CrossRef]
- Hassan, J.; Abbas, M.; Shaukat, M.; Faisal, A.B.; Hassan, D.; Salman, M.; Raheem, A.; Sharif, H. Assessing operative mortality risk in cardiothoracic surgery: Analysis of STS scores—A retrospective study. Cardiothorac. Surg. 2025, 33, 18. [Google Scholar] [CrossRef]
- Kosmopoulos, M.; Drekolias, D.; Zavras, P.D.; Piperi, C.; Papavassiliou, A.G. Impact of advanced glycation end products (AGEs) signaling in coronary artery disease. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2019, 1865, 611–619. [Google Scholar] [CrossRef]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Robles-Rivera, K.; Rivera-Paredez, B.; Quezada-Sanchéz, A.D.; Velázquez-Cruz, R.; Salmerón, J. Advanced glycation end products are associated with cardiovascular risk in the Mexican population. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Stirban, A.; Gawlowski, T.; Roden, M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol. Metab. 2014, 3, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Andrades, M.É.; Lorenzi, R.; Nagai, R.; Moreira, J.C.F.; Ritter, C.; Dal-Pizzol, F. Plasma glycation levels are associated with severity in sepsis. Eur. J. Clin. Investig. 2012, 42, 1055–1060. [Google Scholar] [CrossRef]
- Bronowicka-Szydełko, A.; Gostomska-Pampuch, K.; Kuzan, A.; Pietkiewicz, J.; Krzystek-Korpacka, M.; Gamian, A. Effect of advanced glycation end-products in a wide range of medical problems including COVID-19. Adv. Med. Sci. 2024, 69, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Heidland, A.; Sebekova, K.; Schinzel, R. Advanced glycation end products and the progressive course of renal disease. Am. J. Kidney Dis. 2001, 38, S100–S106. [Google Scholar] [CrossRef]
- Stinghen, A.E.M.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef]
- Busch, M.; Franke, S.; Rüster, C.; Wolf, G. Advanced glycation end-products and the kidney. Eur. J. Clin. Investig. 2010, 40, 742–755. [Google Scholar] [CrossRef]
- Hartog, J.W.; Smit, A.J.; van Son, W.J.; Navis, G.; Gans, R.O.; Wolffenbuttel, B.H.; de Jong, P.E. Advanced glycation end products in kidney transplant patients: A putative role in the development of chronic renal transplant dysfunction. Am. J. Kidney Dis. 2004, 43, 966–975. [Google Scholar] [CrossRef]
- Liu, X.; Liu, K.; Wang, Z.; Liu, C.; Han, Z.; Tao, J.; Lu, P.; Wang, J.; Wu, B.; Huang, Z.; et al. Advanced glycation end products accelerate arteriosclerosis after renal transplantation through the AGE/RAGE/ILK pathway. Exp. Mol. Pathol. 2015, 99, 312–319. [Google Scholar] [CrossRef]
- Patel, S.I.; Chakkera, H.A.; Wennberg, P.W.; Liedl, D.A.; Alrabadi, F.; Cha, S.S.; Hooley, D.D.; Amer, H.; Wadei, H.M.; Shamoun, F.E. Peripheral arterial disease preoperatively may predict graft failure and mortality in kidney transplant recipients. Vasc. Med. 2017, 22, 225–230. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced glycation end products (AGEs): Biochemistry, signaling, analytical methods, and epigenetic effects. Oxidative Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef]
- Ahdi, M.; Gerdes, V.E.; Graaff, R.; Kuipers, S.; Smit, A.J.; Meesters, E.W. Skin autofluorescence and complications of diabetes: Does ethnic background or skin color matter? Diabetes Technol. Ther. 2015, 17, 88–95. [Google Scholar] [CrossRef]
- Corica, D.; Pepe, G.; Currò, M.; Aversa, T.; Tropeano, A.; Ientile, R.; Wasniewska, M. Methods to investigate advanced glycation end-product and their application in clinical practice. Methods 2022, 203, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Bell-Farrow, A.D.; Wang, Z.Q.; Sonntag, W.E.; Fu, M.X.; Baynes, J.W.; Thorpe, S.R. Caloric Restriction Decreases Age-Dependent Accumulation of the Glycoxidation Products, N∈-(Carboxymethyl) lysine and Pentosidine, in Rat Skin Collagen. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995, 50, B337–B341. [Google Scholar] [CrossRef]
- Birlouez-Aragon, I.; Saavedra, G.; Tessier, F.J.; Galinier, A.; Ait-Ameur, L.; Lacoste, F.; Niamba, C.N.; Alt, N.; Somoza, V.; Lecerf, J.M. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am. J. Clin. Nutr. 2010, 91, 1220–1226. [Google Scholar] [CrossRef]
- Liska, D.J.; Cook, C.M.; Wang, D.D.; Szpylka, J. Maillard reaction products and potatoes: Have the benefits been clearly assessed? Food Sci. Nutr. 2016, 4, 234–249. [Google Scholar] [CrossRef]
- Guilbaud, A.; Niquet-Leridon, C.; Boulanger, E.; Tessier, F.J. How can diet affect the accumulation of advanced glycation end-products in the human body? Foods 2016, 5, 84. [Google Scholar] [CrossRef]
- Wellens, J.; Vissers, E.; Dumoulin, A.; Hoekx, S.; Vanderstappen, J.; Verbeke, J.; Vangoitsenhoven, R.; Derrien, M.; Verstockt, B.; Ferrante, M.; et al. Cooking methods affect advanced glycation end products and lipid profiles: A randomized cross-over study in healthy subjects. Cell Rep. Med. 2025, 6, 102091. [Google Scholar] [CrossRef]
- Turner, D.P. Advanced glycation end-products: A biological consequence of lifestyle contributing to cancer disparity. Cancer Res. 2015, 75, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.L.; Borges, J.P.; Lopes, G.D.; Pereira, E.N.; Mediano, M.F.; Farinatti, P.; Tibiriça, E.; Daliry, A. Influence of physical exercise on advanced glycation end products levels in patients living with the human immunodeficiency virus. Front. Physiol. 2018, 9, 1641. [Google Scholar] [CrossRef] [PubMed]
- Koetsier, M.; Nur, E.; Chunmao, H.; Lutgers, H.L.; Links, T.P.; Smit, A.J.; Rakhorst, G.; Graaff, R. Skin color independent assessment of aging using skin autofluorescence. Opt. Express 2010, 18, 14416. [Google Scholar] [CrossRef] [PubMed]
| Article | Month/Year | Type of Study | Method | Results |
|---|---|---|---|---|
| Drenth et al. [16] | October 2018 | Observational cross-sectional study | 5624 participants aged 65 years and older were taken from the LifeLines Cohort Study [17]. Linear regression conducted between sAF, number of physically active days, and physical functioning. | sAF and physical functioning was significantly associated, even when confounding variables considered. |
| Yabuuchi et al. [18] | October 2020 | Observational cross-sectional study | 37 patients undergoing maintenance dialysis at Juntendo University hospital in Japan. Serum AGE measured and frailty assessed using timed up and go test. | Serum AGE was significantly associated with increased frailty status. Slowness and weight loss were the variables with greatest levels of association with higher AGE levels. |
| Waqas et al. [19] | October 2022 | Prospective cohort study | 2521 participants were taken from the Rotterdam study [20] and AGEs measured as sAF using AGE reader. Frailty was measured using both Fried and Rockwood’s criteria. Multivariate regression was carried out, adjusting for confounders like age, renal function, smoking, and socioeconomic status. | sAF was associated with both pre-frailty and a higher frailty index. |
| Butcher et al. [21] | April 2024 | Prospective cohort study | 391 diabetic patients from European cohorts enrolled in the FRAILOMIC project [22], followed over 6 years. Patients were divided into frail and non-frail patients with frailty assessed using Fried’s criteria. Serum sRAGE measured using ELISA. | Stronger association between sRAGE and mortality in those that were frail. Higher sRAGE was associated with higher mortality in both groups. |
| Iida et al. [23] | June 2024 | Observational cross-sectional study | AGE was measured using sAF. 264 participants, from the 2022 Yakumo Study [24], were divided into two groups according to presence or absence of fall risk and factors associated with fall risk were investigated. | Fall risk group had a higher age, sAF, and a higher proportion of locomotive syndrome. sAF was independently associated with fall risk in older adults, even after factoring in confounders. |
| Kuiper et al. [25] | November 2024 | Prospective cohort study | 2382 participants from the Doetinchem Cohort Study [26]—measured sAF, MetaboHealth, frailty index, and frailty phenotype using Fried’s criteria. Confounding variables considered were as follows: age, sex, socioeconomic status, and season. | sAF was associated with higher frailty index scores. Associations between sAF and frailty and pre-frailty were still seen when data was adjusted for confounders. |
| Takei et al. [27] | December 2024 | Observational cross-sectional study | 559 participants visiting Ehime University Hospital in Japan for check-up. Dermal AGE accumulation was measured using sAF. | Risk of cognitive impairment is significantly associated with sAF, especially in men. There is a closer association between sAF and physical frailty in men than in women. |
| Author | Year | Country of Study | Sample Size | Surgery Type | Measurement of AGE | Complications and Outcomes Measured | Results | Summary of Findings |
|---|---|---|---|---|---|---|---|---|
| Cardiac Surgery | ||||||||
| Simm et al. [30] | 2007 | Germany | 75 | Cardiac surgery—CABG | Using ELISA to measure levels of carboxymethyl lysine (an AGE) from pericardial fluid, taken after pericardial incision was made. | Ventilation time, ejection fraction, AF, low cardiac output, MI, respiratory insufficiency, pneumonia, ICU length of stay. | Statistical association between increasing AGE and age of patient (r = 0.379, p = 0.0008). Increased AGEs correlated with increased levels of cardiac complications (p = 0.018) but not pulmonary complications (p = 0.279). | Increased AGEs are statistically associated with increased cardiac complications (AF, low cardiac output, post-operative MI) but not with increased pulmonary complications (respiratory insufficiency, pneumonia and prolonged ventilation time). |
| Creagh-Brown et al. [31] | 2013 | London, UK | 129 | Cardiac surgery—CABG, valve replacement, or more than one valve being repaired/replaced. | sRAGE measured using ELISA before surgery and 2 h after surgery. | Duration of mechanical ventilation, acute lung injury presence, hospital length of stay, ICU length of stay, mortality. | Plasma levels of sRAGE were significantly higher in patients 2 h after surgery (p < 0.0001). Preoperative sRAGE levels appeared to be the strongest predictor of hospital length of stay and other outcomes (p < 0.001). Post-operative sRAGE did not have any such correlations. | Preoperative sRAGE is a strong predictor of cardiac surgical outcomes, but not post-operative sRAGE. |
| Hoffman et al. [32] | 2020 | Germany | 758 | Cardiac surgery—CABG or aortic valve replacement or both. | sAF was measured at time of preoperative patient visit. | Mortality, MI, new AF or VF, reintubation, prolonged ventilation > 24 h, dialysis post-operatively, Cr > 350 μmol/L, neurological deficit > 24 h, wound healing issue, reoperation. | sAF was independently associated with higher morbidity (p < 0.0001)—independent of age or lower LVEF. sAF could predict in-hospital mortality (p = 0.0003) with greater significance compared to other existing scores, e.g., EUROSCORE II (p = 0.12) and STS Score (p = 0.02). | sAF is an independent predictive marker of mortality and morbidity after cardiac surgery and is at least comparable to other predictive scoring systems, if not better. |
| Reichert et al. [33] | 2022 | Germany | 95 | Cardiac surgery—CABG. | Both sRAGE and sAF were measured pre-operatively. | Observed over a period of 3 years post-operatively to monitor early and late cardiovascular and cerebrovascular outcomes. | sAF and sRAGE serum levels were not significantly associated with a poorer cardiovascular outcome after CABG (p = 0.257). | Insufficient evidence to suggest that sAF and sRAGE were associated with poorer cardiovascular outcome after CABG. |
| Smoor et al. [34] | 2023 | 2 centres in Netherlands | 555 | Cardiac surgery—open procedures. | sAF was measured preoperatively in participants aged over 70. | Disability, death, reoperation, reintubation, stroke, readmission to ICU, life-threatening bleeding, renal replacement therapy, respiratory insufficiency. | sAF was associated with dependent living (p < 0.006) and had worse physical health related quality of life (p = 0.0025). Higher sAF was also associated as a predictor of poor outcomes (when taking death or disability as a composite endpoint) 1 year after surgery (p < 0.001). | Higher sAF levels predict death and disability 1 year after cardiac surgery. |
| General Surgery | ||||||||
| Pol et al. [35] | 2011 | Netherlands | 40 | General surgery—elective colorectal surgery for malignancy. | sAF was measured day before operation and every day after until discharge. | Systemic infections, MI, pulmonary complications, anastomotic leakage. | There was an increase in sAF by 19 +/− 2% after surgery. Both increased preoperative sAF and perioperative increases in sAF were correlated with development of post-operative complications (p < 0.01). Changes in sAF correlated to changes with CRP (p = 0.03). | Both increased preoperative sAF and perioperative increases in sAF may be useful to predict post-operative complications. |
| Neto et al. [36] | 2017 | Germany, USA, Netherlands | 242 | General surgery—open abdominal surgery. | sRAGE measured directly after and 5 days after surgery. | Post-operative pulmonary complications measured up to 5 days after surgery, including ARDS, atelectasis, pleural effusion, pulmonary oedema, etc. | Median levels of sRAGE did not change after surgery (p = 0.783). sRAGE levels were not associated with post-operative pulmonary complications (p = 0.132). | sRAGE levels are not statistically associated with post-operative pulmonary complications and so insufficient evidence to suggest that they have prognostic capacity. |
| Choi et al. [37] | 2019 | Seol, South Korea | 46 | General surgery—laparoscopic colectomy. | sRAGE measured using ELISA 20 min after induction of anaesthesia, at skin closure and 24 h after operation. | Complications measured during first six post-operative days including the following: duration of recovery room stays, diet day, duration of hospital and ICU stay, and respiratory complications. | Intra- and post-operative sRAGE was significantly associated with development of post-operative respiratory complications (p < 0.00001). | Intra- and post-operative sRAGE can be used to predict development of post-operative respiratory complications. |
| Krasnodebski et al. [38] | 2021 | Warsaw, Poland | 130 | General surgery—liver resection for suspected malignancy. | AGEs were measured using sAF in the immediate preoperative period. | AKI within seven post-operative days was measured. | 32 patients had an AKI with 9 having a severe AKI. sAF was independently associated with AKI development (p = 0.047) and sAF-predicted operative time (p = 0.046). | sAF is a significant predictor of AKI, even after confounding variables are factored in. |
| Morawski et al. [39] | 2022 | Warsaw, Poland | 54 | General surgery—subcostal laparotomy for suspected GI malignancy. | sAF was measured before and after surgery. | Presence or absence of incisional hernias were measured 1–2 years after surgery. BMI and diabetes was monitored too. | There was no difference in sAF between patients with and without incisional hernias when BMI and diabetes were individually factored in (p = 0.587 and p = 0.669, respectively). | Lack of evidence to suggest that sAF could be used to predict incisional hernias after surgery. |
| Thoracic surgery | ||||||||
| Calfee et al. [40] | 2007 | USA | 20 | Thoracic surgery—lung transplantation or heart lung transplantation. | sRAGE levels measured within mean of 4 h of cross clamp release via ELISA. | Bronchiolitis obliterans syndrome (BOS), mortality (up to 1 year later), duration of mechanical ventilation, ICU length of stay. | Doubling the sRAGE levels, increased mechanical ventilation time by 26 h and ICU stay by 1.76 days, when adjusted for ischemia time (p = 0.018). No association between sRAGE and mortality or presence of BOS at 1 year. Neither PGD score nor ischemia time predicted post-operative complications. | sRAGE levels at 4 h were significantly correlated with both duration of mechanical ventilation and ICU stay, and was a better indicator than primary graft dysfunction score. |
| Shah et al. [41] | 2013 | USA | 106 | Thoracic surgery—lung transplant. | sRAGE measured 6 h and 24 h after surgery. | Development of BOS. | Average time to develop BOS was 3.4 ± 1.8 years. sRAGE measured 6 and 24 h after surgery were associated with increased risk of BOS (p = 0.02 and p = 0.01, respectively). | sRAGE levels were significantly associated with bronchiolitis obliterans syndrome post-operatively. |
| Nakao et al. [42] | 2022 | Japan | 152 | Thoracic surgery—wedge resection, segmentectomy and lobectomy. | sRAGE levels measured using ELISA just before surgery and 1 month after surgery. | Post-operative acute exacerbation of interstitial lung disease was measured. | Post-operative AE-ILD developed in 17 patients. Lower sRAGE levels were significantly associated with development of post-operative AE-ILD, independently of confounding variables (p = 0.024). | Lower levels of sRAGE were associated with occurrence of post-operative AE-ILD, especially in lobectomy patients. |
| Study | Reasoning for Exclusion |
|---|---|
| Shohat et al. [43] | This article was not directly measuring AGEs through sAF or sRAGEs, but was measuring AGEs indirectly as it was measuring fructosamine which becomes degraded into AGEs. |
| Chen et al. [44] | This article was not directly measuring AGEs through sAF or sRAGEs, but was measuring AGEs indirectly as it was measuring glycated albumin which is a precursor of AGE but not an AGE itself. |
| Zhang et al. [45] | This article was not directly measuring AGEs through sAF or sRAGEs, but was measuring AGEs indirectly as it was analysing RIP3K, which is not an AGE but was shown to be interacting with sRAGE instead. |
| Lagier et al. [46] | Upon detailed full text analysis, it was apparent that this article was not looking at post-operative complications but was looking at how different types of ventilation affect sRAGE. |
| Redondo et al. [47] | After a discussion, a consensus was reached between the researchers that since the main intervention examined was coronary angioplasty, which is minimally invasive, this would not be considered as a surgical procedure and, therefore, was excluded. |
| Bohm et al. [48] | After a discussion, a consensus was reached between the researchers that since the main intervention examined was pulmonary vein isolation, which is minimally invasive, this would not be considered as a surgical procedure and, therefore, was excluded. |
| González-Ferrero et al. [49] | After a discussion, a consensus was reached between the researchers that since the main intervention examined was atrial fibrillation ablation, which is minimally invasive, this would not be considered as a surgical procedure, and therefore, was excluded. |
| Hartog et al. [50] | After a discussion, consensus was reached to exclude this since sAF was measured 73 months after surgery. |
| Kottmaier et al. [51] | After a discussion, a consensus was reached between the researchers that since the main intervention examined was atrial fibrillation ablation, which is minimally invasive, this would not be considered as a surgical procedure and, therefore, was excluded. |
| Perkins et al. [52] | Upon detailed full text analysis, this text was not analysing the relationship between sRAGE and acute lung injury, but was assessing the relationship between salmeterol treatment and acute lung injury. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, D.S.; Motta, J.C.; Ali, J.M. The Use of Advanced Glycation End-Product Measurements to Predict Post-Operative Complications After Cardiac Surgery. J. Clin. Med. 2025, 14, 6176. https://doi.org/10.3390/jcm14176176
Agrawal DS, Motta JC, Ali JM. The Use of Advanced Glycation End-Product Measurements to Predict Post-Operative Complications After Cardiac Surgery. Journal of Clinical Medicine. 2025; 14(17):6176. https://doi.org/10.3390/jcm14176176
Chicago/Turabian StyleAgrawal, Divya S., Jose C. Motta, and Jason M. Ali. 2025. "The Use of Advanced Glycation End-Product Measurements to Predict Post-Operative Complications After Cardiac Surgery" Journal of Clinical Medicine 14, no. 17: 6176. https://doi.org/10.3390/jcm14176176
APA StyleAgrawal, D. S., Motta, J. C., & Ali, J. M. (2025). The Use of Advanced Glycation End-Product Measurements to Predict Post-Operative Complications After Cardiac Surgery. Journal of Clinical Medicine, 14(17), 6176. https://doi.org/10.3390/jcm14176176






