Comparative In Vitro Analysis of Root Cementum Surface Alterations Following Various Mechanical and Chemical Treatment Protocols in Gingival Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection and Group Allocation
- Group 1 (Intact control): No treatment was applied to the root surface. These samples served as a baseline reference for morphological and topographical comparison.
- Group 2 (Scaling only): Manual debridement was performed using Gracey curettes (Hu-Friedy, Chicago, IL, USA) under light pressure (~200 g), with vertical and horizontal strokes for 30 s.
- Group 3 (Scaling + EDTA): After mechanical instrumentation, 24% EDTA gel (MD-ChelCream, Meta Biomed, Colmar, PA, USA) was applied to the root surface for 2 min and rinsed with sterile distilled water.
- Group 4 (Scaling + citric acid): A 15% citric acid solution (pH ≈ 1.0) was applied using a microbrush for 2 min following scaling, then thoroughly rinsed.
- Group 5 (Scaling + phosphoric acid): A 37% phosphoric acid gel (Dental Etch, 3M ESPE, Seefeld, Germany) was applied for 15 s post-scaling and rinsed with sterile saline.
- Group 6 (Scaling + tetracycline): A 10% tetracycline hydrochloride solution was applied using a cotton pellet for 3 min and then rinsed.
- Group 7 (Scaling + doxycycline): A 10% doxycycline solution was used following the same protocol as in Group 6.
- Group 8 (Scaling + saline): After mechanical treatment, a 0.9% NaCl solution was applied to the root surface for 2 min to assess potential neutral effects.
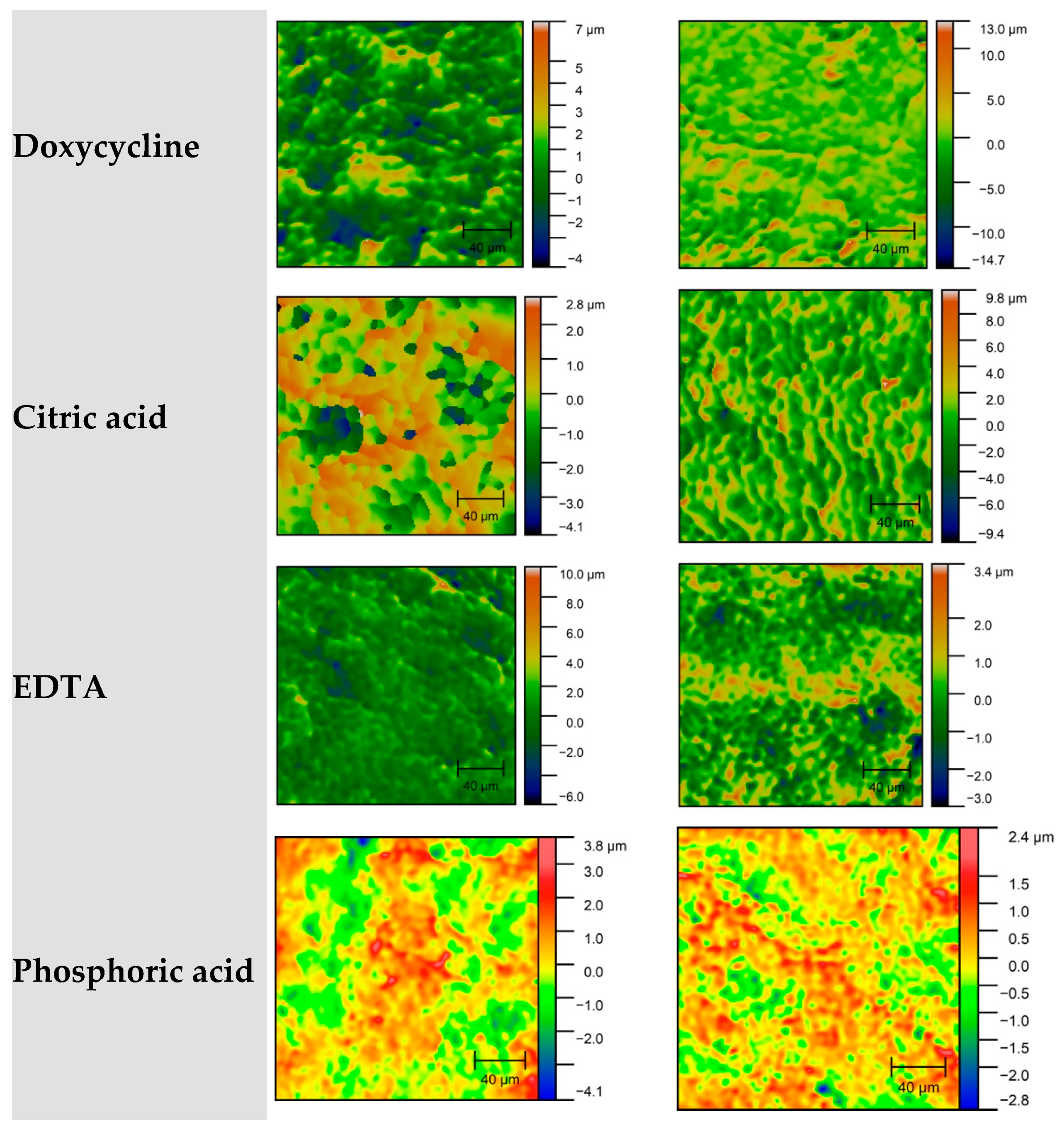
2.2. Surface Roughness Measurement
- Ra—arithmetic mean deviation of the profile;
- Rz—average peak-to-valley height;
- Rq—root mean square roughness.
2.3. Scanning Electron Microscopy (SEM)
2.4. Statistical Analysis
3. Results
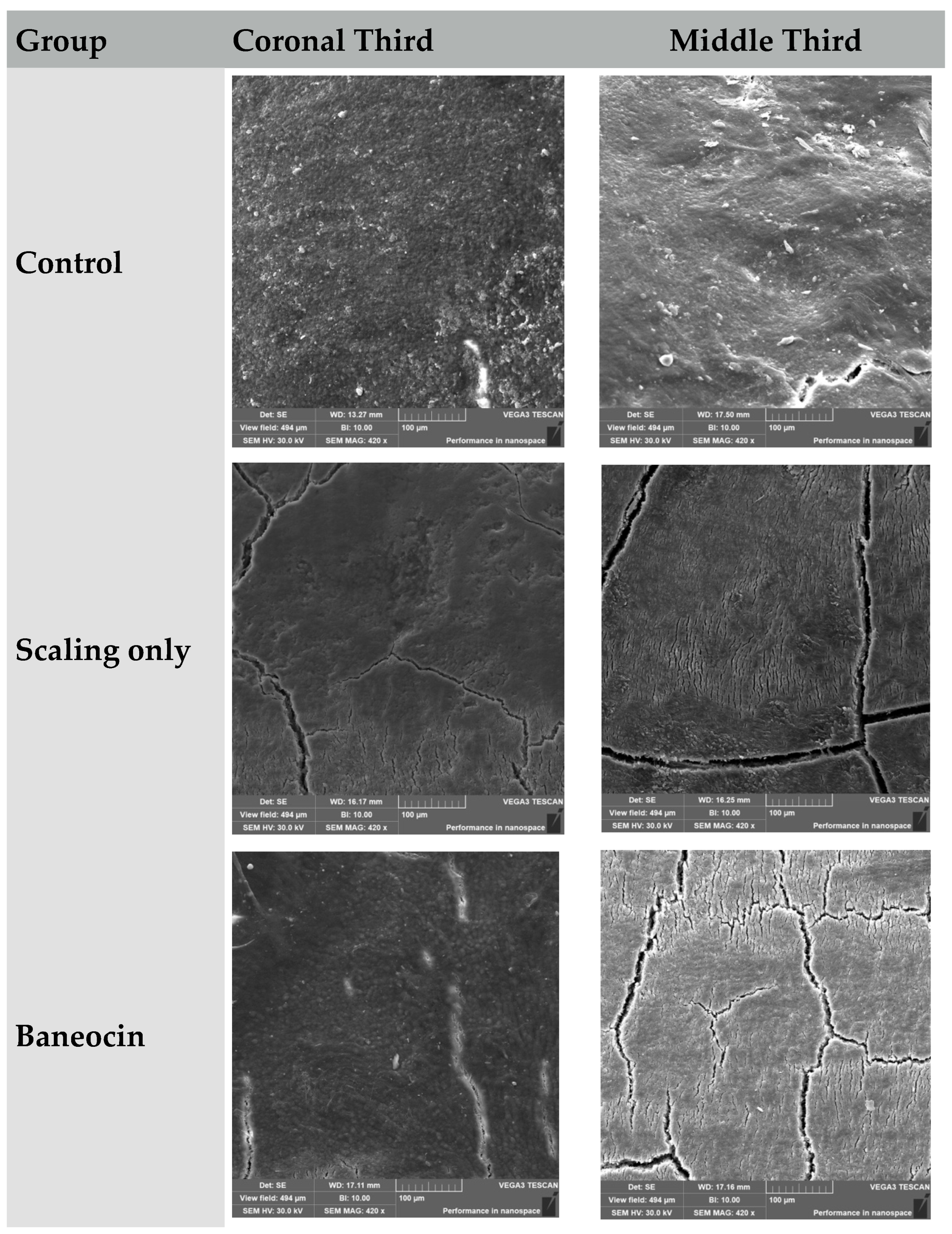
3.1. Scanning Electron Microscopy (Figure 1)
3.2. Control Group (Untreated)
- Coronal third: Preserved morphology with moderate physiological roughness.
- Middle third: Slightly increased granularity without notable structural damage.
3.3. Scaling Only
- Coronal third: Evidence of trauma, linear defects, and partial cementum removal.
- Middle third: Loosely organized surface, structural breakdown of the cementum layer.
3.4. Baneocin
- Coronal third: Granular surface with moderate structural alteration.
- Middle third: Deeper zones of destruction and porosity.
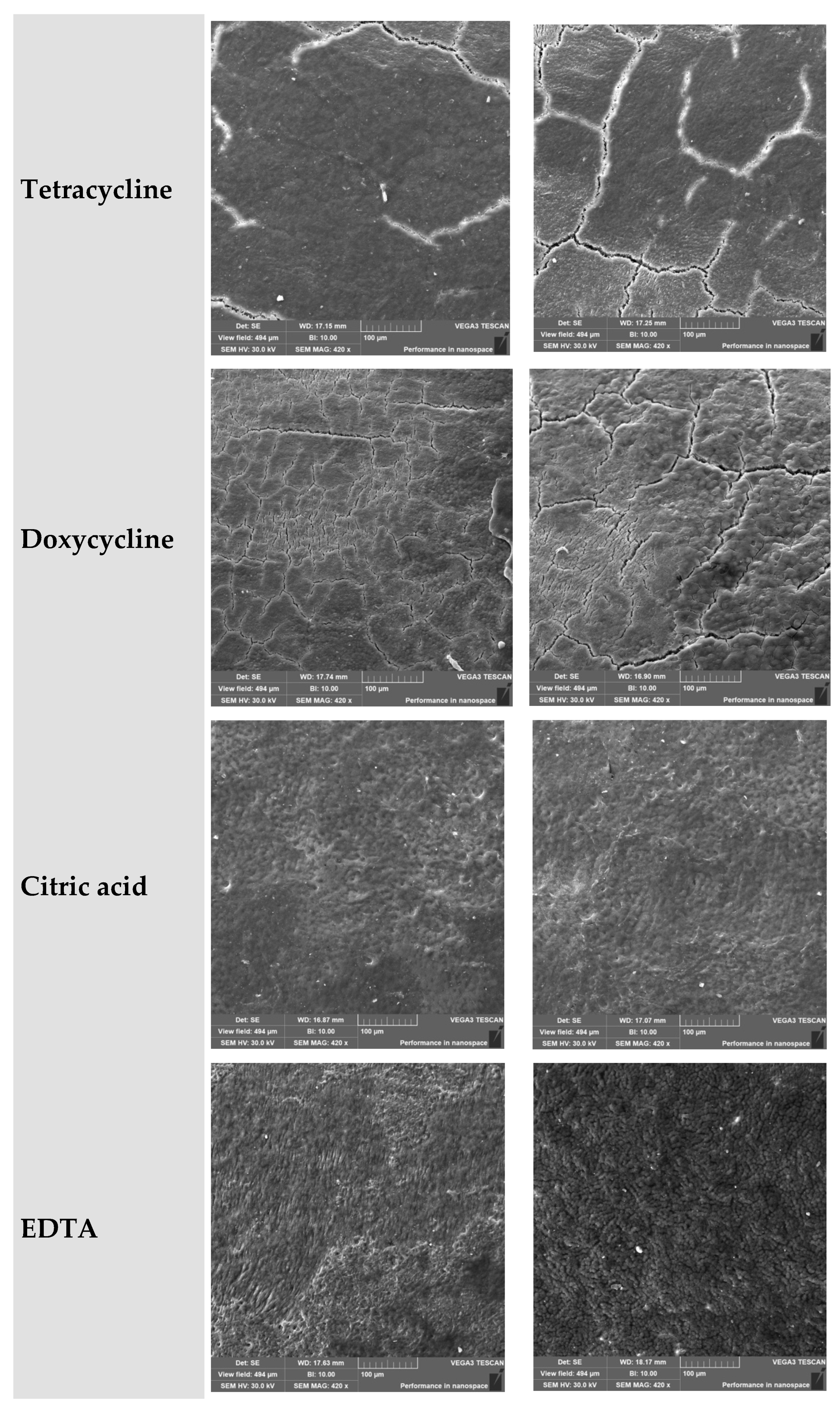
3.5. Tetracycline
- Coronal third: Reticulated microtexture with initial signs of degradation.
- Middle third: Extensive etching and cementum destruction.
3.6. Doxycycline
- Coronal third: Partial structural disruption with remnants of original morphology.
- Middle third: Increased granularity and loss of uniform surface characteristics.
3.7. Citric Acid
- Coronal third: Fine-grained and well-organized surface morphology.
- Middle third: Porosity present, but the general structure remained preserved.
3.8. EDTA
- Coronal third: Surface varied from mild etching to a reticulated pattern.
- Middle third: Loosely arranged and extensively demineralized area.
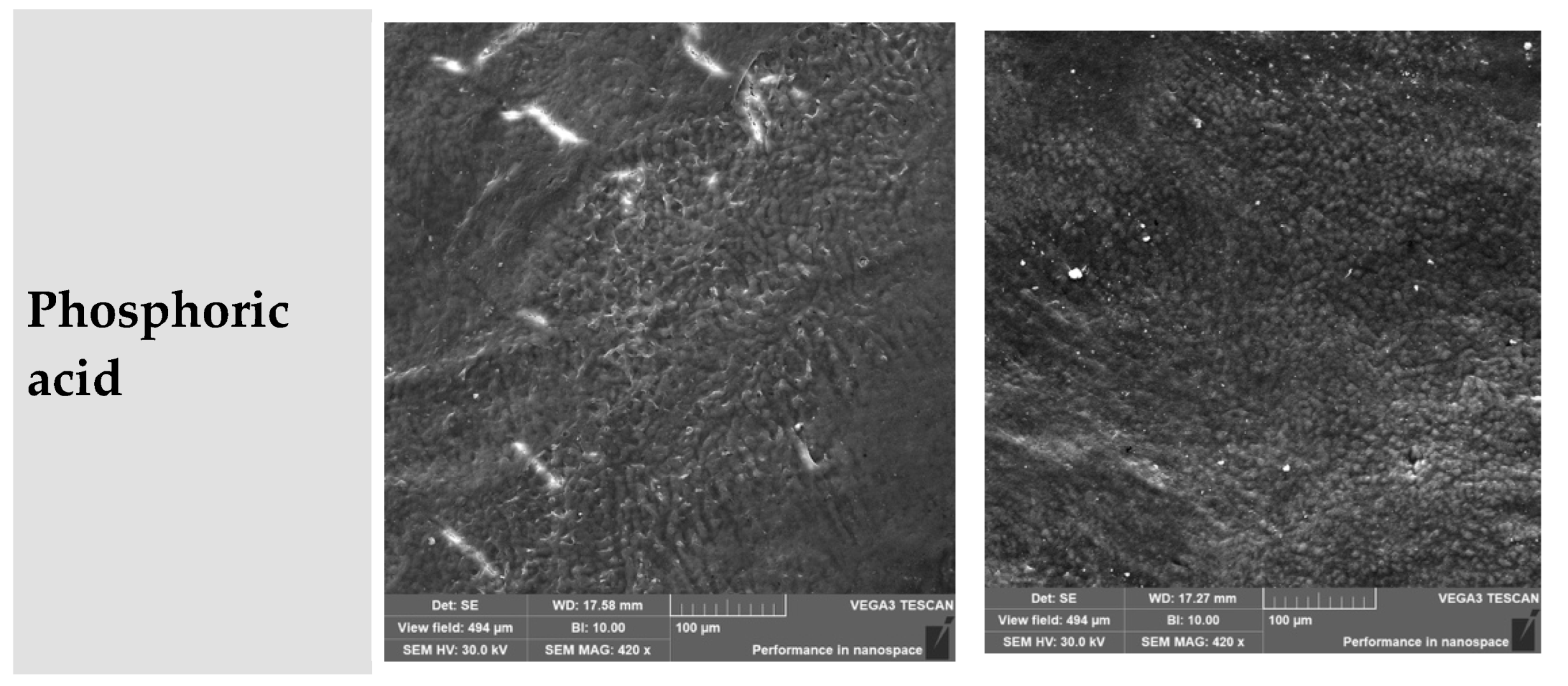
3.9. Phosphoric Acid (H3PO4)
- Coronal third: Ranged from partial to deep etching, depending on the subgroup.
- Middle third: Severely degraded in all specimens.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karam, P.S.B.H.; Sant’ana, A.C.P.; de Rezende, M.L.R.; Greghi, S.L.A.; Damante, C.; Zangrando, M.S.R. Root surface modifiers and subepithelial connective tissue graft for treatment of gingival recessions: A systematic review. J. Periodontal. Res. 2016, 51, 175–185. [Google Scholar] [CrossRef]
- Buggapati, L.; Chava, V.K. Effect of combination of ethylenediaminetetraacetic acid + tetracycline with coronally positioned flap in the treatment of gingival recession: A clinical study. J. Indian Soc. Periodontol. 2016, 20, 57–62. [Google Scholar] [CrossRef]
- Khabadze, Z.S.; Makeeva, M.K.; Gerasimova, M.E.; Chraghyan, L.K.; Begjanov, K.A.; Kirillov, A.S.; Shchetnikova, E.A.; Avetisian, G.G.; Kauk, Y.a.A. Identification of factors contributing to the appearance of inflammatory periodontal diseases preclinical symptoms in prison persons. Endod. Today 2025, 23, 339–346. (In Russian) [Google Scholar] [CrossRef]
- Kumari, K.; Nath, B.; Kumar, A.; Chhabada, A.K.; Kumari, R.; Prakash, G. Comparison of Root Coverage by the Subepithelial Connective Tissue Graft With and Without Root Biomodification: A Comprehensive Clinical Study. Cureus 2023, 15, e44758. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Kiselev, E.G.; Nemtsev, I.V.; Vasiliev, A.D.; Kuzmin, A.P.; Shishatskaya, E.I. Bacterial Cellulose (BC) and BC Composites: Production and Properties. Nanomaterials 2022, 12, 192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cosola, S.; Oldoini, G.; Giammarinaro, E.; Covani, U.; Genovesi, A.; Marconcini, S. The effectiveness of the information-motivation model and domestic brushing with a hypochlorite-based formula on peri-implant mucositis: A randomized clinical study. Clin. Exp. Dent. Res. 2022, 8, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Uspenskaya, O.A.; Fadeeva, I.I.; Shaikhutdinova, A.I.; Galkina, E.S.; Sokolova, V.V.A. Features of periodontal microcirculation in extraoral oncopathology. Endod. Today 2024, 22, 431–435. (In Russian) [Google Scholar] [CrossRef]
- Górski, B.; Szerszeń, M. Effect of Root Surface Biomodification on Multiple Recession Coverage with Modified Coronally Advanced Tunnel Technique and Subepithelial Connective Tissue Graft: A Retrospective Analysis. Gels 2022, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Baher, F. Fibroblast Viability Through Mechanical and Chemical Root Surface Modifications in Periodontal Healing: An In Vitro Comparative Study. Cureus 2023, 15, e50381. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Solt, C.W. A surgical procedure for the treatment of localized gingival recession in conjunction with root surface citric acid conditioning. J. Periodontol. 1980, 51, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Aiuto, R.; Fumei, G.; Lipani, E.; Garcovich, D.; Dioguardi, M.; Re, D. Conservative Therapy of External Invasive Cervical Resorption with Adhesive Systems: A 6-Year Follow-Up Case Report and Literature Review. Case Rep. Dent. 2022, 2022, 9620629. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Aoki, A.; Sasaki, K.M.; Takasaki, A.A.; Iwasaki, K.; Ichinose, S.; Oda, S.; Ishikawa, I.; Izumi, Y. The effect of chemical and/or mechanical conditioning on the Er:YAG laser-treated root cementum: Analysis of surface morphology and periodontal ligament fibroblast attachment. Lasers Surg. Med. 2008, 40, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Carvelli, G.; Caton, J.; Barmak, A.B.; Tsigarida, A. Comparison of Coronally Advanced Flap and Connective Tissue Graft With or Without Enamel Matrix Derivative for Gingival Recession Treatment: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int. J. Periodontics Restor. Dent. 2022, 42, e121–e131. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, S.; Ribeiro, É.; Sallum, E.; Sallum, A.; Nociti, F.; Casati, M. Comparative 6-month clinical study of a semilunar coronally positioned flap and subepithelial connective tissue graft for the treatment of gingival recession. J. Periodontol. 2006, 77, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Barootchi, S.; Mancini, L.; Sabri, H.; Wang, H.-L.; Tavelli, C.; Tavelli, L. Root Coverage and Volumetric Outcomes of a Novel Porcine-Derived Acellular Dermal Matrix with the Tunneled Coronally Advanced Flap (TCAF) for Treatment of Multiple Adjacent Gingival Recessions: A Clinical Prospective Study. Int. J. Periodontics Restor. Dent. 2023, 44, 377. [Google Scholar] [CrossRef] [PubMed]




| Group | Surface Condition | Roughness | Microstructure | Conclusion |
|---|---|---|---|---|
| Control | Intact, smooth | Minimal | Continuous, unchanged | Reference surface for comparison |
| Scaling | Rough, grooves, exposed dentin | High | Cracks, microfractures | Mechanical damage |
| Baneocin | Moderately altered, partially cleaned | Medium | Mixed structure, preserved cement zones | Moderate structural alteration with partial preservation |
| Tetracycline | Rough, partially etched | Moderate | Porous, exposed tubules | Balanced effect |
| Doxycycline | Deeply damaged, loose | High | Severe cementum degradation | Caution required |
| Citric acid | Etched, erosive, collagen exposed | Very high | Porosity, fibrillar texture | Optimal for regeneration |
| EDTA | Smear layer removed, moderate etching | Significant | Organized tubules, preserved architecture | Controlled treatment |
| Phosphoric acid | Destruction, full tubule exposure, deep erosions | Excessive | Matrix breakdown, fiber separation, loss of continuity | Aggressive agent, high damage risk |
| Group | Coronal Third | Middle Third | Comparative Conclusion |
|---|---|---|---|
| Control | Homogeneous, intact cementum structure, moderate roughness | Preserved structure, increased granularity, minor defects | Structure mostly preserved, cementum intact, physiologic surface |
| Scaling | Surface etching, partial cementum exposure, linear defects | Loose, fragmented, signs of leaching and cementum erosion | Traumatic intervention causes structural damage and leaching |
| Baneocin | Segmented structure, presence of granules and roughness | Porous, extensive destruction, granular texture | Antibiotic induces destructive changes in both thirds |
| Tetracycline | Fine-grained with microcracks, surface breakdown | Cementum disintegration, chaotic and porous | Pronounced destruction in middle third, aggressive leaching |
| Doxycycline | Dense with preserved morphology, signs of surface degradation | Granular and loose, signs of demineralization, partial destruction | Progressive degradation from coronal to middle third, moderate effect |
| Citric acid | Fine-grained, uniform, crack-free, moderate roughness | Porous but preserved morphology, uniform roughness | Gentle impact, structure largely preserved |
| EDTA | Segmented or degraded, from ‘orange peel’ texture to complete destruction | Loose to nearly completely degraded surface | Maximum degradation in middle third, cementum lost |
| Phosphoric acid | Ranging from relatively smooth to fully degraded; shallow to deep erosion | Loose, granular, variable depth of cementum destruction | Phosphoric acid induces aggressive demineralization, especially in depth |
| Group | Mean Rq (nm) | SD Rq (nm) | Description |
|---|---|---|---|
| Group 1—Control | 1583.25 | 546.73 | No treatment |
| Group 2—Scaling | 1556.61 | 748.22 | Mechanical instrumentation (second control) |
| Group 3—Baneocin | 753.4 | 55.84 | Scaling + bacitracin |
| Group 4—Tetracycline | 1161.78 | 594.03 | Scaling + tetracycline |
| Group 5—Doxycycline | 1387.44 | 485.4 | Scaling + doxycycline |
| Group 6—Citric Acid | 1305.94 | 439.6 | Scaling + citric acid |
| Group 7—EDTA | 1476.94 | 602.61 | Scaling + EDTA |
| Group 8—Phosphoric Acid | 998.22 | 360.42 | Scaling + H3PO4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khabadze, Z.; Mordanov, O.; Magomedov, O. Comparative In Vitro Analysis of Root Cementum Surface Alterations Following Various Mechanical and Chemical Treatment Protocols in Gingival Surgery. J. Clin. Med. 2025, 14, 6174. https://doi.org/10.3390/jcm14176174
Khabadze Z, Mordanov O, Magomedov O. Comparative In Vitro Analysis of Root Cementum Surface Alterations Following Various Mechanical and Chemical Treatment Protocols in Gingival Surgery. Journal of Clinical Medicine. 2025; 14(17):6174. https://doi.org/10.3390/jcm14176174
Chicago/Turabian StyleKhabadze, Zurab, Oleg Mordanov, and Omargadzhi Magomedov. 2025. "Comparative In Vitro Analysis of Root Cementum Surface Alterations Following Various Mechanical and Chemical Treatment Protocols in Gingival Surgery" Journal of Clinical Medicine 14, no. 17: 6174. https://doi.org/10.3390/jcm14176174
APA StyleKhabadze, Z., Mordanov, O., & Magomedov, O. (2025). Comparative In Vitro Analysis of Root Cementum Surface Alterations Following Various Mechanical and Chemical Treatment Protocols in Gingival Surgery. Journal of Clinical Medicine, 14(17), 6174. https://doi.org/10.3390/jcm14176174






