Effects of Preoperative Exercise Interventions in Patients Undergoing Metabolic and Bariatric Surgery: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Risk Bias Assessment of Included Studies
2.6. Data Synthesis and Statistical Analysis
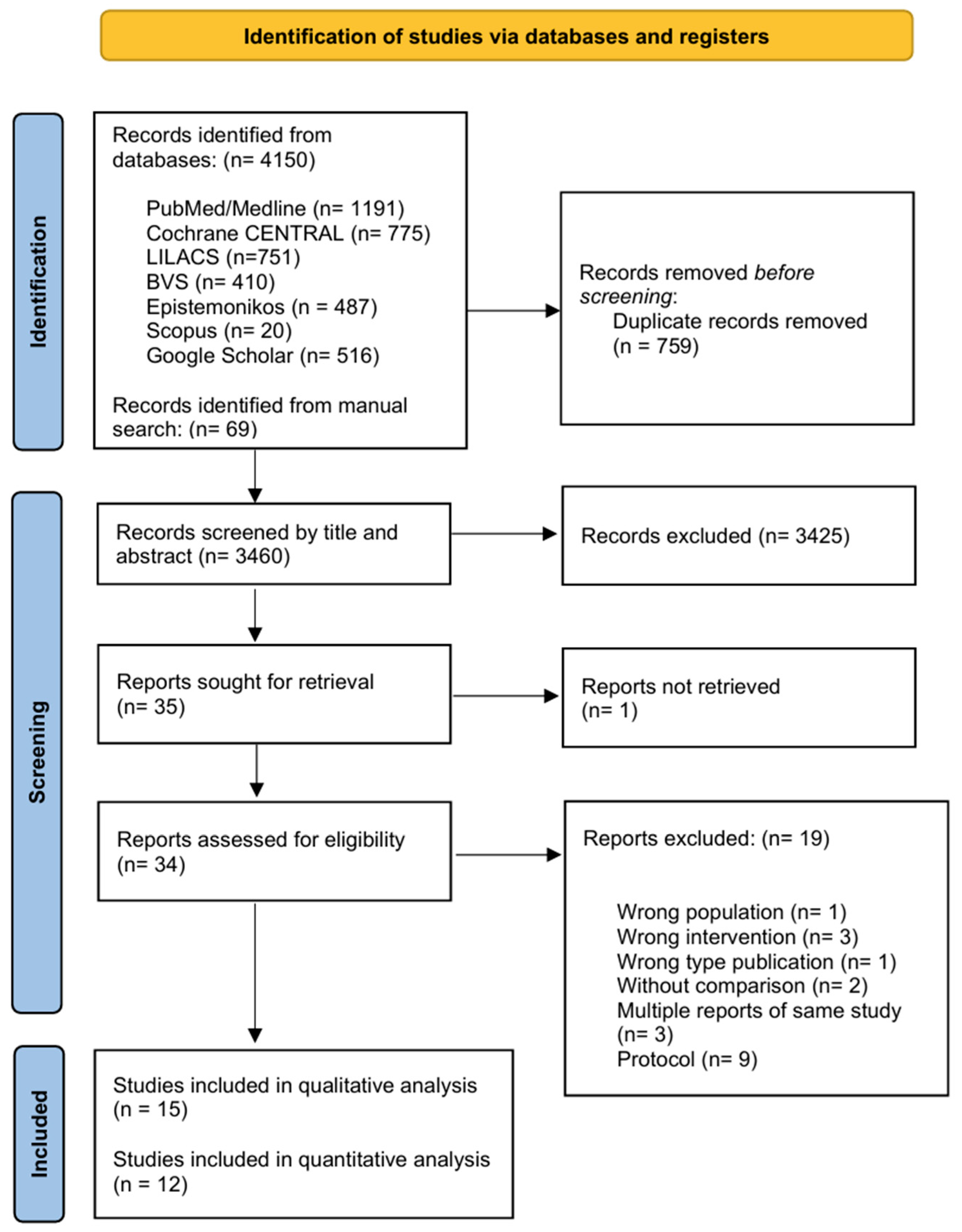
3. Results
3.1. Characteristics of Studies
3.1.1. Geographic Distribution and Settings
3.1.2. Participant Characteristics
3.1.3. Exercise Intervention Characteristics
Exercise Modality and Design
Duration and Frequency
Supervision and Setting
Exercise Intensity
3.1.4. Control Group Characteristics
3.1.5. Funding Sources and Conflicts of Interest
3.2. Qualitative Synthesis of Key Outcomes
3.2.1. Intervention Characteristics
3.2.2. Surgical Parameters
3.2.3. Body Composition
3.2.4. Functional Capacity
3.2.5. Metabolic Parameters
3.2.6. Other Outcomes
| Study (Author, Year) | Study Design | Population (N; BMI kg/m2; Age yrs; % Female) | Intervention (Type; Duration; Frequency; Supervision) | Control Group | Key Outcomes Measured | Follow-Up Duration |
|---|---|---|---|---|---|---|
| Gilbertson et al. (2020) [28] | Controlled pilot study | N = 14 completed (SC = 7, EX + SC = 7); BMI: NR; age: ~39–46 (SC 39.0 ± 5.3, EX + SC 45.6 ± 4.8); female: 13/14 (92.8%) (SC 6/7, EX + SC 7/7) | Aerobic exercise + standard care + Low-calorie diet; 30 days; frequency NR; supervised (inferred from authors) | Standard care (includes diet) | Metabolic health (insulin sensitivity, glucose tAUC), adipokines (mentioned), cardiometabolic health (arterial stiffness surrogate (AIx@75), CRP, CK18), body composition (weight, FFM, and WC), and weight-related quality of life | 30 days pre-op |
| Hardy et al. (2022) [29] | Pilot randomized controlled trial | N = 54 enrolled, N = 52 in tables; BMI: ~45.75 (Cont 45.2, Int 46.3); age: NR; female: Cont 63.0%, Int 88.0% | Supervised exercise program (aerobic + resistance); 12 weeks; 3x/week (inferred from citation); supervised | Standard multidisciplinary preoperative care | Functional capacity (6MWT primary, GXT, VO2max), strength (chair stand, handgrip), flexibility (sit and reach), quality of life (Laval questionnaire: activity, symptoms, hygiene, emotions, social, and sex), anthropometric (Wt, BMI, neck, waist, and hip), and balance | 12 weeks pre-op |
| Arman et al. (2021) [57] | Randomized controlled trial (RCT) | N = 21 analyzed (EG = 10, CG = 11); BMI: NR; age: NR; female: NR | Core stabilization exercise program + physical activity counseling (CSEP); 8 weeks; 2x/week; supervised | Physical activity counseling only | Body composition (using Tanita BC420MA), balance (PST, FRT, and BCT with Biodex Balance System), fatigue (Fatigue Severity Scale), and functional capacity (6MWT) | 8 weeks pre-op |
| Baillot et al. (2016) [58] | Randomized controlled trial (RCT) | N = 30 randomized; N = 21 completers; BMI: 47.5 ± 8.1, median ~45; age: 43.2 ± 9.2; female: NR in (percentages per group NR) | Pre-surgical exercise training (PreSET) (endurance and strength training) + interdisciplinary lifestyle management; 12 weeks; 3x/week; supervised | Usual care (includes counseling) | Physical fitness (symptom-limited exercise test, 6MWT, sit-to-stand, half-squat, and arm curl), Quality of life (Laval questionnaire), PA barriers (physical exercise belief questionnaire), anthropometric parameters (BMI, weight), body composition, blood pressure and HR, and satisfaction | 12 weeks pre-op |
| Bond et al. (2015) [59] | Randomized controlled trial (RCT) | N = 75 (analysis); BMI: 45.0 ± 6.5; age: 46.0 (8.9); female: 86.7% (sample N = 36: age 47.0 (8.2), female 86.1%) | Physical activity counseling (PAI) individual face-to-face with behavioral strategies (e.g., self-monitoring, goal setting); 6 weeks; weekly; counseling | Standard pre-surgical care (SC) | Physical activity (objective MVPA, bout-related and total), barriers, self-efficacy, and weight | 6 weeks pre-op + post-op follow-up |
| Creel et al. (2016) [60] | Randomized controlled trial (RCT) | N = 150 randomized, N = 107 (ITT analysis); BMI: NR; age: 43.2 ± 11.2 (randomized); female: 84.1% (ITT) | Counseling (collaborative vs. prescriptive) vs. pedometer use; focus on 6 weeks pre-op; frequency NR; supervision NR | Standard care (SC) | Physical activity (accelerometry, self-report), Physical capacity (submaximal exercise test—METs), sedentary and light PA %, and steps | 6 weeks pre-op + 2, 4, and 6 months post-op |
| Funderburk et al. (2010) [65] | Randomized controlled pilot trial (quasi-experimental pre-post design) | N = 7 (4 intervention, 3 control). Individuals with morbid obesity (BMI > 40). Age: 28–54 years (mean ± SD: intervention: 37.25 ± 5.6; control: 49.3 ± 3.8). % female: 57% (4 females total: 3 control and 1 intervention) | Aquatic therapy (warm-up, strength/endurance exercise). Duration: 12 weeks. Frequency: 2 sessions/week (1 h/session). Supervision: supervised by aquatic leaders. | Continued normal routine (no intervention) | Health-related quality of life (SF-36v2), depression (Beck Depression Inventory, BDI), adjustment to obesity (Obesity Adjustment Scale, OAS), physical status (weight, blood pressure, 6-min walk test [6MWT], and rate of perceived exertion [RPE]). | 2 weeks (pre- and post-intervention assessments) |
| Garcia-Delgado et al. (2021) [61] | RCT protocol and controlled pilot study | N = 15 randomized (pilot: Int = 7, Cont = 8); BMI: ~46.7 (Int 44.7 ± 4.5, Cont 48.5 ± 6.7); age: ~39.5 (Int 38 ± 10, Cont 41 ± 10); female: 14/15 (93.3%) | Physical conditioning + respiratory physiotherapy + standard group intervention; minimum 4 months; 2x/week (inferred from context); supervised (inferred from authors) | Standard treatment (education + cognitive behavioral therapy) | Preoperative weight-loss, functional capacity (6MWT, handgrip strength), quality of Life (EQ–5D–5L, HAD Scale), physical activity (IPAQ), adherence, body composition (weight, BMI, FM, and PA angle), and postoperative complications (Clavien–Dindo) | Minimum 4 months pre-op + 30 days post-op |
| Li et al. (2013) [62] | Three-arm unblinded pilot randomized controlled trial (RCT) | N: 22 patients (8 home-based, 7 gym-based, and 7 non-intervention). BMI: 47 ± 6 kg/m2 (similar across groups). Age: Similar across groups (exact values not reported); % female: not reported. | Home-based group: aerobic + resistance training. Duration: 2 months preoperatively. Frequency: 3 × 30-min aerobic + 2 × resistance sessions/week. Supervision: unsupervised. Gym-based group: Same type, duration, and frequency as home-based. Supervision: supervised by trainer. | Non-intervention group: received standard care without exercise program | Primary: percent excess weight loss (%EWL), maximal aerobic capacity (VO2max) pre- and post-surgery. Secondary: Not explicitly detailed beyond %EWL and VO2max. | Baseline pre-surgery (2 months post-randomization); 10 weeks post-surgery |
| Marc-Hernandez et al. (2019) [66] | Controlled pilot study (non-randomized) | N = 23 enrolled (EG = 12, CG = 11); BMI: ~44.5 (EG 47.5 ± 7.1, CG 41.5 ± 2.7); age: ~45.4 (EG 45.4 ± 8.2, CG 45.4 ± 8.2); female: 83% (19/23) | Monitored and supervised exercise program (aerobic + resistance, progressive); 12 weeks; 3x/week; monitored and supervised | Usual pre-surgical care + advice to follow active lifestyle | Body composition (weight, BMI, FM, and FFM), anthropometric measures (waist/hip circ., visceral fat), physical fitness (VO2peak, strength), and cardiometabolic risk factors (glucose, TC, and LDL-C) | 12 weeks pre-op |
| Marcon et al. (2016) [63] | Randomized controlled trial (RCT) | N = 57 analyzed; BMI: NR; age: NR; female: ~96.5% (55/57), per group: CONTROL 100%, EXER 95.5%, and EXER + CBT 94.1% | Exercise (EXER) vs. exercise + cognitive behavioral therapy (EXER + CBT); 18–21 weeks; 2–3x/week; supervised | Control (usual care) | Functional capacity (VO2max estimated, 6MWT), weight, resting heart rate (HRrest), blood pressure (SBP), HDL, total cholesterol, triglycerides, and glucose | 18–21 weeks pre-op |
| Parikh et al. (2012) [64] | Randomized controlled trial (RCT) | N = 55 enrolled, N = 29/26 ITT, N = 10/13 completers; BMI: ~45.4 (ITT baseline MSWM 45.82, UC 44.99); age: ~45.15 (ITT baseline); female: 100% (55/55) | Medically supervised weight management (MSWM) (combined individual/group monthly visits for analysis); 6 months; monthly; medically supervised | Usual care (UC) | BMI, physical activity, adherence, eating behavior, patient activation, weight loss (implied by BMI changes) | 6 months pre-op + 6 months post-op |
| Pico-Sirvent et al. (2019) [67] | Controlled pilot study (non-randomized) | N = 6 (EG = 3, CG = 3); BMI: 38.78 ± 1.18; age: 38.17 ± 12.06; female: 5/6 (83.3%) (EG = 2/3, CG = 3/3) | Combined exercise training program (ETP) (HIIT + resistance, progressive); 6 months; 3x/week; supervised | Control group (followed usual medical indications) | Body composition (weight, BMI, FM, FFM, and visceral fat), physical fitness (VO2peak), cardiometabolic health (SBP, DBP, and HRrest), anthropometric measures (waist/hip circumference | 6 months pre-op |
| Pico-Sirvent et al. (2022) [68] | Controlled study (non-randomized) | N = 20 (EG = 10, CG = 10); BMI: NR (weight given: EG 125.3 ± 13.9, CG 115.8 ± 15.1); age: ~42.5 (EG 43 ± 5, CG 42 ± 9); female: 100% (20/20) | Combined aerobic training (at Fatmax) + resistance (low intensity); 12 weeks; frequency NR; supervised | Conventional care | Body composition (weight, FM, FFM, visceral fat—via bioimpedance), fat oxidation (resting RFO, exercise MFO), resting metabolic rate (RMR), physical fitness (VO2peak, strength (POpeak)), RCHO, VT2, and Fatmax intensity/HR/VO2 | 12 weeks pre-op |
| Still et al. (2007) [69] | Cohort study | N = 884; BMI: 51.3 ± 8.0; age: 45 ± 10; female: 78% | Standard multidisciplinary preoperative program (medical, psychological, nutritional, surgical, education, behavioral modules, support groups, and attempt to lose 10% EBW) | Patients within the program observed (comparison based on preoperative weight loss groups) | Hospital length of stay, postoperative complications, weight/BMI changes, preoperative weight loss, and % EBW loss at 6–12 months post-op based on pre-op weight loss groups | Up to 27 months post-op (data shown for 6 and 12 mos) |
3.3. Risk of Bias Assessment of Included Studies
3.3.1. Risk of Bias in Included Randomized Controlled Trials
3.3.2. Risk of Bias in Included Non-Randomized Studies
3.4. Quantitative Meta-Analysis
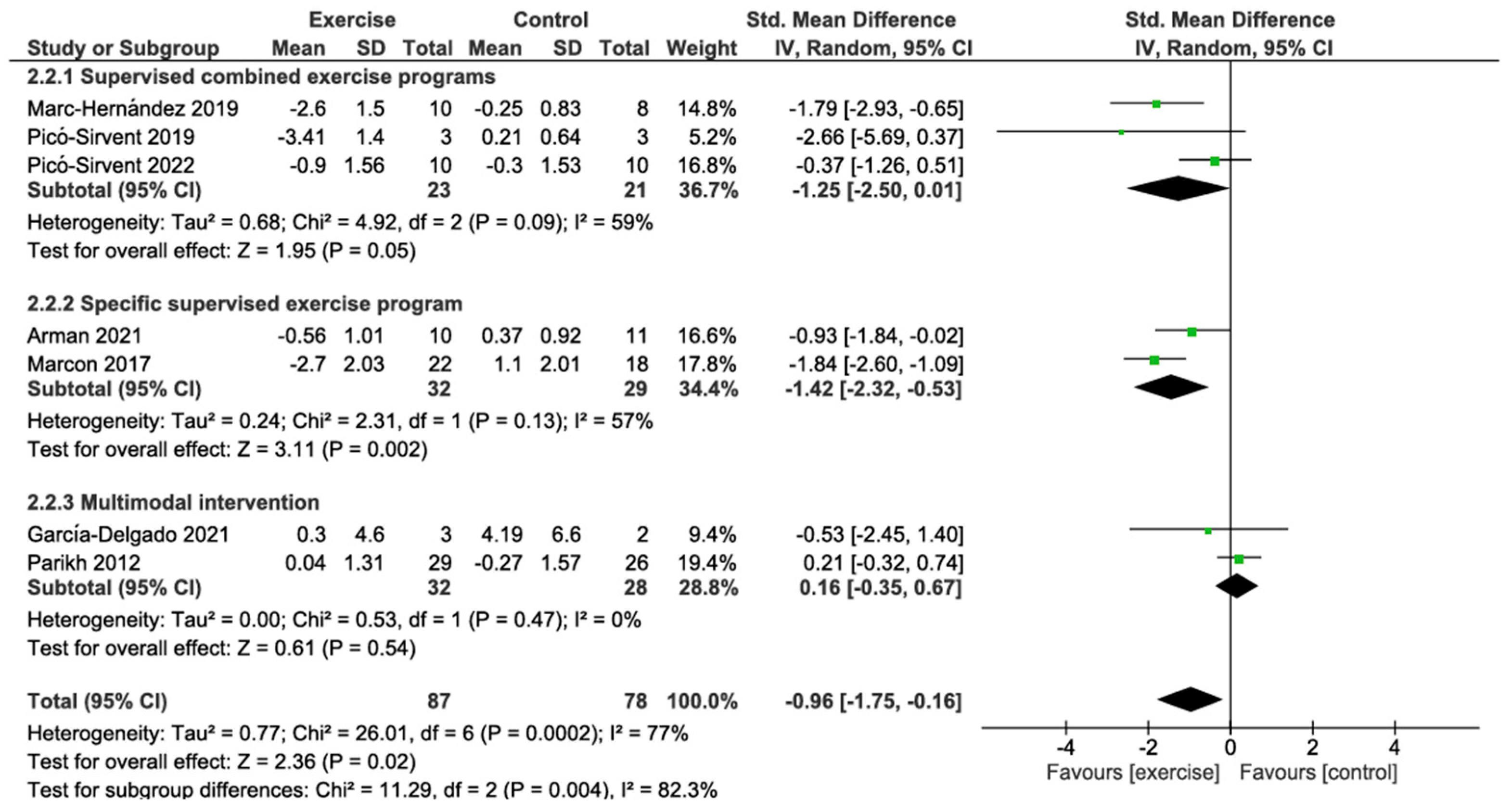
3.4.1. Weight Change
3.4.2. BMI Change
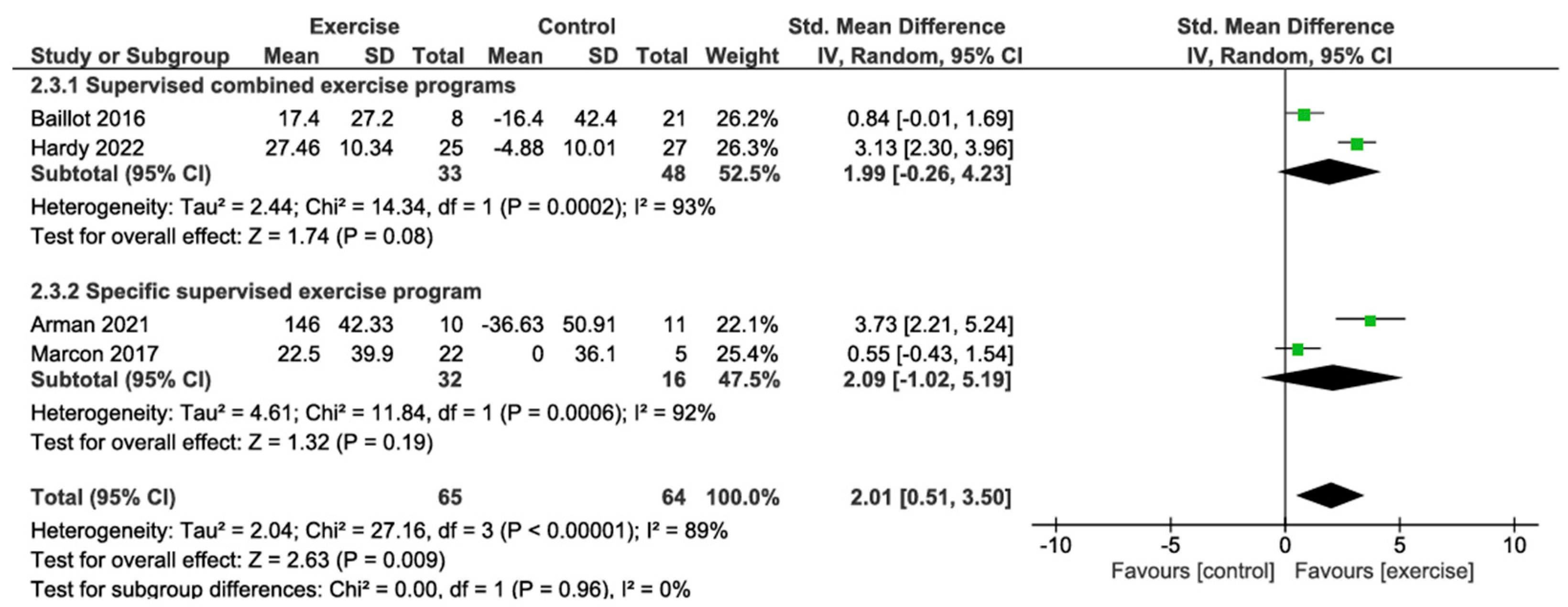
3.4.3. Six-Minute Walk Test Change
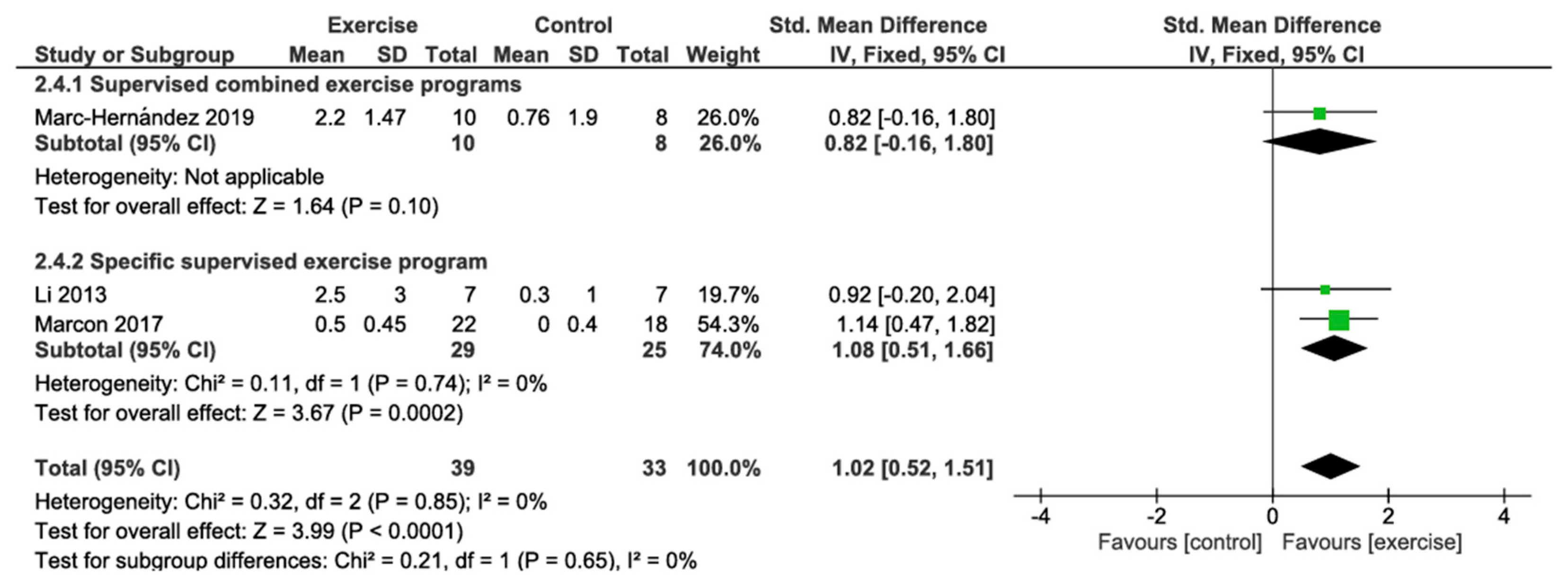
3.4.4. VO2 Peak Change
3.4.5. Length Hospital Stay (Days)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2024.
- Alem, A.; Yeshaw, Y.; Liyew, A.; Tessema, Z.; Worku, M.; Tesema, G.; Alamneh, T.; Teshale, A.; Chilot, D.; Ayalew, H.; et al. Double burden of malnutrition and its associated factors among women in low and middle income countries: Findings from 52 nationally representative data. BMC Public Health 2023, 23, 1479. [Google Scholar] [CrossRef]
- Fuchs-Tarlovsky, V.; Castillo Pineda, J.; Rodríguez Veintimilla, D.; Calvo Higuera, I.; Grijalva Guerrero, P.; Gómez García, A.; Frias-Toral, E.; Santana Porbén, S. Cancer-Related Malnutrition: Epidemiological Results from the Latin American Study of Malnutrition in the Oncology Practice. Nutr. Cancer 2022, 74, 2479–2488. [Google Scholar] [CrossRef]
- Jaacks, L.; Vandevijvere, S.; Pan, A.; McGowan, C.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The obesity transition: Stages of the global epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef]
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef]
- Kurtgil, S.; Pekcan, A.G. Determination of breakfast habits, food pattern and quality among adults. Med. J. Nutr. Metab. 2023, 16, 281–291. [Google Scholar] [CrossRef]
- Verde, L.; Barrea, L.; Bowman-Busato, J.; Yumuk, V.D.; Colao, A.; Muscogiuri, G. Obesogenic environments as major determinants of a disease: It is time to re-shape our cities. Diabetes Metab. Res. Rev. 2024, 40, e3748. [Google Scholar] [CrossRef]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.; Poirier, P.; Burke, L.; Després, J.; Gordon-Larsen, P.; Lavie, C.; Lear, S.; Ndumele, C.; Neeland, I.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Karra, P.; Winn, M.; Pauleck, S.; Bulsiewicz-Jacobsen, A.; Peterson, L.; Coletta, A.; Doherty, J.; Ulrich, C.; Summers, S.; Gunter, M.; et al. Metabolic dysfunction and obesity-related cancer: Beyond obesity and metabolic syndrome. Obesity 2022, 30, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Reytor-González, C.; Zambrano, A.K.; Frias-Toral, E.; Campuzano-Donoso, M.; Simancas-Racines, D. Mediterranean diet and breast cancer: A narrative review. Medwave 2025, 25, e3027. [Google Scholar] [CrossRef]
- Martiniakova, M.; Biro, R.; Penzes, N.; Sarocka, A.; Kovacova, V.; Mondockova, V.; Omelka, R. Links among Obesity, Type 2 Diabetes Mellitus, and Osteoporosis: Bone as a Target. Int. J. Mol. Sci. 2024, 25, 4827. [Google Scholar] [CrossRef]
- Santamaría-Ulloa, C.; Chinnock, A.; Montero-López, M. Association between obesity and mortality in the Costa Rican elderly: A cohort study. BMC Public Health 2022, 22, 1007. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.; De Luca, M.; Faria, S.; Goodpaster, K.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- O’Brien, P.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2019, 29, 3–14. [Google Scholar] [CrossRef]
- Crozet, J.; Pasquer, A.; Pelascini, E.; Robert, M. Factors influencing bariatric surgery outcomes. J. Visc. Surg. 2023, 160, S7–S11. [Google Scholar] [CrossRef]
- Aljabri, D. Associations Between Obesity, Physical Inactivity, Healthcare Capacity, and the Built Environment: Geographic Information System Analysis. J. Multidiscip. Healthc. 2022, 15, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, G.; Ibrahim, R.; Bragazzi, N. Preoperative Physical Activity Level and Exercise Prescription in Adults With Obesity: The Effect on Post-Bariatric Surgery Outcomes. Front. Physiol. 2022, 13, 869998. [Google Scholar] [CrossRef] [PubMed]
- Simancas-Racines, D.; Reytor-González, C.; Parise-Vasco, J.; Angamarca-Iguago, J.; Garcia-Velasquez, E.; Cuzco-Macias, A.; Frias-Toral, E.; Schiavo, L. Effectiveness and Safety of Preoperative Nutritional Interventions on Surgical Outcomes in Patients Undergoing Metabolic and Bariatric Surgery: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 1533. [Google Scholar] [CrossRef] [PubMed]
- Reytor-González, C.; Frias-Toral, E.; Nuñez-Vásquez, C.; Parise-Vasco, J.; Zambrano-Villacres, R.; Simancas-Racines, D.; Schiavo, L. Preventing and Managing Pre- and Postoperative Micronutrient Deficiencies: A Vital Component of Long-Term Success in Bariatric Surgery. Nutrients 2025, 17, 741. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Frias-Toral, E.; Campuzano-Donoso, M.; Ramos-Sarmiento, D.; Zambrano-Villacres, R.; Reytor-González, C.; Schiavo, L. Preoperative Nutrition in Bariatric Surgery: A Narrative Review on Enhancing Surgical Success and Patient Outcomes. Nutrients 2025, 17, 566. [Google Scholar] [CrossRef]
- Sarno, G.; Calabrese, P.; Frias-Toral, E.; Ceriani, F.; Fuchs-Tarlovsky, V.; Spagnuolo, M.; Cucalón, G.; Álvarez Córdova, L.; Schiavo, L.; Pilone, V. The relationship between preoperative weight loss and intra and post-bariatric surgery complications: An appraisal of the current preoperative nutritional strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 10230–10238. [Google Scholar] [CrossRef]
- Chapela, S.; Martinuzzi, A.; Llobera, N.; Ceriani, F.; Gonzalez, V.; Montalvan, M.; Verde, L.; Frias-Toral, E. Obesity and micronutrients deficit, when and how to suplement. Food Agric. Immunol. 2024, 35, 2381725. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Reytor-González, C.; Zambrano, A.; Annunziata, G.; Carella, A.; Verde, L.; Frias-Toral, E.; Guerra, C.; Hidalgo, R. Unlocking the potential: Very-low-energy ketogenic therapy in obesity-related disorders. Food Agric. Immunol. 2025, 36, 2442368. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Barbarisi, A.; Cesaretti, M.; Iannelli, A. A 4-Week Preoperative Ketogenic Micronutrient-Enriched Diet Is Effective in Reducing Body Weight, Left Hepatic Lobe Volume, and Micronutrient Deficiencies in Patients Undergoing Bariatric Surgery: A Prospective Pilot Study. Obes. Surg. 2018, 28, 2215–2224. [Google Scholar] [CrossRef]
- Schiavo, L.; De Stefano, G.; Persico, F.; Gargiulo, S.; Di Spirito, F.; Griguolo, G.; Petrucciani, N.; Fontas, E.; Iannelli, A.; Pilone, V. A Randomized, Controlled Trial Comparing the Impact of a Low-Calorie Ketogenic vs a Standard Low-Calorie Diet on Fat-Free Mass in Patients Receiving an ElipseTM Intragastric Balloon Treatment. Obes. Surg. 2021, 31, 1514–1523. [Google Scholar] [CrossRef]
- Gilbertson, N.; Gaitán, J.; Osinski, V.; Rexrode, E.; Garmey, J.; Mehaffey, J.; Hassinger, T.; Kranz, S.; McNamara, C.; Weltman, A.; et al. Pre-operative aerobic exercise on metabolic health and surgical outcomes in patients receiving bariatric surgery: A pilot trial. PLoS ONE 2020, 15, e0239130. [Google Scholar] [CrossRef]
- Hardy, K.; Kwok, K.; Bouchard, D.R.; Bharti, N.; Gamey, D.; Vergis, A. Impact of a Preoperative Exercise Program on General Fitness in Patients Awaiting Bariatric Surgery: A Pilot Randomized Trial. Cureus 2022, 14, e22566. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-Schmidt, J.; Mautner Molina, C.; Kalazich Rosales, M.; Muñoz, M.; Ruiz-Uribe, M.; Fuentes Leal, F.; Monrroy Uarca, M.; Cárcamo Ibaceta, C.; Fazakerley, D.; Larance, M.; et al. Moderate-intensity constant or high-intensity interval training? Metabolic effects on candidates to undergo bariatric surgery. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1681–1691. [Google Scholar] [CrossRef]
- Fernández-Alonso, M.; Bejarano, G.; Creel, D.; Kohl, H.; Messiah, S.; Altieri, M.; Papasavas, P.; Horn, C.; Marroquin, E. Expert-based physical activity guidelines for metabolic and bariatric surgery patients: A systematic review of randomized controlled trials. Surg. Obes. Relat. Dis. 2025, 21, 606–614. [Google Scholar] [CrossRef]
- Durey, B.J.; Fritche, D.; Martin, D.S.; Best, L.M.J. The Effect of Pre-operative Exercise Intervention on Patient Outcomes Following Bariatric Surgery: A Systematic Review and Meta-analysis. Obes. Surg. 2022, 32, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ghannadi, S.; Selk-Ghaffari, M.; Ejtahed, H.; Khalaji, K.; Hoseini Tavassol, Z.; Pourgharib Shahi, M.; Hasani-Ranjbar, S. Evaluation of the Effect of the Pre-Operative Exercise Training on Weight Loss, Quality of Life, and Cardiopulmonary Parameter in Bariatric Metabolic Surgery: A Systematic Review and Meta-Analysis. Obes. Surg. 2024, 34, 2670–2684. [Google Scholar] [CrossRef] [PubMed]
- Bellicha, A.; van Baak, M.; Battista, F.; Beaulieu, K.; Blundell, J.; Busetto, L.; Carraça, E.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of exercise training before and after bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 2021, 22, e13296. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 6.5; Cochrane: London, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 16 March 2025).
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.; Hernán, M.; Reeves, B.; Savović, J.; Berkman, N.; Viswanathan, M.; Henry, D.; Altman, D.; Ansari, M.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.; Akl, E.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; de Beer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Rzepa, A.; Karpińska, I.; Wierdak, M.; Pisarska-Adamczyk, M.; Stefura, T.; Kawa, I.; Pędziwiatr, M.; Major, P. Effect of preoperative intragastric balloon treatment on perioperative and postoperative outcomes after laparoscopic sleeve gastrectomy: A retrospective cohort study. Pol. J. Surg. 2024, 96, 56–62. [Google Scholar] [CrossRef]
- Fennig, U.; Snir, A.; Halifa-Kurzman, I.; Sela, A.; Hadas, A.; Fennig, S. Pre-surgical Weight Loss Predicts Post-surgical Weight Loss Trajectories in Adolescents Enrolled in a Bariatric Program. Obes. Surg. 2019, 29, 1154–1163. [Google Scholar] [CrossRef]
- Lemanu, D.; Singh, P.; Shao, R.; Pollock, T.; MacCormick, A.; Arroll, B.; Hill, A. Text messaging improves preoperative exercise in patients undergoing bariatric surgery. ANZ J. Surg. 2018, 88, 733–738. [Google Scholar] [CrossRef]
- Pouwels, S.; Sanches, E.E.; Cagiltay, E.; Severin, R.; Philips, S.A. Perioperative Exercise Therapy in Bariatric Surgery: Improving Patient Outcomes. Diabetes Metab. Syndr. Obes. 2020, 13, 1813–1823. [Google Scholar] [CrossRef]
- Daniels, P.; Burns, R.; Brusseau, T.; Hall, M.; Davidson, L.; Adams, T.; Eisenman, P. Effect of a randomised 12-week resistance training programme on muscular strength, cross-sectional area and muscle quality in women having undergone Roux-en-Y gastric bypass. J. Sports Sci. 2018, 36, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Baillot, A.; Mampuya, W.M.; Comeau, E.; Méziat-Burdin, A.; Langlois, M.F. Feasibility and Impacts of Supervised Exercise Training in Subjects with Obesity Awaiting Bariatric Surgery: A Pilot Study. Obes. Surg. 2013, 23, 882–891. [Google Scholar] [CrossRef]
- Bond, D.; Thomas, J.; Vithiananthan, S.; Unick, J.; Webster, J.; Roye, G.; Ryder, B.; Sax, H. Intervention-related increases in preoperative physical activity are maintained 6-months after Bariatric surgery: Results from the bari-active trial. Int. J. Obes. 2017, 41, 467–470. [Google Scholar] [CrossRef]
- Martinez-Huenchullan, S.; Ehrenfeld-Slater, I.P.; Ehrenfeld-Slater, I.P. Effects of Exercise on Physical and Metabolic Function of Candidates Before Undergoing Bariatric Surgery; London, UK. 2023. Available online: http://isrctn.com/ (accessed on 16 March 2025). [CrossRef]
- Herrera-Santelices, A.; Tabach-Apraiz, A.; Andaur-Cáceres, K.; Zamunér, A.R. Effect of physical exercise in bariatric surgery patients: Protocol of a randomized controlled clinical trial. Trials 2021, 22, 107. [Google Scholar] [CrossRef]
- Mills, J.; Liebert, C.; Pratt, J.; Earley, M.; Eisenberg, D. Complete Telehealth for Multidisciplinary Preoperative Workup Does Not Delay Time to Metabolic and Bariatric Surgery: A Pilot Study. Obes. Surg. 2022, 32, 3605–3610. [Google Scholar] [CrossRef]
- Mazowita, A.E. A Nonrandomized Trial of a Pre-Operative Physical Activity Program on Bariatric Surgery Candidates as Evaluated by Pre- and Post-Operative Physical Activity and Obesity-Related Biomarkers. 2020. Available online: http://hdl.handle.net/1993/34679 (accessed on 16 March 2025).
- NCT01452230. Prebariatric Surgery Physical Activity Program. 2011. Available online: https://clinicaltrials.gov/show/NCT01452230. (accessed on 16 March 2025).
- NCT03976674. A Preoperative Cognitive Behavioural Therapy Program Based on Self-Determination Theory for Bariatric Surgery Candidates. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03976674. (accessed on 16 March 2025).
- NCT00830440. A Multicenter Study for Pre-Surgical Weight Loss. 2009. Available online: https://clinicaltrials.gov/show/NCT00830440. (accessed on 16 March 2025).
- NCT04046367. Prehabilitation in Bariatric Surgery: A Randomized Controlled Clinical Trial. 2019. Available online: https://clinicaltrials.gov/study/NCT04046367 (accessed on 7 August 2025).
- NCT00623792. Study on Impact of Lifestyle Change and Weight Loss Before Bariatric Surgery. 2008. Available online: https://clinicaltrials.gov/show/NCT00623792. (accessed on 16 March 2025).
- NCT03963986. Impacts of Remote Digital Support on Physical Activity for Patients in Bariatric Surgery (STIMUL). 2018. Available online: https://clinicaltrials.gov/study/NCT03963986 (accessed on 7 August 2025).
- NCT03666481. Physical Activity in Bariatric Patients. 2011. Available online: https://clinicaltrials.gov/study/NCT03666481 (accessed on 7 August 2025).
- Arman, N.; Tokgoz, G.; Seyit, H.; Karabulut, M. The effects of core stabilization exercise program in obese people awaiting bariatric surgery: A randomized controlled study. Complement. Ther. Clin. Pract. 2021, 43, 101342. [Google Scholar] [CrossRef]
- Baillot, A.; Mampuya, W.M.; Dionne, I.J.; Comeau, E.; Méziat-Burdin, A.; Langlois, M.-F. Impacts of Supervised Exercise Training in Addition to Interdisciplinary Lifestyle Management in Subjects Awaiting Bariatric Surgery: A Randomized Controlled Study. Obes. Surg. 2016, 26, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.; Vithiananthan, S.; Thomas, J.; Trautvetter, J.; Unick, J.; Jakicic, J.; Pohl, D.; Ryder, B.; Roye, G.; Sax, H.; et al. Bari-Active: A randomized controlled trial of a preoperative intervention to increase physical activity in bariatric surgery patients. Surg. Obes. Relat. Dis. 2015, 11, 169–177. [Google Scholar] [CrossRef]
- Creel, D.; Schuh, L.; Reed, C.; Gomez, A.; Hurst, L.; Stote, J.; Cacucci, B. A randomized trial comparing two interventions to increase physical activity among patients undergoing bariatric surgery. Obesity 2016, 24, 1660–1668. [Google Scholar] [CrossRef]
- García-Delgado, Y.; López-Madrazo-Hernández, M.; Alvarado-Martel, D.; Miranda-Calderín, G.; Ugarte-Lopetegui, A.; González-Medina, R.; Hernández-Lázaro, A.; Zamora, G.; Pérez-Martín, N.; Sánchez-Hernández, R.; et al. Prehabilitation for Bariatric Surgery: A Randomized, Controlled Trial Protocol and Pilot Study. Nutrients 2021, 13, 2903. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zavorsky, G.S.; Kim, D.J.; Christou, N.V.; Feldman, L.S.; Carli, F. Effects of a bariatric preoperative exercise program: A pilot randomized study. Scientific Session of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Baltimore, Maryland, USA, 17–20 April 2013. Surg. Endosc. 2013, 27, 304–503. [Google Scholar] [CrossRef]
- Marcon, E.R.; Baglioni, S.; Bittencourt, L.; Lopes, C.L.N.; Neumann, C.R.; Trindade, M.R.M. What Is the Best Treatment before Bariatric Surgery? Exercise, Exercise and Group Therapy, or Conventional Waiting: A Randomized Controlled Trial. Obes. Surg. 2017, 27, 763–773. [Google Scholar] [CrossRef]
- Parikh, M.; Dasari, M.; McMacken, M.; Ren, C.; Fielding, G.; Ogedegbe, G. Does a preoperative medically supervised weight loss program improve bariatric surgery outcomes? A pilot randomized study. Surg. Endosc. 2012, 26, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Funderburk, J.A.; Callis, S. Aquatic intervention effect on quality of life prior to obesity surgery: A pilot study. Annu. Ther. Recreat. 2010, 18, 66–79. Available online: https://link.gale.com/apps/doc/A252446796/AONE?u=anon~90a8d526&sid=googleScholar&xid=95612b50 (accessed on 16 March 2025).
- Marc-Hernández, A.; Ruiz-Tovar, J.; Aracil, A.; Guillén, S.; Moya-Ramón, M. Impact of Exercise on Body Composition and Cardiometabolic Risk Factors in Patients Awaiting Bariatric Surgery. Obes. Surg. 2019, 29, 3891–3900. [Google Scholar] [CrossRef]
- Picó-Sirvent, I.; Aracil-Marco, A.; Pastor, D.; Moya-Ramón, M. Effects of a Combined High-Intensity Interval Training and Resistance Training Program in Patients Awaiting Bariatric Surgery: A Pilot Study. Sports 2019, 7, 72. [Google Scholar] [CrossRef]
- Picó-Sirvent, I.; Manresa-Rocamora, A.; Aracil-Marco, A.; Moya-Ramón, M. A Combination of Aerobic Exercise at Fatmax and Low Resistance Training Increases Fat Oxidation and Maintains Muscle Mass, in Women Waiting for Bariatric Surgery. Obes. Surg. 2022, 32, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Still, C.D. Outcomes of Preoperative Weight Loss in High-Risk Patients Undergoing Gastric Bypass Surgery. Arch. Surg. 2007, 142, 994. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.M.; Gordon, A.; Hyett, J.; de Vries, B.; Carberry, A.E.; Morris, J. Planned early delivery versus expectant management of the term suspected compromised baby for improving outcomes. Cochrane Database Syst. Rev. 2015, 11, CD009433. [Google Scholar] [CrossRef]
- Herrera-Santelices, A.; Argüello-Florencio, G.; Westphal, G.; Nardo Junior, N.; Zamunér, A.R. Effects of Supervised Physical Exercise as Prehabilitation on Body Composition, Functional Capacity and Quality of Life in Bariatric Surgery Candidates: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5091. [Google Scholar] [CrossRef]
- Lodewijks, Y.; Luyer, M.; van Montfort, G.; de Zoete, J.; Smulders, F.; Nienhuijs, S. Additional preparation program for bariatric surgery: Two-year results of a large cohort study. Obes. Sci. Pract. 2023, 9, 493–500. [Google Scholar] [CrossRef] [PubMed]
| Exercise compared to control for patients undergoing metabolic and bariatric surgery | |||||
| Patient or population: Patients undergoing metabolic and bariatric surgery. Intervention: Exercise. Comparison: Control. | |||||
| Outcomes | № of participants (studies) Follow-up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with control | Risk difference with exercise | ||||
| Weight change (Kg) | 183 (9 studies) | ⨁◯◯◯ Very low a,b,c,d | - | - | SMD 0.81 lower (1.72 lower to 0.09 higher) |
| BMI change (Kg/m2) | 165 (7 studies) | ⨁◯◯◯ Very low a,b,c,d | - | - | SMD 0.96 lower (1.75 lower to 0.16 lower) |
| 6-min walk test change (m) | 129 (4 studies) | ⨁◯◯◯ Very low a,b,c,d | - | - | SMD 2.01 higher (0.51 higher to 3.5 higher) |
| VO2 peak change | 72 (3 studies) | ⨁◯◯◯ Very low a,c,d | - | - | SMD 1.02 higher (0.52 higher to 1.51 higher) |
| Length of hospital stay (days) | 14 (1 study) | ⨁⨁◯◯ Low a,d | - | The mean length of hospital stay (days) was 2.36 days | MD 0.64 days lower (0.86 lower to 0.42 lower) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simancas-Racines, D.; Parise-Vasco, J.M.; Angamarca-Iguago, J.; Cuzco-Macias, A.C.; Soria, C.; Tramontano, S.; Rossetti, G.; Cobellis, F.; Cobellis, L.; Pilone, V.; et al. Effects of Preoperative Exercise Interventions in Patients Undergoing Metabolic and Bariatric Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 6170. https://doi.org/10.3390/jcm14176170
Simancas-Racines D, Parise-Vasco JM, Angamarca-Iguago J, Cuzco-Macias AC, Soria C, Tramontano S, Rossetti G, Cobellis F, Cobellis L, Pilone V, et al. Effects of Preoperative Exercise Interventions in Patients Undergoing Metabolic and Bariatric Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(17):6170. https://doi.org/10.3390/jcm14176170
Chicago/Turabian StyleSimancas-Racines, Daniel, Juan Marcos Parise-Vasco, Jaime Angamarca-Iguago, Ashley Carolina Cuzco-Macias, Carlos Soria, Salvatore Tramontano, Gianluca Rossetti, Francesco Cobellis, Luigi Cobellis, Vincenzo Pilone, and et al. 2025. "Effects of Preoperative Exercise Interventions in Patients Undergoing Metabolic and Bariatric Surgery: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 17: 6170. https://doi.org/10.3390/jcm14176170
APA StyleSimancas-Racines, D., Parise-Vasco, J. M., Angamarca-Iguago, J., Cuzco-Macias, A. C., Soria, C., Tramontano, S., Rossetti, G., Cobellis, F., Cobellis, L., Pilone, V., Barrea, L., Frias-Toral, E., Reytor-González, C., & Schiavo, L. (2025). Effects of Preoperative Exercise Interventions in Patients Undergoing Metabolic and Bariatric Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(17), 6170. https://doi.org/10.3390/jcm14176170