Assessing Endovenous Heat-Induced Thrombosis in Flush Endovenous Laser Ablation: A Study on Incidence, Risk Factors, and Patient Outcomes
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamann, S.A.S.; van der Velden, S.K.; De Maeseneer, M. Safety and Effectiveness of Endovenous Thermal Ablation for Incompetent Saphenous Veins with an Aneurysm Close to the Junction. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 244–248. [Google Scholar] [CrossRef]
- Hamann, S.A.S.; Giang, J.; Maeseneer MDe Nijsten, T.; van den Bos, R.R. Editor’s Choice—Five Year Results of Great Saphenous Vein Treatment: A Meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Rass, K.; Frings, N.; Glowacki, P.; Gräber, S.; Tilgen, W.; Vogt, T. Same Site Recurrence is More Frequent After Endovenous Laser Ablation Compared with High Ligation and Stripping of the Great Saphenous Vein: 5 year Results of a Randomized Clinical Trial (RELACS Study). Eur. J. Vasc. Endovasc. Surg. 2015, 50, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.H.; Lawaetz, M.; Bjoern, L.; Blemings, A.; Eklof, B. Randomized clinical trial comparing endovenous laser ablation and stripping of the great saphenous vein with clinical and duplex outcome after 5 years. J. Vasc. Surg. 2013, 58, 421–426. [Google Scholar] [CrossRef]
- Malskat, W.S.J.; Giang, J.; Maeseneer MGRDe Nijsten, T.; van den Bos, R.R. Randomized clinical trial of 940- versus 1470-nm endovenous laser ablation for great saphenous vein incompetence. Br. J. Surg. 2016, 103, 192–198. [Google Scholar] [CrossRef]
- Bontinis, V.; Bontinis, A.; Giannopoulos, A.; Manaki, V.; Kontes, I.; Pitoulias, A.G.; Chorti, A.; Ktenidis, K. Mid-term and Long-term Outcomes of Endovenous Laser Ablation Utilizing a 1470 nm Laser a Systematic Review and Meta-Analysis. J. Endovasc. Ther. 2024. [Google Scholar] [CrossRef] [PubMed]
- Rits, J.; Maurins, U.; Rabe, E.; Kadiss, A.; Prave, S.; Vigants, R.; Brunenieks, I.; Pannier, F. Lower prevalence of stump reflux after endovenous laser flush ablation of the great saphenous vein. Vasa 2022, 51, 222–228. [Google Scholar] [CrossRef]
- Meissner, M.; Boyle, E.M.; Labropoulos, N.; Caggiati, A.; Drgastin, R.; Doganci, S.; Gasparis, A. The anterior saphenous vein. Part 1. A position statement endorsed by the American Vein and Lymphatic Society, the American Venous Forum, and the International Union of Phlebology. Phlebol. J. Venous Dis. 2024, 39, 310–312. [Google Scholar] [CrossRef]
- Kabnick, L.S.; Sadek, M.; Bjarnason, H.; Coleman, D.M.; Dillavou, E.D.; Hingorani, A.; Lal, B.K.; Lawrence, P.F.; Malgor, R.D.; Puggioni, A. Classification and treatment of endothermal heat-induced thrombosis: Recommendations from the American Venous Forum and the Society for Vascular Surgery. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 9, 6–22. [Google Scholar] [CrossRef]
- Hirokawa, M.; Ogawa, T.; Sugawara, H.; Shokoku, S.; Sato, S. Comparison of 1470 nm Laser and Radial 2ring Fiber with 980 nm Laser and Bare-Tip Fiber in Endovenous Laser Ablation of Saphenous Varicose Veins: A Multicenter, Prospective, Randomized, Non-Blind Study. Ann. Vasc. Dis. 2015, 8, 282–289. [Google Scholar] [CrossRef]
- Hirokawa, M.; Kurihara, N. Comparison of Bare-Tip and Radial Fiber in Endovenous Laser Ablation with 1470 nm Diode Laser. Ann. Vasc. Dis. 2014, 7, 239–245. [Google Scholar] [CrossRef]
- Spinedi, L.; Stricker, H.; Keo, H.H.; Staub, D.; Uthoff, H. Feasibility and safety of flush endovenous laser ablation of the great saphenous vein up to the saphenofemoral junction. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 1006–1013. [Google Scholar] [CrossRef]
- Jun, J.; Yoon, M.; Jung, H.; Jun, H. Feasibility and Safety of Flush Endovenous Thermal Ablation of the Great Saphenous Vein with Consecutive Foam Sclerotherapy of Saphenofemoral Junction Tributaries: A Single-Center Experience. J. Clin. Med. 2024, 13, 7148. [Google Scholar] [CrossRef]
- Bontinis, V.; Bontinis, A.; Giannopoulos, A.; Pitoulias, A.G.; Chorti, A.; Ktenidis, K. Flush Endovenous Laser Ablation (fEVLA) in the Treatment of Lower Limb Venous Insufficiency a Systematic Review and Meta-Analysis. Ann. Vasc. Surg. 2024, 112, 352–362. [Google Scholar] [CrossRef]
- Alozai, T.; Oud, S.; Eggen, C.A.M.; Pullens, R.; Schreve, M.A.; Ünlü, Ç.; Mooij, M.C.; van Vlijmen, C.J. Editor’s Choice—A Randomised, Single Blind, Controlled Trial Comparing Flush Endovenous Laser Ablation with Standard Endovenous Laser Ablation of the Great Saphenous Vein. Eur. J. Vasc. Endovasc. Surg. 2025, 69, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Grassi, C.J.; Khilnani, N.M.; Fanelli, F.; Kalva, S.P.; Khan, A.A.; McGraw, J.K.; Maynar, M.; Millward, S.F.; Owens, C.A.; et al. Multi-disciplinary quality improvement guidelines for the treatment of lower extremity superficial venous insufficiency with ambulatory phlebectomy from the Society of Interventional Radiology, Cardiovascular Interventional Radiological Society of Europe, American College of Phlebology and Canadian Interventional Radiology Association. J. Vasc. Interv. Radiol. 2010, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Khilnani, N.M.; Grassi, C.J.; Kundu, S.; D’Agostino, H.R.; Khan, A.A.; McGraw, J.K.; Miller, D.L.; Millward, S.F.; Osnis, R.B.; Postoak, D.; et al. Multi-society consensus quality improvement guidelines for the treatment of lower-extremity superficial venous insufficiency with endovenous thermal ablation from the Society of Interventional Radiology, Cardiovascular Interventional Radiological Society of Europe, American College of Phlebology and Canadian Interventional Radiology Association. J. Vasc. Interv. Radiol. 2010, 21, 14–31. [Google Scholar] [CrossRef]
- van der Velden, S.K.; van den Bos, R.R.; Pichot, O.; Nijsten, T.; De Maeseneer, M. Towards an individualized management strategy for patients with chronic venous disease: Results of a Delphi consensus. Phlebol. J. Venous Dis. 2017, 33, 492–499. [Google Scholar] [CrossRef]
- De Maeseneer, M.G.; Kakkos, S.K.; Aherne, T.; Baekgaard, N.; Black, S.; Blomgren, L.; Giannoukas, A.; Gohel, M.; de Graaf, R.; Hamel-Desnos, C.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 184–267. [Google Scholar] [CrossRef] [PubMed]
- Van Der Geld, C.W.M.; Van Den Bos, R.R.; Van Ruijven, P.W.M.; Nijsten, T.; Neumann, H.A.M.; Van Gemert, M.J.C. The heat-pipe resembling action of boiling bubbles in endovenous laser ablation. Lasers Med Sci. 2010, 25, 907–909. [Google Scholar] [CrossRef][Green Version]
- Pannier, F.; Rabe, E.; Rits, J.; Kadiss, A.; Maurins, U. Endovenous laser ablation of great saphenous veins using a 1470 nm diode laser and the radial fibre--follow-up after six months. Phlebol. J. Venous Dis. 2010, 26, 35–39. [Google Scholar] [CrossRef]
- Disselhoff, B.C.V.M.; Rem, A.I.; Verdaasdonk, R.M.; Der Kinderen, D.J.; Moll, F.L. Endovenous laser ablation: An experimental study on the mechanism of action. Phlebol. J. Venous Dis. 2008, 23, 69–76. [Google Scholar] [CrossRef]
- Rabe, E.; Breu, F.X.; Cavezzi, A.; Frullini, A.; Smith, P.D.C.; Frullini, A.; Gillet, J.; Kern, P.; Guex, J.J.; Hamel-Desnos, C.; et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebol. J. Venous Dis. 2013, 29, 338–354. [Google Scholar] [CrossRef]
- Uthoff, H.; Holtz, D.; Brož, P.; Staub, D.; Spinedi, L. Rivaroxaban for thrombosis prophylaxis in endovenous laser ablation with and without phlebectomy. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 515–523. [Google Scholar] [CrossRef]
- Eroglu, E.; Yasim, A. A Randomised Clinical Trial Comparing N-Butyl Cyanoacrylate, Radiofrequency Ablation and Endovenous Laser Ablation for the Treatment of Superficial Venous Incompetence: Two Year Follow up Results. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 553–560. [Google Scholar] [CrossRef]
- Karam, B.; Moussally, M.; Nassar, H.; Ataya, K.; Jaafar, R.; Haddad, F. Long-term results of endovenous laser ablation of saphenous vein reflux: Up to nine years of follow-up. Phlebol. J. Venous Dis. 2020, 36, 43–47. [Google Scholar] [CrossRef]
- Kibrik, P.; Chait, J.; Arustamyan, M.; Alsheekh, A.; Rajaee, S.; Marks, N.; Hingorani, A.; Ascher, E. Safety and efficacy of endovenous ablations in octogenarians, nonagenarians, and centenarians. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, H.; Mo, M.; Ito, T.; Inoue, Y.; Obitsu, Y.; Kichikawa, K.; Yamaki, T.; Ogawa, T. Venous thromboembolism complications after endovenous laser ablation for varicose veins and role of duplex ultrasound scan. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Theivacumar, N.S.; Darwood, R.; Gough, M.J. Neovascularisation and recurrence 2 years after varicose vein treatment for sapheno-femoral and great saphenous vein reflux: A comparison of surgery and endovenous laser ablation. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Kane, K.; Fisher, T.; Bennett, M.; Hicks, T.D.; Shutze, W.P.; Grimsley, B.; Hicks, T.D.; Grimsley, B.; Gable, D.; Pearl, G.; et al. The Incidence and Outcome of Endothermal Heat-induced Thrombosis after Endovenous Laser Ablation. Ann. Vasc. Surg. 2014, 28, 1744–1750. [Google Scholar] [CrossRef]
- Sufian, S.; Arnez, A.; Labropoulos, N.; Lakhanpal, S. Endovenous heat-induced thrombosis after ablation with 1470 nm laser: Incidence, progression, and risk factors. Phlebol. J. Venous Dis. 2014, 30, 325–330. [Google Scholar] [CrossRef]
- Ryer, E.J.; Elmore, J.R.; Garvin, R.P.; Cindric, M.C.; Dove, J.T.; Kekulawela, S.; Franklin, D.P. Value of delayed duplex ultrasound assessment after endothermal ablation of the great saphenous vein. J. Vasc. Surg. 2016, 64, 446–451.e1. [Google Scholar] [CrossRef]
- Dermody, M.; Schul, M.W.; O’Donnell, T.F. Thromboembolic complications of endovenous thermal ablation and foam sclerotherapy in the treatment of great saphenous vein insufficiency. Phlebol. J. Venous Dis. 2014, 30, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.A.; Kimura, S.; Power, D.; Elhaj, A.; Abdeldaim, Y.; Cross, K.S.; McGreal, G.T.; Burke, P.E.; Moloney, T.; Manning, B.J.; et al. A Systematic Review and Meta-analysis of Thrombotic Events Following Endovenous Thermal Ablation of the Great Saphenous Vein. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Sermsathanasawadi, N.; Voravitvet, T.Y.; Chinsakchai, K.; Wongwanit, C.; Ruangsetakit, C.; Mutirangura, P. Risk factors for endovenous heat-induced thrombosis after endovenous radiofrequency ablation performed in Thailand. Phlebol. J. Venous Dis. 2016, 31, 582–587. [Google Scholar] [CrossRef]
- Harlander-Locke, M.; Jimenez, J.C.; Lawrence, P.F.; Derubertis, B.G.; Rigberg, D.A.; Gelabert, H.A.; Farley, S.M. Management of endovenous heat-induced thrombus using a classification system and treatment algorithm following segmental thermal ablation of the small saphenous vein. J. Vasc. Surg. 2013, 58, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.J.; Cantelmo, N.L.; Conrad, M.F.; Stoughton, J. Factors Influencing the Incidence of Endovenous Heat-Induced Thrombosis (EHIT). Vasc. Endovasc. Surg. 2013, 47, 207–212. [Google Scholar] [CrossRef]
- Jacobs, C.E.; Pinzon, M.M.; Orozco, J.; Hunt, P.J.B.; Rivera, A.; McCarthy, W.J. Deep venous thrombosis after saphenous endovenous radiofrequency ablation: Is it predictable? Ann. Vasc. Surg. 2014, 28, 679–685. [Google Scholar] [CrossRef]
- Tan, M.; Sadek, M.; Kabnick, L.; Parsi, K.; Davies, A.H. Management of endothermal heat-induced thrombosis. Phlebol. J. Venous Dis. 2023, 39, 214–217. [Google Scholar] [CrossRef]
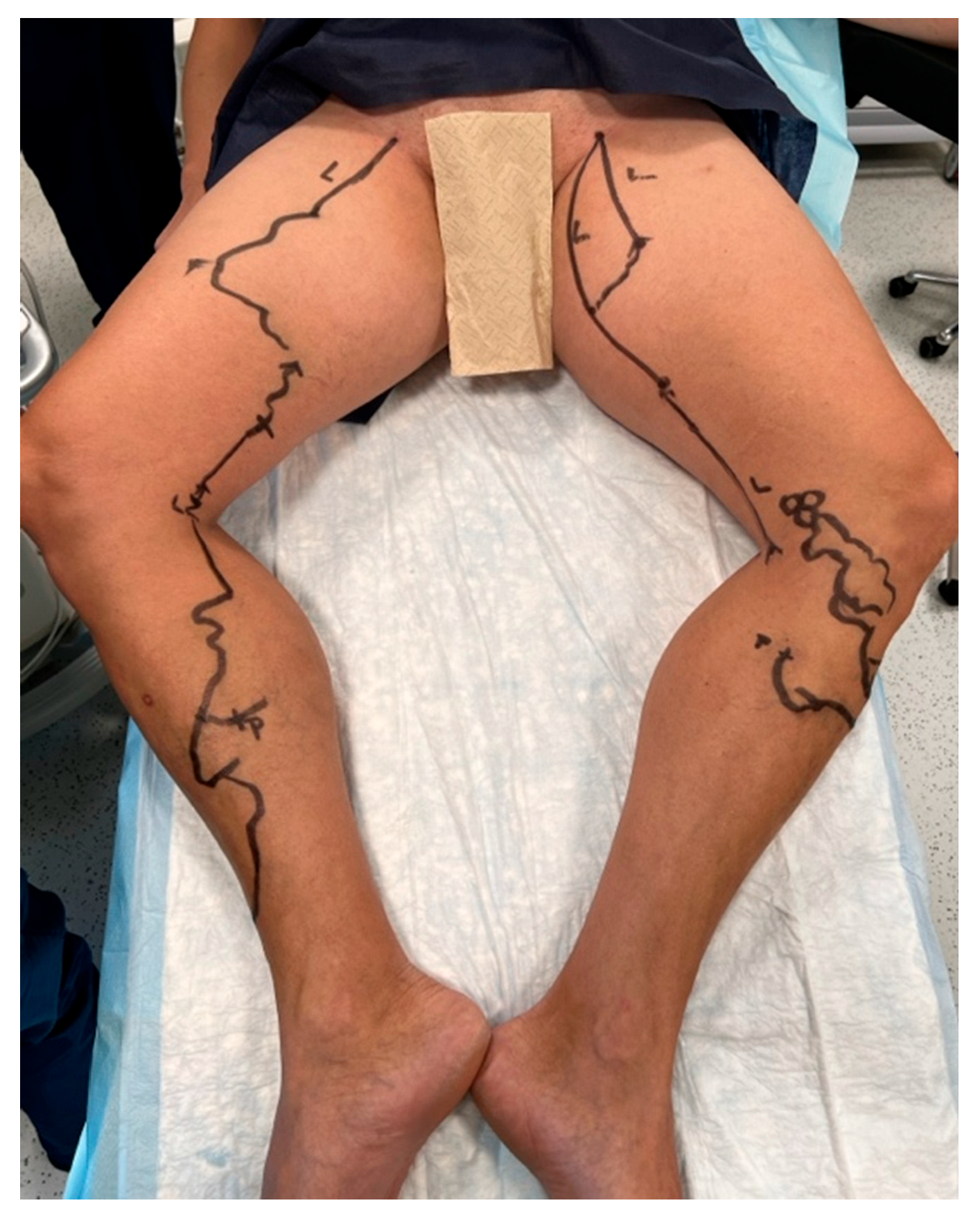
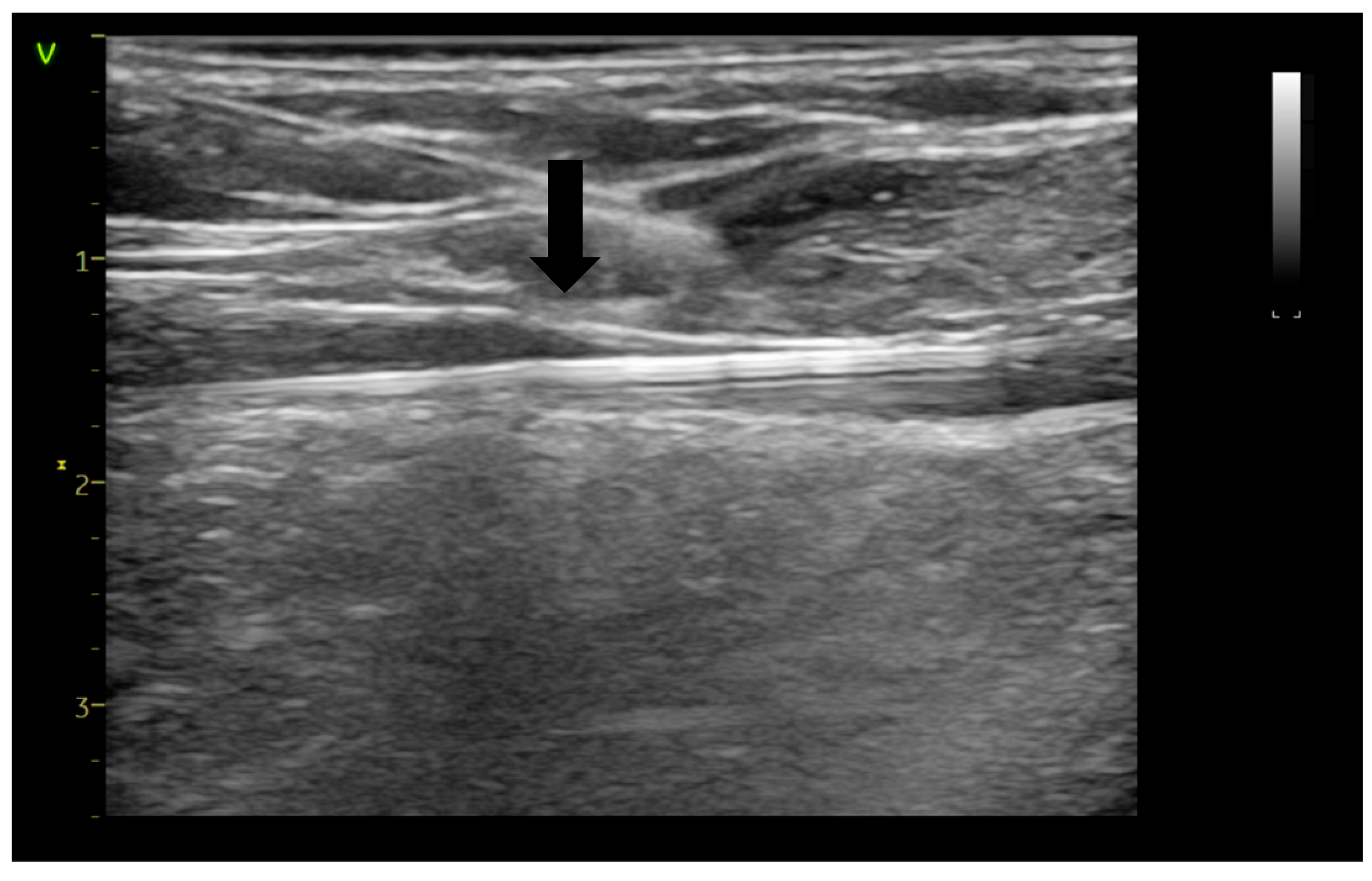
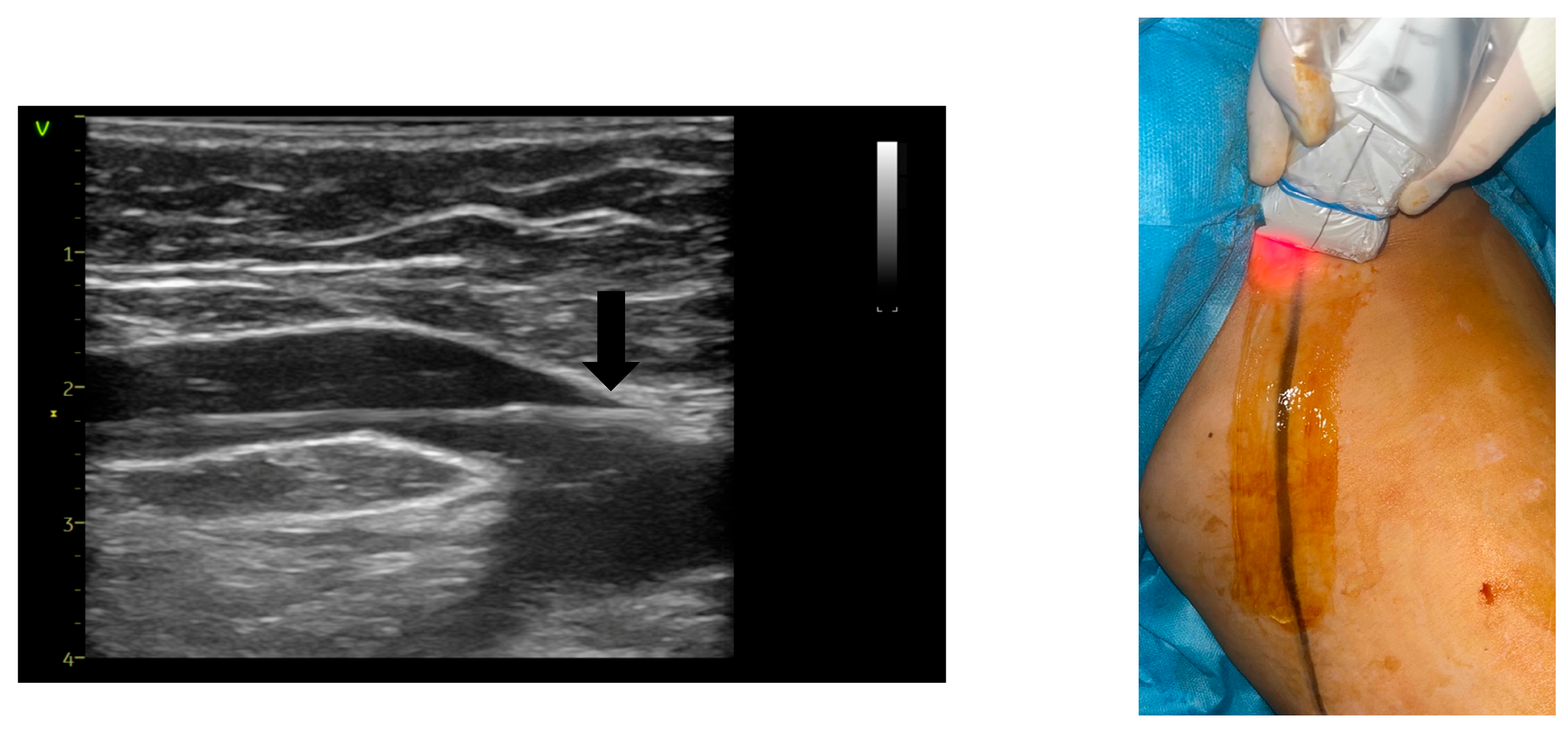
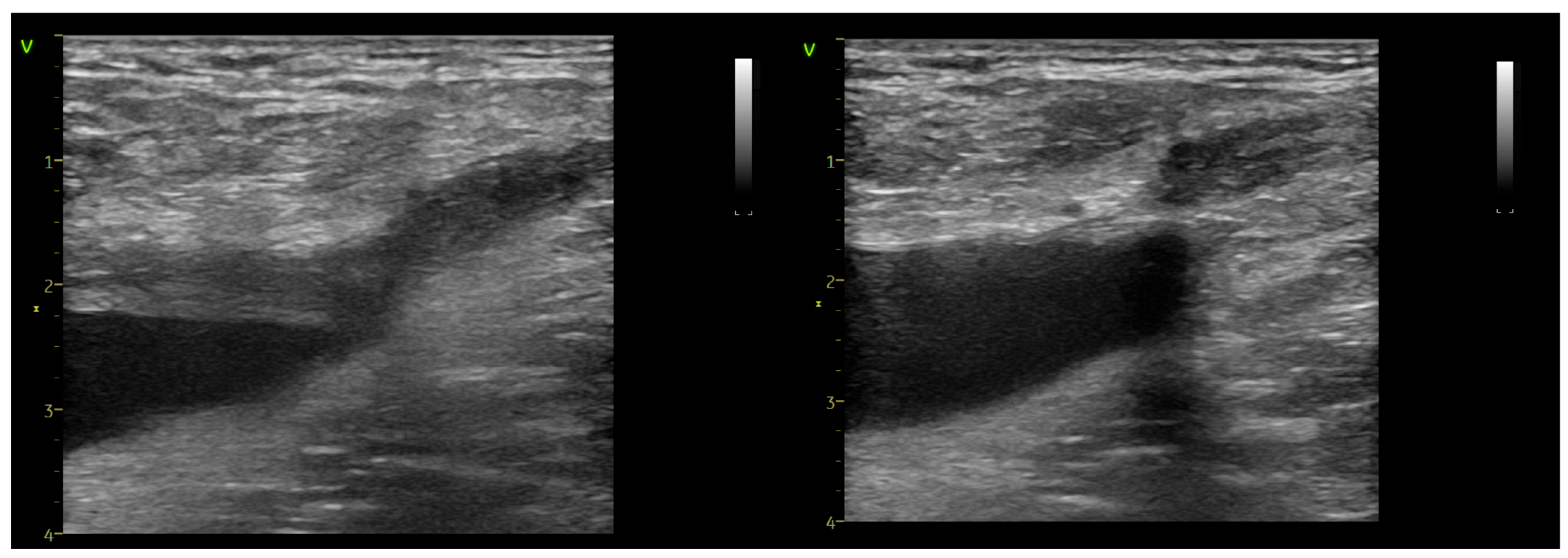
| AVF-EHIT CLASS | Definition | Treatment Recommendation |
|---|---|---|
| I | Thrombus without propagation into deep vein a. Peripheral to superficial epigastric vein b. Central to superficial epigastric vein, up to and including the deep vein junction | No treatment or surveillance. |
| II | Thrombus propagation into the adjacent deep vein, but comprising < 50% of the deep vein lumen | No treatment, weekly surveillance until thrombus resolution. In high-risk patients consider antiplatelet therapy vs. anticoagulation. Discontinue treatment following thrombus retraction or resolution. |
| III | Thrombus propagation into the adjacent deep vein, but comprising > 50% of the deep vein lumen | Therapeutic anticoagulation, weekly surveillance. Discontinue treatment following thrombus retraction or resolution. |
| IV | Occlusive deep vein thrombosis contiguous with the treated superficial vein | Treatment should be individualized, taking into account risks and benefits to patient. |
| CEAP Classification | Number of Limbs |
|---|---|
| 1 | 1 |
| 2 | 24 |
| 3 | 147 |
| 4 | 164 |
| 5 | 13 |
| 6 | 56 |
| Variable | Mean ± SD | Range |
|---|---|---|
| Age | 53.27 ± 13.28 | 21.00–92.00 |
| SFJ Diameter (mm) | 9.75 ± 3.06 | 3.50–27.00 |
| Vein Diameter (mm) (Mid Tigh) | 10.06 ± 5.54 | 1.00–30.00 |
| BMI (kg/m2) | 27.90 ± 3.93 | 19.03–42.17 |
| Power (W) | 10.62 ± 1.71 | 5.00–14.00 |
| EHIT Classification | Frequency | Percent |
|---|---|---|
| 1 | 327 | 80.7% |
| 2 | 65 | 16.0% |
| 3 | 10 | 2.5% |
| 4 | 1 | 0.2% |
| Total | 404 | 99.5% |
| Missing | 1 | 0.5% |
| Total | 405 | 100.0% |
| Venous Stump Length (mm) | Frequency | Percent |
|---|---|---|
| 0 | 380 | 93.8% |
| 6 | 3 | 0.5% |
| 7 | 1 | 0.2% |
| 8 | 1 | 0.2% |
| 9 | 4 | 1.0% |
| 10 | 4 | 1.0% |
| 11 | 2 | 0.5% |
| 12 | 3 | 0.7% |
| 13 | 4 | 1.0% |
| 15 | 2 | 0.5% |
| 16 | 1 | 0.2% |
| Total | 405 | 100.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burta, M.C.; Avram, A.; Avram, R.F.; Rogers, S.K.; Bowling, F.L.; Ionac, S.; Ionac, M.E. Assessing Endovenous Heat-Induced Thrombosis in Flush Endovenous Laser Ablation: A Study on Incidence, Risk Factors, and Patient Outcomes. J. Clin. Med. 2025, 14, 6165. https://doi.org/10.3390/jcm14176165
Burta MC, Avram A, Avram RF, Rogers SK, Bowling FL, Ionac S, Ionac ME. Assessing Endovenous Heat-Induced Thrombosis in Flush Endovenous Laser Ablation: A Study on Incidence, Risk Factors, and Patient Outcomes. Journal of Clinical Medicine. 2025; 14(17):6165. https://doi.org/10.3390/jcm14176165
Chicago/Turabian StyleBurta, Mihai Cosmin, Adela Avram, Radu Florian Avram, Steven Kristofor Rogers, Frank Lee Bowling, Stefan Ionac, and Mihai Edmond Ionac. 2025. "Assessing Endovenous Heat-Induced Thrombosis in Flush Endovenous Laser Ablation: A Study on Incidence, Risk Factors, and Patient Outcomes" Journal of Clinical Medicine 14, no. 17: 6165. https://doi.org/10.3390/jcm14176165
APA StyleBurta, M. C., Avram, A., Avram, R. F., Rogers, S. K., Bowling, F. L., Ionac, S., & Ionac, M. E. (2025). Assessing Endovenous Heat-Induced Thrombosis in Flush Endovenous Laser Ablation: A Study on Incidence, Risk Factors, and Patient Outcomes. Journal of Clinical Medicine, 14(17), 6165. https://doi.org/10.3390/jcm14176165






