Stress Septal Sign (Triple S) Preexists in Hypertensive Hearts and Clarifies Critical Diagnostic Strategies
Abstract
1. Introduction
1.1. Real-Time Three-Dimensional Imaging
1.2. Stressed Heart Morphology
1.3. Third-Generation Microscopic Imaging
1.4. Segmental Tissue Function of LV Base
1.5. Triple S and Takotsubo Syndrome
2. Why Triple S for Multiple Stressors in Clinical Practice
3. The Neuro-Cardiac Anatomical and Clinical Importance of Segmental Examination of the Interventricular Septum
4. Conclusions
5. Future Insights
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
| LV | left ventricle |
| RT3DE | real-time three-dimensional echocardiography |
| BSH | basal septal hypertrophy |
| SHM | stressed heart morphology |
| Triple S | stress septal sign |
| LV | LV hypertrophy |
References
- Maron, B.J.; Edwards, J.E.; Epstein, S.E. Disproportionate Ventricular Septal Thickening in Patients with Systemic Hypertension. Chest 1978, 73, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Gaudron, P.D.; Liu, D.; Scholz, F.; Hu, K.; Florescu, C.; Herrmann, S.; Bijnens, B.; Ertl, G.; Störk, S.; Weidemann, F. The Septal Bulge—An Early Echocardiographic Sign in Hypertensive Heart Disease. J. Am. Soc. Hypertens. 2016, 10, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Frielingsdorf, J.; Franke, A.; Kühl, H.P.; Hess, O.M.; Flachskampf, F.A. Evaluation of Septal Hypertrophy and Systolic Function in Diseases That Cause Left Ventricular Hypertrophy: A 3-Dimensional Echocardiography Study. J. Am. Soc. Echocardiogr. 2001, 14, 370–377. [Google Scholar] [CrossRef]
- Yalçin, F.; Shiota, T.; Odabashian, J.; Agler, D.; Greenberg, N.L.; Garcia, M.J.; Lever, H.M.; Thomas, J.D. Comparison by Real-Time Three-Dimensional Echocardiography of Left Ventricular Geometry in Hypertrophic Cardiomyopathy versus Secondary Left Ventricular Hypertrophy. Am. J. Cardiol. 2000, 85, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Shiota, M.; Greenberg, N.; Thomas, J.D.; Shiota, T. Real Time Three-Dimensional Echocardiography Evaluation of Mitral Annular Characteristics in Patients with Myocardial Hypertrophy. Echocardiography 2008, 25, 424–428. [Google Scholar] [CrossRef]
- Yalçin, F.; Muderrisoǧlu, H. Tako-Tsubo Cardiomyopathy May Be Associated with Cardiac Geometric Features as Observed in Hypertensive Heart Disease. Int. J. Cardiol. 2009, 135, 251–252. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Abraham, T. Stress-Induced Regional Features of Left Ventricle Is Related to Pathogenesis of Clinical Conditions with Both Acute and Chronic Stress. Int. J. Cardiol. 2010, 145, 367–368. [Google Scholar] [CrossRef]
- Yalçin, F.; Yiǧit, F.; Erol, T.; Baltali, M.; Korkmaz, M.E.; Müderrisoǧu, H. Effect of Dobutamine Stress on Basal Septal Tissue Dynamics in Hypertensive Patients with Basal Septal Hypertrophy. J. Hum. Hypertens. 2006, 20, 628–630. [Google Scholar] [CrossRef]
- Yalçin, F.; Abraham, M.R.; Garcia, M.J. Stress and Heart in Remodeling Process: Multiple Stressors at the Same Time Kill. J. Clin. Med. 2024, 13, 2597. [Google Scholar] [CrossRef]
- Yalçin, F.; Kucukler, N.; Cingolani, O.; Mbiyangandu, B.; Sorensen, L.; Pinherio, A.; Abraham, M.R.; Abraham, T.P. Evolution of Ventricular Hypertrophy and Myocardial Mechanics in Physiological and Pathological Hypertrophy. J. Appl. Physiol. 2019, 126, 354–362. [Google Scholar] [CrossRef]
- Yalcin, F.; Kucukler, N.; Cingolani, O.; Mbiyangandu, B.; Sorensen, L.L.; Pinheiro, A.C.; Abraham, M.R.; Abraham, T.P. Intracavitary Gradients in Mice With Early Regional Remodeling at the Compensatory Hyperactive Stage Prior To Lv Tissue Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 1585. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Abraham, T.P. Exercise Hypertension Should Be Recalled in Basal Septal Hypertrophy as the Early Imaging Biomarker in Patients with Stressed Heart Morphology. Blood Press. Monit. 2020, 25, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, F.; Schindler, T.; Abraham, T.P. Hypertension Should Be Ruled out in Patients with Hyperdynamic Left Ventricle on Radionuclide Myocardial Perfusion Imaging, Diastolic Dysfunction and Dyspnea on Exertion. Int. J. Cardiol. Heart Vasc. 2015, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, F.; Abraham, T.P.; Gottdiener, J.S. Regarding Article, “Left Ventricular Wall Thickness and the Presence of Asymmetric Hypertrophy in Healthy Young Army Recruits: Data from the LARGE Heart Study. Circ. Cardiovasc. Imaging 2013, 6, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Gottdiener, J.S. Left Ventricular Hypertrophy in Men with Normal Blood Pressure: Relation to Exaggerated Blood Pressure Response to Exercise. Ann. Intern. Med. 1990, 112, 161. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Küçükler, N.; Arslan, S.; Akkuş, O.; Kurtul, A.; Abraham, M.R. Basal Septal Hypertrophy as the Early Imaging Biomarker for Adaptive Phase of Remodeling Prior to Heart Failure. J. Clin. Med. 2021, 11, 75. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Abraham, M.R.; Abraham, T.P. Ultimate Phases of Hypertensive Heart Disease and Stressed Heart Morphology by Conventional and Novel Cardiac Imaging. Am. J. Cardiovasc. Dis. 2021, 11, 628. [Google Scholar]
- Yalçin, F.; Abraham, R.; Abraham, T.P. Basal Septal Hypertrophy: Extremely Sensitive Region to Variety of Stress Stimuli and Stressed Heart Morphology. J. Hypertens. 2022, 40, 626–627. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Abraham, R.; Abraham, T.P. Hemodynamic Stress and Microscopic Remodeling. Int. J. Cardiol. Cardiovasc. Risk Prev. 2021, 11, 200115. [Google Scholar] [CrossRef]
- Yalçin, F.; Abraham, R.; Abraham, T.P. Myocardial Aspects in Aortic Stenosis and Functional Increased Afterload Conditions in Patients with Stressed Heart Morphology. Ann. Thorac. Cardiovasc. Surg. 2021, 27, 332–334. [Google Scholar] [CrossRef]
- Yalçin, F.; Abraham, M.R.; Abraham, T.P. It Is Time to Assess Left Ventricular Segmental Remodelling in Aortic Stenosis. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e299–e300. [Google Scholar] [CrossRef]
- Tsuda, D.; Mori, S.; Izawa, Y.; Toh, H.; Suzuki, M.; Takahashi, Y.; Toba, T.; Fujiwara, S.; Tanaka, H.; Watanabe, Y.; et al. Diversity and Determinants of the Sigmoid Septum and Its Impact on Morphology of the Outflow Tract as Revealed Using Cardiac Computed Tomography. Echocardiography 2022, 39, 248–259. [Google Scholar] [CrossRef]
- Korhonen, P.E.; Kautiainen, H.; Järvenpää, S.; Kantola, I. Target Organ Damage and Cardiovascular Risk Factors among Subjects with Previously Undiagnosed Hypertension. Eur. J. Prev. Cardiol. 2014, 21, 980–988. [Google Scholar] [CrossRef]
- Yalçin, F.; Yalçin, H.; Küçükler, N.; Abraham, T.P. Quantitative Left Ventricular Contractility Analysis under Stress: A New Practical Approach in Follow-up of Hypertensive Patients. J. Hum. Hypertens. 2011, 25, 578–584. [Google Scholar] [CrossRef][Green Version]
- Yalçin, F.; Topaloglu, C.; Kuçukler, N.; Ofgeli, M.; Abraham, T.P. Could Early Septal Involvement in the Remodeling Process Be Related to the Advance Hypertensive Heart Disease? IJC Heart Vasc. 2015, 7, 141–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vieira, M.L.C.; Silva Filho, R.M.; Brito Filho, F.S.; Leal, S.B.; Lira Filho, E.B.; Fischer, C.H.; De Souza, J.A.; Perin, M.A. Selective Contrast Echocardiography in Percutaneous Transluminal Septal Myocardial Ablation in an Elderly Patient with Left Ventricular Concentric Hypertrophy. Echocardiography 2003, 20, 563–566. [Google Scholar] [CrossRef] [PubMed]
- ANGELAKOS, E.T.; Millard, R.W. Regional Distribution of Catecholamines in the Dog Heart. Circ. Res. 1965, 16, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kimata, S.-I. Chemical Evaluation of the Distribution of Catecholamines in the Heart. Jpn. Circ. J. 1965, 29, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Ino, H.; Okeie, K.; Emoto, Y.; Yamaguchi, M.; Yasuda, T.; Fujino, N.; Fujii, H.; Fujita, S.; Mabuchi, H.; et al. Cardiac Sympathetic Activity in the Asymmetrically Hypertrophied Septum in Patients with Hypertension or Hypertrophic Cardiomyopathy. Clin. Cardiol. 2000, 23, 365–370. [Google Scholar] [CrossRef]
- Yalcin, F.; Topaloglu, C.; Kucukler, N.; Yeral, N.; Vefali, H.; Abraham, T.P.; Garcia, M.J. Abstract 11796: Determination of Regional Myocardial Performance in Hypertensive Patients with/without Left Ventricular Hypertrophy. Circulation 2011, 124, A11796. [Google Scholar]
- Narayanan, A.; Aurigemma, G.P.; Chinali, M.; Hill, J.C.; Meyer, T.E.; Tighe, D.A. Cardiac Mechanics in Mild Hypertensive Heart Disease: A Speckle-Strain Imaging Study. Circ. Cardiovasc. Imaging 2009, 2, 382–390. [Google Scholar] [CrossRef]
- Plante, G.E. Predisease Biological Markers: Early Diagnosis and Prevention of Arterial Hypertension. Metabolism 2008, 57, S36–S39. [Google Scholar] [CrossRef]
- Huseynov, A.; El-Battrawy, I.; Ansari, U.; Schramm, K.; Zhou, X.; Lang, S.; Borggrefe, M.; Akin, I. Age Related Differences and Outcome of Patients with Takotsubo Syndrome. J. Geriatr. Cardiol. JGC 2017, 14, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Khan, H.; Gamble, D.T.; Scally, C.; Newby, D.E.; Dawson, D. Takotsubo Syndrome: Pathophysiology, Emerging Concepts, and Clinical Implications. Circulation 2022, 145, 1002–1019. [Google Scholar] [CrossRef]
- Wilkhoo, H.S.; Visvanathan, H.; Chatterjee, S.; Hussain, S.; Gundaniya, P.; Francis, V.; Singh, B.; Pillai, A.A. Current Clinical Perspectives on Takotsubo Syndrome: Comprehensive Analysis of Diagnosis, Management, and Pathophysiology. Int. J. Cardiovasc. Acad. 2024, 10, 82–90. [Google Scholar] [CrossRef]
- Schlossbauer, S.A.; Ghadri, J.-R.; Scherff, F.; Templin, C. The Challenge of Takotsubo Syndrome: Heterogeneity of Clinical Features. Swiss Med. Wkly. 2017, 147, w14490. [Google Scholar] [CrossRef] [PubMed]
- Prokudina, E.S.; Kurbatov, B.K.; Zavadovsky, K.V.; Vrublevsky, A.V.; Naryzhnaya, N.V.; Lishmanov, Y.B.; Maslov, L.N.; Oeltgen, P.R. Takotsubo Syndrome: Clinical Manifestations, Etiology and Pathogenesis. Curr. Cardiol. Rev. 2021, 17, 188–203. [Google Scholar] [CrossRef]
- Lee, P.T.; Dweck, M.R.; Prasher, S.; Shah, A.; Humphries, S.E.; Pennell, D.J.; Montgomery, H.E.; Payne, J.R. Left Ventricular Wall Thickness and the Presence of Asymmetric Hypertrophy in Healthy Young Army Recruits. Circ. Cardiovasc. Imaging 2013, 6, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, F.; Melek, I.; Mutlu, T. Stressed Heart Morphology and Neurologic Stress Score Effect Beyond Hemodynamic Stress on Focal Geometry. J. Hypertens. 2022, 40, e79. [Google Scholar] [CrossRef]
- Schlaich, M.P.; Kaye, D.M.; Lambert, E.; Sommerville, M.; Socratous, F.; Esler, M.D. Relation Between Cardiac Sympathetic Activity and Hypertensive Left Ventricular Hypertrophy. Circulation 2003, 108, 560–565. [Google Scholar] [CrossRef]
- Holmgren, S.; Abrahamsson, T.; Almgren, O. Adrenergic Innervation of Coronary Arteries and Ventricular Myocardium in the Pig: Fluorescence Microscopic Appearance in the Normal State and after Ischemia. Basic Res. Cardiol. 1985, 80, 18–26. [Google Scholar] [CrossRef]
- Kawano, H.; Okada, R.; Yano, K. Histological Study on the Distribution of Autonomic Nerves in the Human Heart. Heart Vessels 2003, 18, 32–39. [Google Scholar] [CrossRef]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.C.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral Features of Myocardial Stunning Due to Sudden Emotional Stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef]
- Smelley, M.P.; Virnich, D.E.; Williams, K.A.; Ward, R.P. A Hypertensive Response to Exercise Is Associated with Transient Ischemic Dilation on Myocardial Perfusion SPECT Imaging. J. Nucl. Cardiol. 2007, 14, 537–543. [Google Scholar] [CrossRef]
- Semánová, C. NCD Risk Factor Collaboration (NCD-RisC) Worldwide Trends in Hypertension Prevalence and Progress in Treatment and Control from 1990 to 2019: A Pooled Analysis of 1201 Population-Representative Studies with 104 Million Participants. Lancet Lond. Engl. 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Azzarelli, S.; Galassi, A.R.; Amico, F.; Giacoppo, M.; Argentino, V.; Fiscella, A. Intraventricular Obstruction in a Patient with Tako-Tsubo Cardiomyopathy. Int. J. Cardiol. 2007, 121, e22–e24. [Google Scholar] [CrossRef] [PubMed]
- Krasnow, N. Subaortic Septal Bulge Simulates Hypertrophic Cardiomyopathy by Angulation of the Septum with Age, Independent of Focal Hypertrophy. An Echocardiographic Study. J. Am. Soc. Echocardiogr. 1997, 10, 545–555. [Google Scholar] [CrossRef]
- Yalçin, F.; Muderrisoglu, H.; Korkmaz, M.E.; Ozin, B.; Baltali, M.; Yigit, F. The Effect of Dobutamine Stress on Left Ventricular Outflow Tract Gradients in Hypertensive Patients with Basal Septal Hypertrophy. Angiology 2004, 55, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Smart, S.C.; Knickelbine, T.; Malik, F.; Sagar, K.B. Dobutamine-Atropine Stress Echocardiography for the Detection of Coronary Artery Disease in Patients With Left Ventricular Hypertrophy. Circulation 2000, 101, 258–263. [Google Scholar] [CrossRef]
- Ratey, J.; Hagerman, E. Spark: The Revolutionary New Science of Exercise and the Brain. Psychiatr. Serv. Psychiatr. Serv. 2008, 59, 304. [Google Scholar]
- Zandstra, T.E.; Notenboom, R.G.E.; Wink, J.; Kiès, P.; Vliegen, H.W.; Egorova, A.D.; Schalij, M.J.; De Ruiter, M.C.; Jongbloed, M.R.M. Asymmetry and Heterogeneity: Part and Parcel in Cardiac Autonomic Innervation and Function. Front. Physiol. 2021, 12, 665298. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, K.; So, E.L.; Rajendran, P.S.; Hoang, J.D.; Makkar, N.; Mahajan, A.; Shivkumar, K.; Vaseghi, M. Electrophysiological Effects of Right and Left Vagal Nerve Stimulation on the Ventricular Myocardium. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H722–H731. [Google Scholar] [CrossRef]
- Brack, K.E.; Coote, J.H.; Ng, G.A. Interaction between Direct Sympathetic and Vagus Nerve Stimulation on Heart Rate in the Isolated Rabbit Heart. Exp. Physiol. 2004, 89, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Chen, J.; Zhao, B.; Jiang, J.; Tang, A.; Chen, Y. Real Role of β-Blockers in Regression of Left Ventricular Mass in Hypertension Patients: Bayesian Network Meta-Analysis. Medicine 2017, 96, e6290. [Google Scholar] [CrossRef] [PubMed]
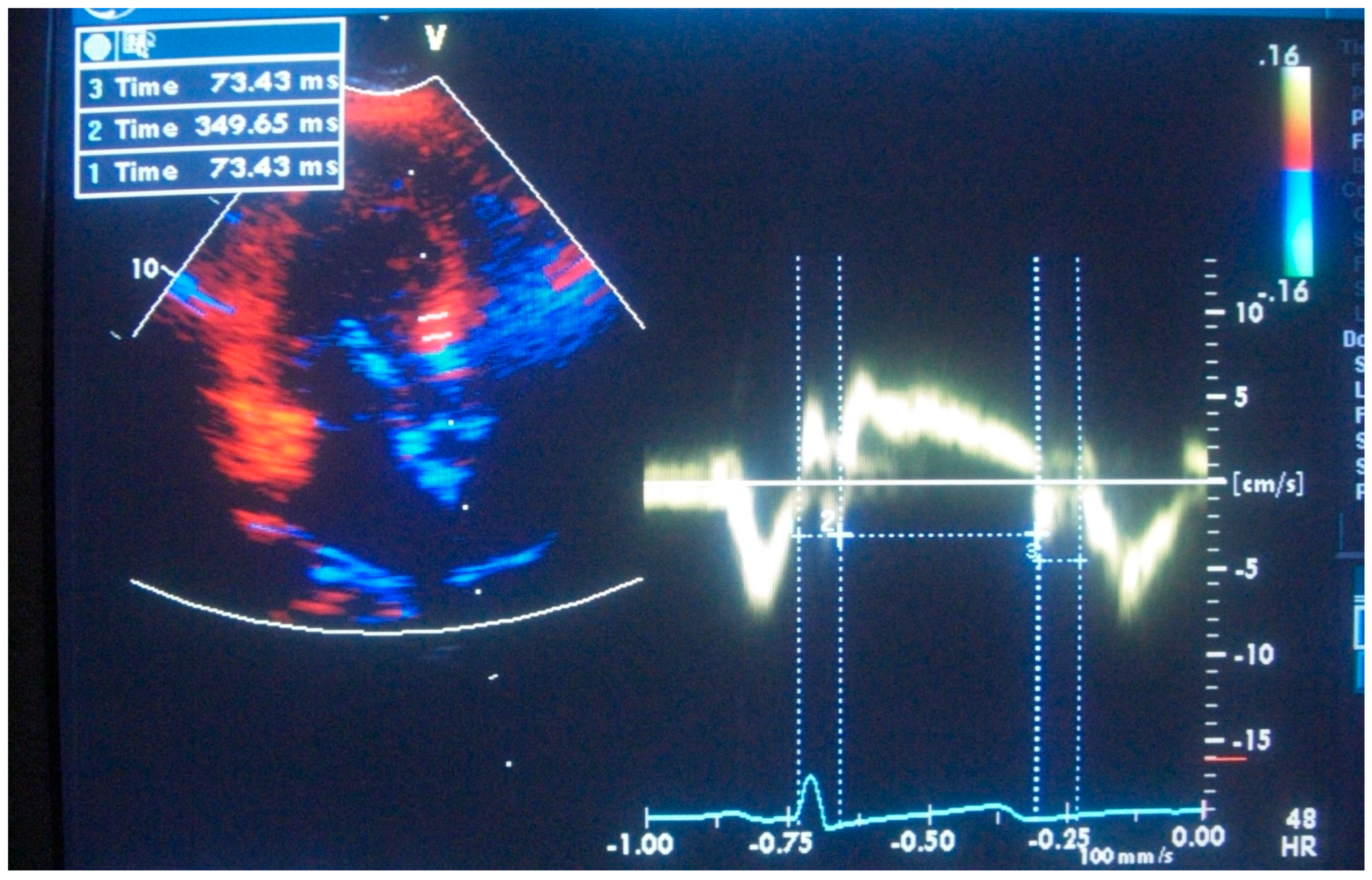
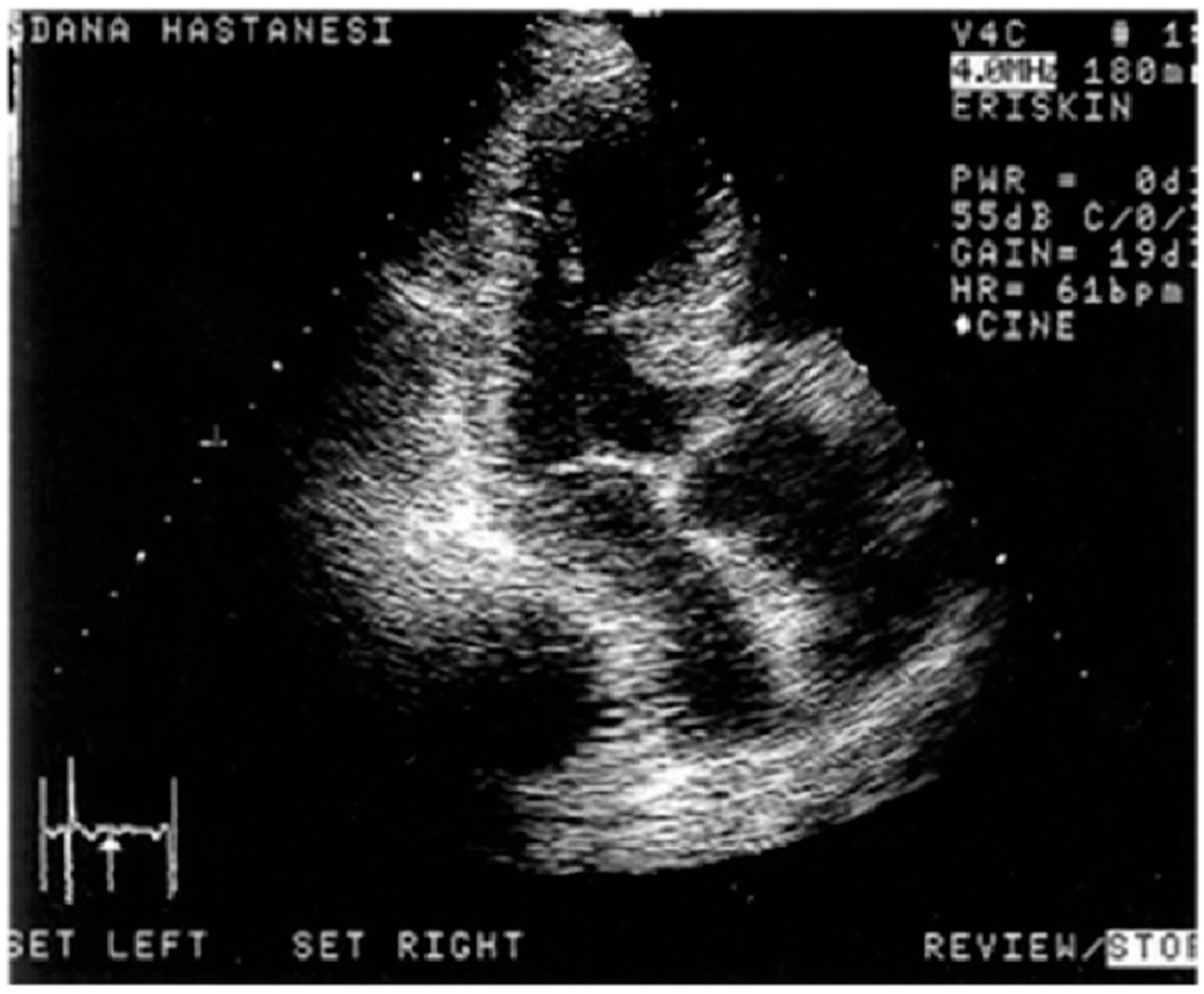

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalçin, F.; Cagatay, B.; Abraham, M.R.; Garcia, M.J. Stress Septal Sign (Triple S) Preexists in Hypertensive Hearts and Clarifies Critical Diagnostic Strategies. J. Clin. Med. 2025, 14, 6143. https://doi.org/10.3390/jcm14176143
Yalçin F, Cagatay B, Abraham MR, Garcia MJ. Stress Septal Sign (Triple S) Preexists in Hypertensive Hearts and Clarifies Critical Diagnostic Strategies. Journal of Clinical Medicine. 2025; 14(17):6143. https://doi.org/10.3390/jcm14176143
Chicago/Turabian StyleYalçin, Fatih, Boran Cagatay, M. Roselle Abraham, and Mario J. Garcia. 2025. "Stress Septal Sign (Triple S) Preexists in Hypertensive Hearts and Clarifies Critical Diagnostic Strategies" Journal of Clinical Medicine 14, no. 17: 6143. https://doi.org/10.3390/jcm14176143
APA StyleYalçin, F., Cagatay, B., Abraham, M. R., & Garcia, M. J. (2025). Stress Septal Sign (Triple S) Preexists in Hypertensive Hearts and Clarifies Critical Diagnostic Strategies. Journal of Clinical Medicine, 14(17), 6143. https://doi.org/10.3390/jcm14176143





