Hybrid Surgical Guidance in Urologic Robotic Oncological Surgery
Abstract
1. Introduction
2. Methods
3. Results
3.1. Tracer Designs
3.1.1. Radiotracers
3.1.2. Fluorescent Dyes
3.1.3. Tracer Design That Facilitates Combined Preoperative and Intraoperative Guidance
3.2. Devices for Intraoperative Detection
3.2.1. Gamma Probes
3.2.2. Fluorescence Laparoscopes
3.3. Navigation Techniques
3.3.1. Electromagnetic Tracking
3.3.2. Near Infra-Red Optical Tracking
3.4. Clinical Indications
3.4.1. Prostate Cancer
3.4.2. Sentinel Lymph Node Biopsy
3.4.3. PSMA Targeted Surgery
3.5. Kidney Cancer
3.5.1. Sentinel Lymph Node Biopsy
3.5.2. Tumor Targeted Surgery
3.6. Bladder Cancer
3.6.1. Sentinel Lymph Node Biopsy
3.6.2. Tumor Targeted Tracers
4. Discussion
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| SLNB | sentinel lymph node biopsy |
| SN | sentinel node |
| RA | Robot-assisted |
| ICG | indocyanine green |
| PSMA | prostate specific membrane antigen |
| PET | positron emission tomography |
| SPECT | single photon emission computed tomography |
| CT | computed tomography |
| CAIX | carbonic anhydrase IX |
| CLI | Cerenkov luminescence imaging |
| NIR | Near infrared |
| 68Ga | Gallium-68 |
| 99mTc | Technetium-99m |
| 111 In | Indium-111 |
| uPAR | urokinase plasminogen activator receptor |
| LN | lymph node |
| ePLND | extended pelvic lymph node dissection |
References
- Stolzenburg, J.U.; Holze, S.; Arthanareeswaran, V.K.; Neuhaus, P.; Do, H.M.; Haney, C.M.; Dietel, A.; Truss, M.C.; Stützel, K.D.; Teber, D.; et al. Robotic-assisted Versus Laparoscopic Radical Prostatectomy: 12-month Outcomes of the Multicentre Randomised Controlled LAP-01 Trial. Eur. Urol. Focus. 2022, 8, 1583–1590. [Google Scholar] [CrossRef]
- Menon, M.; Shrivastava, A.; Tewari, A.; Sarle, R.; Hemal, A.; Peabody, J.O.; Vallancien, G. Laparoscopic and robot assisted radical prostatectomy: Establishment of a structured program and preliminary analysis of outcomes. J. Urol. 2002, 168, 945–949. [Google Scholar] [CrossRef]
- KleinJan, G.H.; van den Berg, N.S.; de Jong, J.; Wit, E.M.; Thygessen, H.; Vegt, E.; van der Poel, H.G.; van Leeuwen, F.W. Multimodal hybrid imaging agents for sentinel node mapping as a means to (re)connect nuclear medicine to advances made in robot-assisted surgery. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1278–1287. [Google Scholar] [CrossRef]
- Valdés Olmos, R.A.; Rietbergen, D.D.D.; Rubello, D.; Pereira Arias-Bouda, L.M.; Collarino, A.; Colletti, P.M.; Vidal-Sicart, S.; van Leeuwen, F.W.B. Sentinel Node Imaging and Radioguided Surgery in the Era of SPECT/CT and PET/CT: Toward New Interventional Nuclear Medicine Strategies. Clin. Nucl. Med. 2020, 45, 771–777. [Google Scholar] [CrossRef]
- van der Poel, H.G.; Buckle, T.; Brouwer, O.R.; Valdes Olmos, R.A.; van Leeuwen, F.W. Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: Clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur. Urol. 2011, 60, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Abascal Junquera, J.M.; Mestre-Fusco, A.; Grootendorst, M.R.; Vidal-Sicart, S.; Fumado, L. Sentinel Lymph Node Biopsy in Prostate Cancer Using the SENSEI® Drop-In Gamma Probe. Clin. Nucl. Med. 2022, 47, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Mazzone, E.; Stabile, A.; Pellegrino, A.; Cucchiara, V.; Barletta, F.; Scuderi, S.; Robesti, D.; Leni, R.; Samanes Gajate, A.M.; et al. Prostate-specific membrane antigen Radioguided Surgery to Detect Nodal Metastases in Primary Prostate Cancer Patients Undergoing Robot-assisted Radical Prostatectomy and Extended Pelvic Lymph Node Dissection: Results of a Planned Interim Analysis of a Prospective Phase 2 Study. Eur. Urol. 2022, 82, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Collamati, F.; van Oosterom, M.N.; De Simoni, M.; Faccini, R.; Fischetti, M.; Mancini Terracciano, C.; Mirabelli, R.; Moretti, R.; Heuvel, J.o.; Solfaroli Camillocci, E.; et al. A DROP-IN beta probe for robot-assisted 68Ga-PSMA radioguided surgery: First ex vivo technology evaluation using prostate cancer specimens. EJNMMI Res. 2020, 10, 92. [Google Scholar] [CrossRef]
- Darr, C.; Harke, N.N.; Radtke, J.P.; Yirga, L.; Kesch, C.; Grootendorst, M.R.; Fendler, W.P.; Costa, P.F.; Rischpler, C.; Praus, C.; et al. Intraoperative (68)Ga-PSMA Cerenkov Luminescence Imaging for Surgical Margins in Radical Prostatectomy: A Feasibility Study. J. Nucl. Med. 2020, 61, 1500–1506. [Google Scholar] [CrossRef]
- Wit, E.M.K.; KleinJan, G.H.; Berrens, A.C.; van Vliet, R.; van Leeuwen, P.J.; Buckle, T.; Donswijk, M.L.; Bekers, E.M.; van Leeuwen, F.W.B.; van der Poel, H.G. A hybrid radioactive and fluorescence approach is more than the sum of its parts; outcome of a phase II randomized sentinel node trial in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2861–2871. [Google Scholar] [CrossRef]
- van Leeuwen, F.W.B.; Schottelius, M.; Brouwer, O.R.; Vidal-Sicart, S.; Achilefu, S.; Klode, J.; Wester, H.J.; Buckle, T. Trending: Radioactive and Fluorescent Bimodal/Hybrid Tracers as Multiplexing Solutions for Surgical Guidance. J. Nucl. Med. 2020, 61, 13–19. [Google Scholar] [CrossRef]
- Berrens, A.C.; Knipper, S.; Marra, G.; van Leeuwen, P.J.; van der Mierden, S.; Donswijk, M.L.; Maurer, T.; van Leeuwen, F.W.B.; van der Poel, H.G. State of the Art in Prostate-specific Membrane Antigen-targeted Surgery—A Systematic Review. Eur. Urol. Open Sci. 2023, 54, 43–55. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, J.; Wen, W.; Qin, W.; Chen, X. Prostate-specific membrane antigen-targeted surgery in prostate cancer: Accurate identification, real-time diagnosis, and precise resection. Theranostics 2024, 14, 2736–2756. [Google Scholar] [CrossRef]
- KleinJan, G.H.; van den Berg, N.S.; Brouwer, O.R.; de Jong, J.; Acar, C.; Wit, E.M.; Vegt, E.; van der Noort, V.; Valdés Olmos, R.A.; van Leeuwen, F.W.; et al. Optimisation of fluorescence guidance during robot-assisted laparoscopic sentinel node biopsy for prostate cancer. Eur. Urol. 2014, 66, 991–998. [Google Scholar] [CrossRef]
- KleinJan, G.H.; van den Berg, N.S.; van Oosterom, M.N.; Wendler, T.; Miwa, M.; Bex, A.; Hendricksen, K.; Horenblas, S.; van Leeuwen, F.W. Toward (Hybrid) Navigation of a Fluorescence Camera in an Open Surgery Setting. J. Nucl. Med. 2016, 57, 1650–1653. [Google Scholar] [CrossRef] [PubMed]
- van Gennep, E.J.; Pisano, G.; KleinJan, G.H.; Rietbergen, D.D.D.; Hendricksen, K.; Mertens, L.S.; Vd Kamp, M.W.; Wit, E.M.K.; van Montfoort, M.L.; Donswijk, M.; et al. Prospective clinical study of sentinel node detection in bladder cancer using a hybrid tracer—Towards replacement of pelvic lymph node dissection in cases with sentinel node visualization on SPECT/CT? Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 3653–3661. [Google Scholar] [CrossRef]
- Vermeeren, L.; Valdés Olmos, R.A.; Meinhardt, W.; Bex, A.; van der Poel, H.G.; Vogel, W.V.; Sivro, F.; Hoefnagel, C.A.; Horenblas, S. Value of SPECT/CT for detection and anatomic localization of sentinel lymph nodes before laparoscopic sentinel node lymphadenectomy in prostate carcinoma. J. Nucl. Med. 2009, 50, 865–870. [Google Scholar] [CrossRef]
- Maurer, T.; Graefen, M.; van der Poel, H.; Hamdy, F.; Briganti, A.; Eiber, M.; Wester, H.-J.; van Leeuwen, F.W.B. Prostate-Specific Membrane Antigen–Guided Surgery. J. Nucl. Med. 2020, 61, 6. [Google Scholar] [CrossRef] [PubMed]
- Berrens, A.-C.; Scheltema, M.; Maurer, T.; Hermann, K.; Hamdy, F.C.; Knipper, S.; Dell’Oglio, P.; Mazzone, E.; de Barros, H.A.; Sorger, J.M.; et al. Delphi consensus project on prostate-specific membrane antigen (PSMA)–targeted surgery—Outcomes from an international multidisciplinary panel. Eur. J. Nucl. Med. Mol. Imaging 2023, 51, 2893–2902. [Google Scholar] [CrossRef]
- Taneja, S.S. ProstaScint(R) Scan: Contemporary Use in Clinical Practice. Rev. Urol. 2004, 6 (Suppl. S10), S19–S28. [Google Scholar] [PubMed]
- de Barros, H.A.; van Oosterom, M.N.; Donswijk, M.L.; Hendrikx, J.; Vis, A.N.; Maurer, T.; van Leeuwen, F.W.B.; van der Poel, H.G.; van Leeuwen, P.J. Robot-assisted Prostate-specific Membrane Antigen-radioguided Salvage Surgery in Recurrent Prostate Cancer Using a DROP-IN Gamma Probe: The First Prospective Feasibility Study. Eur. Urol. 2022, 82, 97–105. [Google Scholar] [CrossRef]
- Martiniova, L.; Palatis, L.; Etchebehere, E.; Ravizzini, G. Gallium-68 in Medical Imaging. Curr. Radiopharm. 2016, 9, 187–207. [Google Scholar] [CrossRef]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef]
- Collamati, F.; Morganti, S.; van Oosterom, M.N.; Campana, L.; Ceci, F.; Luzzago, S.; Mancini-Terracciano, C.; Mirabelli, R.; Musi, G.; Nicolanti, F.; et al. First-in-human validation of a DROP-IN β-probe for robotic radioguided surgery: Defining optimal signal-to-background discrimination algorithm. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3098–3108. [Google Scholar] [CrossRef] [PubMed]
- Boykoff, N.; Grimm, J. Current clinical applications of Cerenkov luminescence for intraoperative molecular imaging. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2931–2940. [Google Scholar] [CrossRef] [PubMed]
- Pratt, E.C.; Skubal, M.; Mc Larney, B.; Causa-Andrieu, P.; Das, S.; Sawan, P.; Araji, A.; Riedl, C.; Vyas, K.; Tuch, D.; et al. Prospective testing of clinical Cerenkov luminescence imaging against standard-of-care nuclear imaging for tumour location. Nat. Biomed. Eng. 2022, 6, 559–568. [Google Scholar] [CrossRef]
- Oderda, M.; Grimaldi, S.; Rovera, G.; Delsedime, L.; D’Agate, D.; Lavagno, F.; Marquis, A.; Marra, G.; Molinaro, L.; Deandreis, D.; et al. Robot-assisted PSMA-radioguided Surgery to Assess Surgical Margins and Nodal Metastases in Prostate Cancer Patients: Report on Three Cases Using an Intraoperative PET-CT Specimen Imager. Urology 2023, 182, e257–e261. [Google Scholar] [CrossRef]
- van Beurden, F.; van Willigen, D.M.; Vojnovic, B.; van Oosterom, M.N.; Brouwer, O.R.; der Poel, H.G.V.; Kobayashi, H.; van Leeuwen, F.W.B.; Buckle, T. Multi-Wavelength Fluorescence in Image-Guided Surgery, Clinical Feasibility and Future Perspectives. Mol. Imaging 2020, 19, 1536012120962333. [Google Scholar] [CrossRef]
- Giulioni, C.; Mulawkar, P.M.; Castellani, D.; De Stefano, V.; Nedbal, C.; Gadzhiev, N.; Pirola, G.M.; Law, Y.X.T.; Wroclawski, M.L.; Keat, W.O.L.; et al. Near-Infrared Fluorescence Imaging with Indocyanine Green for Robot-Assisted Partial Nephrectomy: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 5560. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Buffi, N.M.; Lughezzani, G.; Dell’Oglio, P.; Mazzone, E.; Porter, J.; Mottrie, A. The Role of Intraoperative Indocyanine Green in Robot-assisted Partial Nephrectomy: Results from a Large, Multi-institutional Series. Eur. Urol. 2020, 78, 743–749. [Google Scholar] [CrossRef]
- Harke, N.; Schoen, G.; Schiefelbein, F.; Heinrich, E. Selective clamping under the usage of near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: A single-surgeon matched-pair study. World J. Urol. 2014, 32, 1259–1265. [Google Scholar] [CrossRef]
- Manny, T.B.; Patel, M.; Hemal, A.K. Fluorescence-enhanced robotic radical prostatectomy using real-time lymphangiography and tissue marking with percutaneous injection of unconjugated indocyanine green: The initial clinical experience in 50 patients. Eur. Urol. 2014, 65, 1162–1168. [Google Scholar] [CrossRef]
- Grimes, C.L.; Patankar, S.; Ryntz, T.; Philip, N.; Simpson, K.; Truong, M.; Young, C.; Advincula, A.; Madueke-Laveaux, O.S.; Walters, R.; et al. Evaluating ureteral patency in the post-indigo carmine era: A randomized controlled trial. Am. J. Obstet. Gynecol. 2017, 217, 601.e1–601.e10. [Google Scholar] [CrossRef]
- Kunitsky, K.; Lec, P.M.; Brisbane, W.; Lenis, A.T.; Chamie, K. Sodium Fluorescein for Identification of Intraoperative Urine Leaks During Partial Nephrectomy. Urology 2020, 142, 249. [Google Scholar] [CrossRef] [PubMed]
- Buckle, T.; Rietbergen, D.D.D.; de Wit-van der Veen, L.; Schottelius, M. Lessons learned in application driven imaging agent design for image-guided surgery. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3040–3054. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Sicart, S.; Seva, A.; Campos, F.; Sánchez, N.; Alonso, I.; Pahisa, J.; Caparrós, X.; Perissinotti, A.; Paredes, P.; van Leeuwen, F.W. Clinical use of an opto-nuclear probe for hybrid sentinel node biopsy guidance: First results. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, M.T.A.; Azargoshasb, S.; van Willigen, D.; Molenaar, T.; van Oosterom, M.N.; Buckle, T.; Slof, L.J.; Klop, M.; Karakullukcu, B.; Donswijk, M.; et al. Comparison of two hybrid sentinel node tracers: Indocyanine green (ICG)-(99m)Tc-nanocolloid vs. ICG-(99m)Tc-nanoscan from a nuclear medicine and surgical perspective. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2282–2291. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Buckle, T.; Vermeeren, L.; Klop, W.M.; Balm, A.J.; van der Poel, H.G.; van Rhijn, B.W.; Horenblas, S.; Nieweg, O.E.; van Leeuwen, F.W.; et al. Comparing the hybrid fluorescent-radioactive tracer indocyanine green-99mTc-nanocolloid with 99mTc-nanocolloid for sentinel node identification: A validation study using lymphoscintigraphy and SPECT/CT. J. Nucl. Med. 2012, 53, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- KleinJan, G.H.; van Werkhoven, E.; van den Berg, N.S.; Karakullukcu, M.B.; Zijlmans, H.; van der Hage, J.A.; van de Wiel, B.A.; Buckle, T.; Klop, W.M.C.; Horenblas, S.; et al. The best of both worlds: A hybrid approach for optimal pre- and intraoperative identification of sentinel lymph nodes. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1915–1925. [Google Scholar] [CrossRef]
- Mazzone, E.; Dell’Oglio, P.; Grivas, N.; Wit, E.; Donswijk, M.; Briganti, A.; Leeuwen, F.V.; Poel, H.V. Diagnostic Value, Oncologic Outcomes, and Safety Profile of Image-Guided Surgery Technologies During Robot-Assisted Lymph Node Dissection with Sentinel Node Biopsy for Prostate Cancer. J. Nucl. Med. 2021, 62, 1363–1371. [Google Scholar] [CrossRef]
- Hekman, M.C.; Boerman, O.C.; de Weijert, M.; Bos, D.L.; Oosterwijk, E.; Langenhuijsen, J.F.; Mulders, P.F.; Rijpkema, M. Targeted Dual-Modality Imaging in Renal Cell Carcinoma: An Ex Vivo Kidney Perfusion Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 4634–4642. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Buckle, T.; Bunschoten, A.; Kuil, J.; Vahrmeijer, A.L.; Wendler, T.; Valdes-Olmos, R.A.; van der Poel, H.G.; van Leeuwen, F.W. Image navigation as a means to expand the boundaries of fluorescence-guided surgery. Phys. Med. Biol. 2012, 57, 3123–3136. [Google Scholar] [CrossRef]
- van Oosterom, M.N.; Meershoek, P.; KleinJan, G.H.; Hendricksen, K.; Navab, N.; van de Velde, C.J.H.; van der Poel, H.G.; van Leeuwen, F.W.B. Navigation of Fluorescence Cameras during Soft Tissue Surgery-Is it Possible to Use a Single Navigation Setup for Various Open and Laparoscopic Urological Surgery Applications? J. Urol. 2018, 199, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, S.; Beri, A.; Grüll, M.; Ziegerhofer, J.; Prammer, P.; Leeb, K.; Sega, W.; Janetschek, G. Laparoscopic radioisotope-guided sentinel lymph node dissection in staging of prostate cancer. Eur. Urol. 2008, 53, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, W.; Valdés Olmos, R.A.; van der Poel, H.G.; Bex, A.; Horenblas, S. Laparoscopic sentinel node dissection for prostate carcinoma: Technical and anatomical observations. BJU Int. 2008, 102, 714–717. [Google Scholar] [CrossRef]
- van Oosterom, M.N.; Simon, H.; Mengus, L.; Welling, M.M.; van der Poel, H.G.; van den Berg, N.S.; van Leeuwen, F.W. Revolutionizing (robot-assisted) laparoscopic gamma tracing using a drop-in gamma probe technology. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 1–17. [Google Scholar] [PubMed]
- Dell’Oglio, P.; Meershoek, P.; Maurer, T.; Wit, E.M.K.; van Leeuwen, P.J.; van der Poel, H.G.; van Leeuwen, F.W.B.; van Oosterom, M.N. A DROP-IN Gamma Probe for Robot-assisted Radioguided Surgery of Lymph Nodes During Radical Prostatectomy. Eur. Urol. 2021, 79, 124–132. [Google Scholar] [CrossRef]
- Van Oosterom, M.N.; Rietbergen, D.D.D.; Welling, M.M.; Van Der Poel, H.G.; Maurer, T.; Van Leeuwen, F.W.B. Recent advances in nuclear and hybrid detection modalities for image-guided surgery. Expert. Rev. Med. Devices 2019, 16, 711–734. [Google Scholar] [CrossRef]
- Pisano, G.; van Oosterom, M.N.; Berrens, A.-C.; Slof, L.J.; Rietbergen, D.D.D.; van der Poel, H.G.; van Leeuwen, P.J.; van Leeuwen, F.W.B. Freehand SPECT Combined with 3-Dimensional Light Detection and Ranging as Alternative Means of Specimen Scanning During Prostate Cancer Surgery. J. Nucl. Med. 2024, 65, 1816–1817. [Google Scholar] [CrossRef]
- van Oosterom, M.N.; van Leeuwen, S.I.; Mazzone, E.; Dell’Oglio, P.; Buckle, T.; van Beurden, F.; Boonekamp, M.; van de Stadt, H.; Bauwens, K.; Simon, H.; et al. Click-on fluorescence detectors: Using robotic surgical instruments to characterize molecular tissue aspects. J. Robot. Surg. 2022, 17, 131–140. [Google Scholar] [CrossRef]
- KleinJan, G.H.; Hellingman, D.; van den Berg, N.S.; van Oosterom, M.N.; Hendricksen, K.; Horenblas, S.; Valdes Olmos, R.A.; van Leeuwen, F.W. Hybrid Surgical Guidance: Does Hardware Integration of γ- and Fluorescence Imaging Modalities Make Sense? J. Nucl. Med. 2017, 58, 646–650. [Google Scholar] [CrossRef]
- van Leeuwen, F.W.B.; Buckle, T.; van Oosterom, M.N.; Rietbergen, D.D.D. The Rise of Molecular Image-Guided Robotic Surgery. J. Nucl. Med. 2024, 65, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Meershoek, P.; KleinJan, G.H.; van Oosterom, M.N.; Wit, E.M.; van Willigen, D.M.; Bauwens, K.P.; van Gennep, E.J.; Mottrie, A.M.; van der Poel, H.G.; van Leeuwen, F. Multispectral fluorescence imaging as a tool to separate healthy and disease related lymphatic anatomies during robot-assisted laparoscopic procedures. J. Nucl. Med. 2018, 59, 1757–1760. [Google Scholar] [CrossRef]
- Aguilera Saiz, L.; Heerink, W.J.; Groen, H.C.; Hiep, M.A.J.; van der Poel, H.G.; Wit, E.M.K.; Nieuwenhuijzen, J.A.; Roeleveld, T.A.; Vis, A.N.; Donswijk, M.L.; et al. Feasibility of Image-guided Navigation with Electromagnetic Tracking During Robot-assisted Sentinel Node Biopsy: A Prospective Study. Eur. Urol. 2025, 87, 482–490. [Google Scholar] [CrossRef]
- Wendler, T.; van Leeuwen, F.W.B.; Navab, N.; van Oosterom, M.N. How molecular imaging will enable robotic precision surgery : The role of artificial intelligence, augmented reality, and navigation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4201–4224. [Google Scholar] [CrossRef]
- van Oosterom, M.N.; Engelen, M.A.; van den Berg, N.S.; KleinJan, G.H.; van der Poel, H.G.; Wendler, T.; van de Velde, C.J.; Navab, N.; van Leeuwen, F.W. Navigation of a robot-integrated fluorescence laparoscope in preoperative SPECT/CT and intraoperative freehand SPECT imaging data: A phantom study. J. Biomed. Opt. 2016, 21, 86008. [Google Scholar] [CrossRef] [PubMed]
- Hinsenveld, F.J.; Wit, E.M.K.; van Leeuwen, P.J.; Brouwer, O.R.; Donswijk, M.L.; Tillier, C.N.; Vegt, E.; van Muilekom, E.; van Oosterom, M.N.; van Leeuwen, F.W.B.; et al. Prostate-Specific Membrane Antigen PET/CT Combined with Sentinel Node Biopsy for Primary Lymph Node Staging in Prostate Cancer. J. Nucl. Med. 2020, 61, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, H.; Chen, X.; Shen, Q.; Chen, X.; Zhang, M.; Li, Z.; Zhang, Z.; Hao, H.; Yu, W.; et al. First-in-human Study of a Dual-modality Prostate-specific Membrane Antigen-targeted Probe for Preoperative Positron Emission Tomography/Computed Tomography Imaging and Intraoperative Fluorescence Imaging in Prostate Cancer. Eur. Urol. 2025, 87, 717–727. [Google Scholar] [CrossRef]
- Eder, A.C.; Omrane, M.A.; Stadlbauer, S.; Roscher, M.; Khoder, W.Y.; Gratzke, C.; Kopka, K.; Eder, M.; Meyer, P.T.; Jilg, C.A.; et al. The PSMA-11-derived hybrid molecule PSMA-914 specifically identifies prostate cancer by preoperative PET/CT and intraoperative fluorescence imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2057–2058. [Google Scholar] [CrossRef] [PubMed]
- Aras, O.; Demirdag, C.; Kommidi, H.; Guo, H.; Pavlova, I.; Aygun, A.; Karayel, E.; Pehlivanoglu, H.; Yeyin, N.; Kyprianou, N.; et al. Small Molecule, Multimodal, [(18)F]-PET and Fluorescence Imaging Agent Targeting Prostate-Specific Membrane Antigen: First-in-Human Study. Clin. Genitourin. Cancer 2021, 19, 405–416. [Google Scholar] [CrossRef]
- Michalik, B.; Engels, S.; Otterbach, M.C.; Frerichs, J.; Suhrhoff, P.E.; van Oosterom, M.N.; Maurer, M.H.; Wawroschek, F.; Winter, A. A new bimodal approach for sentinel lymph node imaging in prostate cancer using a magnetic and fluorescent hybrid tracer. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2922–2928. [Google Scholar] [CrossRef]
- Wit, E.M.K.; Acar, C.; Grivas, N.; Yuan, C.; Horenblas, S.; Liedberg, F.; Valdes Olmos, R.A.; van Leeuwen, F.W.B.; van den Berg, N.S.; Winter, A.; et al. Sentinel Node Procedure in Prostate Cancer: A Systematic Review to Assess Diagnostic Accuracy. Eur. Urol. 2017, 71, 596–605. [Google Scholar] [CrossRef] [PubMed]
- de Barros, H.A.; Duin, J.J.; Mulder, D.; van der Noort, V.; Noordzij, M.A.; Wit, E.M.K.; Pos, F.J.; Vogel, W.V.; Schaake, E.E.; van Leeuwen, F.W.B.; et al. Sentinel Node Procedure to Select Clinically Localized Prostate Cancer Patients with Occult Nodal Metastases for Whole Pelvis Radiotherapy. Eur. Urol. Open Sci. 2023, 49, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Shimbo, M.; Okawa, D.; Sakamoto, M.; Sugihara, N.; Sawada, T.; Haga, S.; Arai, H.; Nishida, K.; Arai, O.; et al. Extended Lymph Node Dissection May Not Provide a Therapeutic Benefit in Patients with Intermediate-to High-Risk Prostate Cancer Treated with Robotic-Assisted Radical Prostatectomy. Cancers 2025, 17, 655. [Google Scholar] [CrossRef]
- Nguyen, H.G.; van den Berg, N.S.; Antaris, A.L.; Xue, L.; Greenberg, S.; Rosenthal, J.W.; Muchnik, A.; Klaassen, A.; Simko, J.P.; Dutta, S.; et al. First-in-human Evaluation of a Prostate-specific Membrane Antigen-targeted Near-infrared Fluorescent Small Molecule for Fluorescence-based Identification of Prostate Cancer in Patients with High-risk Prostate Cancer Undergoing Robotic-assisted Prostatectomy. Eur. Urol. Oncol. 2023, 7, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Stibbe, J.A.; de Barros, H.A.; Linders, D.G.J.; Bhairosingh, S.S.; Bekers, E.M.; van Leeuwen, P.J.; Low, P.S.; Kularatne, S.A.; Vahrmeijer, A.L.; Burggraaf, J.; et al. First-in-patient study of OTL78 for intraoperative fluorescence imaging of prostate-specific membrane antigen-positive prostate cancer: A single-arm, phase 2a, feasibility trial. Lancet Oncol. 2023, 24, 457–467. [Google Scholar] [CrossRef]
- Dell’Oglio, P.; van Willigen, D.M.; van Oosterom, M.N.; Bauwens, K.; Hensbergen, F.; Welling, M.M.; van der Stadt, H.; Bekers, E.; Pool, M.; van Leeuwen, P.; et al. Feasibility of fluorescence imaging at microdosing using a hybrid PSMA tracer during robot-assisted radical prostatectomy in a large animal model. EJNMMI Res. 2022, 12, 14. [Google Scholar] [CrossRef]
- van Gennep, E.J.; KleinJan, G.H.; Kuusk, T.; Verdijk, R.W.A.; Wit, E.M.K.; van Rhijn, B.W.G.; Bex, A.; Mertens, L.S. Sentinel lymph node staging in urological cancer surgery: Advances in imaging, intra-operative detection and translational research. BJU Int. Early View. 2025. [Google Scholar] [CrossRef]
- Bex, A.; Kuusk, T.; Brouwer, O.R.; Valdés Olmos, R.A. Preoperative and Intraoperative Lymphatic Mapping for Radioguided Sentinel Lymph Node Biopsy in Kidney and Bladder Cancers. In Atlas of Lymphoscintigraphy and Sentinel Node Mapping: A Pictorial Case-Based Approach; Mariani, G., Vidal-Sicart, S., Valdés Olmos, R.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 357–372. [Google Scholar]
- Mahesan, T.; Coscione, A.; Ayres, B.; Watkin, N. Sentinel lymph node biopsy in renal malignancy: The past, present and future. World J. Nephrol. 2016, 5, 182–188. [Google Scholar] [CrossRef]
- Tobis, S.; Knopf, J.K.; Silvers, C.; Messing, E.; Yao, J.; Rashid, H.; Wu, G.; Golijanin, D. Robot-assisted and laparoscopic partial nephrectomy with near infrared fluorescence imaging. J. Endourol. 2012, 26, 797–802. [Google Scholar] [CrossRef]
- Manny, T.B.; Krane, L.S.; Hemal, A.K. Indocyanine green cannot predict malignancy in partial nephrectomy: Histopathologic correlation with fluorescence pattern in 100 patients. J. Endourol. 2013, 27, 918–921. [Google Scholar] [CrossRef]
- Shum, C.F.; Bahler, C.D.; Low, P.S.; Ratliff, T.L.; Kheyfets, S.V.; Natarajan, J.P.; Sandusky, G.E.; Sundaram, C.P. Novel Use of Folate-Targeted Intraoperative Fluorescence, OTL38, in Robot-Assisted Laparoscopic Partial Nephrectomy: Report of the First Three Cases. J. Endourol. Case Rep. 2016, 2, 189–197. [Google Scholar] [CrossRef]
- Sulek, J.E.; Steward, J.E.; Bahler, C.D.; Jacobsen, M.H.; Sundaram, A.; Shum, C.F.; Sandusky, G.E.; Low, P.S.; Sundaram, C.P. Folate-targeted intraoperative fluorescence, OTL38, in robotic-assisted laparoscopic partial nephrectomy. Scand. J. Urol. 2021, 55, 331–336. [Google Scholar] [CrossRef]
- Rietbergen, D.D.D.; van Gennep, E.J.; KleinJan, G.H.; Donswijk, M.; Valdés Olmos, R.A.; van Rhijn, B.W.; van der Poel, H.G.; van Leeuwen, F.W.B. Evaluation of the Hybrid Tracer Indocyanine Green- 99m Tc-Nanocolloid for Sentinel Node Biopsy in Bladder Cancer-A Prospective Pilot Study. Clin. Nucl. Med. 2022, 47, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Liedberg, F.; Chebil, G.; Davidsson, T.; Gudjonsson, S.; Månsson, W. Intraoperative Sentinel Node Detection Improves Nodal Staging in Invasive Bladder Cancer. J. Urol. 2006, 175, 84–88. [Google Scholar] [CrossRef]
- Liss, M.A.; Noguchi, J.; Lee, H.J.; Vera, D.R.; Kader, A.K. Sentinel lymph node biopsy in bladder cancer: Systematic review and technology update. Indian J. Urol. 2015, 31, 170–175. [Google Scholar] [CrossRef]
- Zarifmahmoudi, L.; Ghorbani, H.; Sadri, K.; Tavakkoli, M.; Keshvari, M.; Salehi, M.; Sadeghi, R. Sentinel Node Biopsy in Urothelial Carcinoma of the Bladder: Systematic Review and Meta-Analysis. Urol. Int. 2019, 103, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Baart, V.M.; van der Horst, G.; Deken, M.M.; Bhairosingh, S.S.; Schomann, T.; Sier, V.Q.; van der Mark, M.H.; Iamele, L.; de Jonge, H.; Resnati, M.; et al. A multimodal molecular imaging approach targeting urokinase plasminogen activator receptor for the diagnosis, resection and surveillance of urothelial cell carcinoma. Eur. J. Cancer 2021, 146, 11–20. [Google Scholar] [CrossRef]
- Meershoek, P.; Buckle, T.; van Oosterom, M.N.; KleinJan, G.H.; van der Poel, H.G.; van Leeuwen, F.W.B. Can Intraoperative Fluorescence Imaging Identify All Lesions While the Road Map Created by Preoperative Nuclear Imaging Is Masked? J. Nucl. Med. 2020, 61, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Wit, E.M.K.; van Beurden, F.; Kleinjan, G.H.; Grivas, N.; de Korne, C.M.; Buckle, T.; Donswijk, M.L.; Bekers, E.M.; van Leeuwen, F.W.B.; van der Poel, H.G. The impact of drainage pathways on the detection of nodal metastases in prostate cancer: A phase II randomized comparison of intratumoral vs intraprostatic tracer injection for sentinel node detection. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1743–1753. [Google Scholar] [CrossRef]
- Kuusk, T.; Donswijk, M.L.; Valdés Olmos, R.A.; De Bruijn, R.E.; Brouwer, O.R.; Hendricksen, K.; Horenblas, S.; Jóźwiak, K.; Prevoo, W.; Van Der Poel, H.G.; et al. An analysis of SPECT/CT non-visualization of sentinel lymph nodes in renal tumors. EJNMMI Res. 2018, 8, 105. [Google Scholar] [CrossRef]
- Haj-Mirzaian, A.; Mahmood, U.; Heidari, P. Targeted Molecular Imaging as a Biomarker in Urologic Oncology. Urol. Clin. North. Am. 2023, 50, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Hensbergen, A.W.; van Willigen, D.M.; van Beurden, F.; van Leeuwen, P.J.; Buckle, T.; Schottelius, M.; Maurer, T.; Wester, H.J.; van Leeuwen, F.W.B. Image-Guided Surgery: Are We Getting the Most Out of Small-Molecule Prostate-Specific-Membrane-Antigen-Targeted Tracers? Bioconjug Chem. 2020, 31, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Weirich, G.; Schottelius, M.; Weineisen, M.; Frisch, B.; Okur, A.; Kübler, H.; Thalgott, M.; Navab, N.; Schwaiger, M.; et al. Prostate-specific Membrane Antigen–radioguided Surgery for Metastatic Lymph Nodes in Prostate Cancer. Eur. Urol. 2015, 68, 530–534. [Google Scholar] [CrossRef]
- Azargoshasb, S.; Boekestijn, I.; Roestenberg, M.; KleinJan, G.H.; van der Hage, J.A.; van der Poel, H.G.; Rietbergen, D.D.D.; van Oosterom, M.N.; van Leeuwen, F.W.B. Quantifying the Impact of Signal-to-background Ratios on Surgical Discrimination of Fluorescent Lesions. Mol. Imaging Biol. 2023, 25, 180–189. [Google Scholar] [CrossRef]
- Meershoek, P.; van Oosterom, M.N.; Simon, H.; Mengus, L.; Maurer, T.; van Leeuwen, P.J.; Wit, E.M.K.; van der Poel, H.G.; van Leeuwen, F.W.B. Robot-assisted laparoscopic surgery using DROP-IN radioguidance: First-in-human translation. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 49–53. [Google Scholar] [CrossRef] [PubMed]


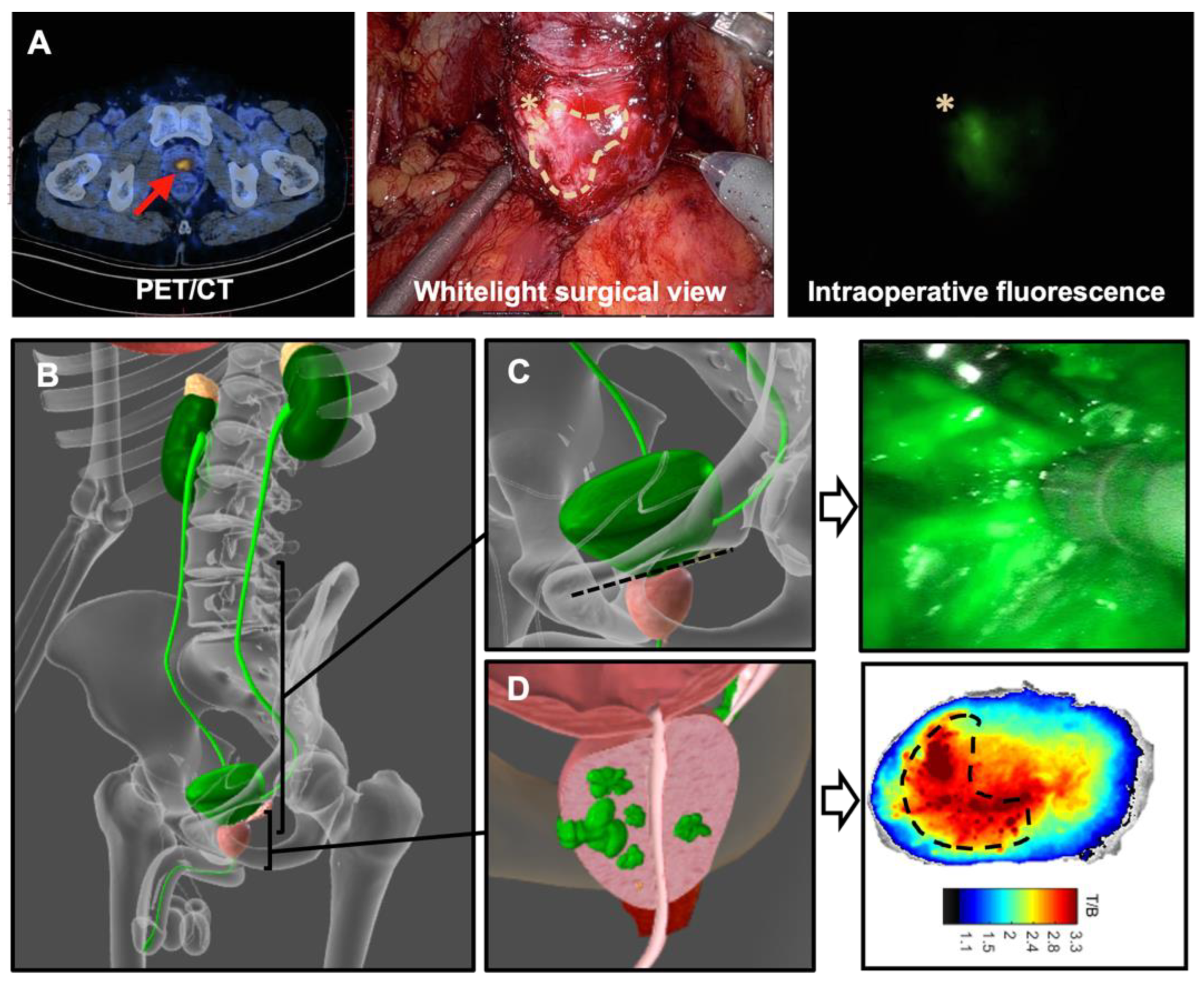
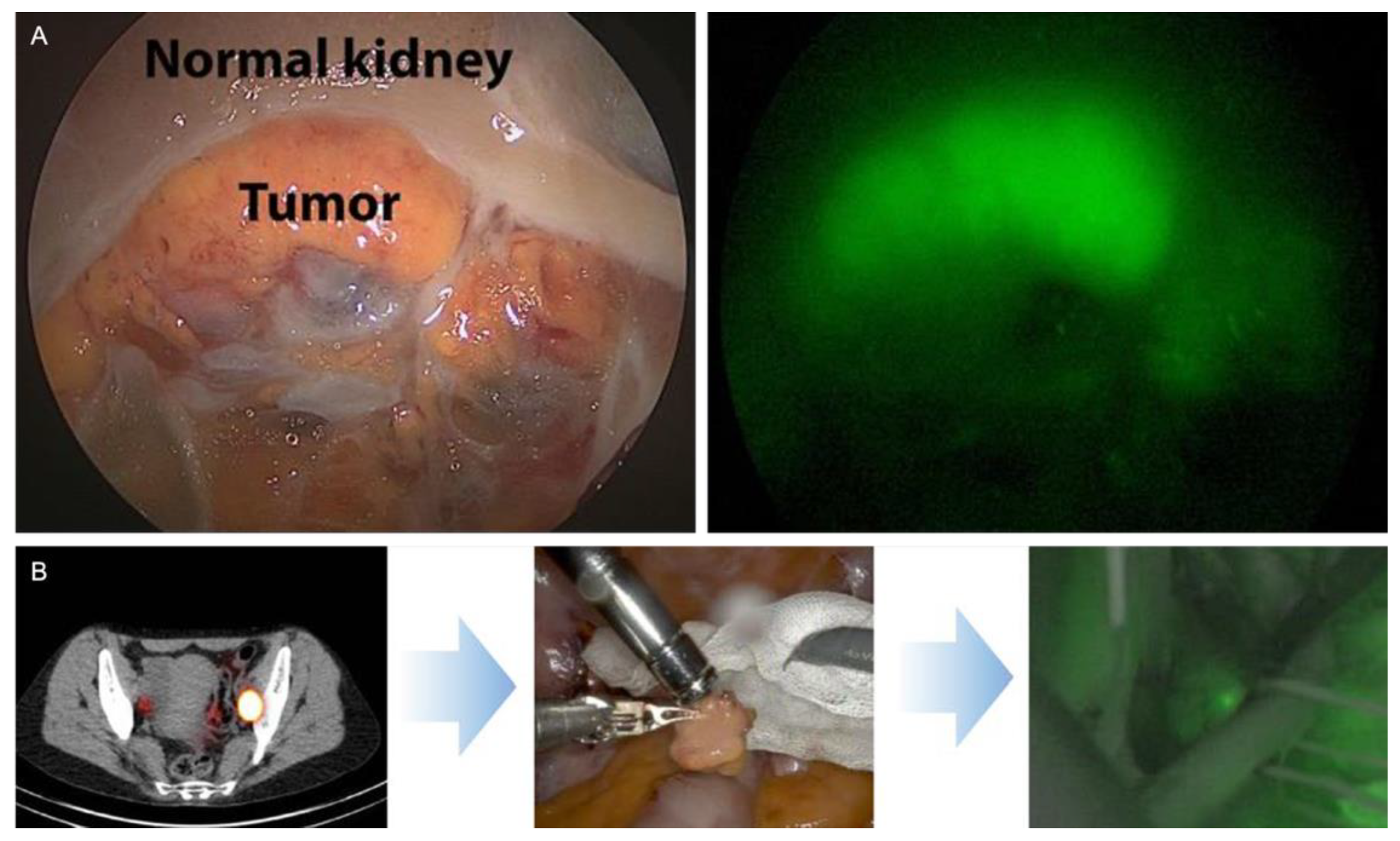
| Reference | N | Surgical Procedure | Tracer | Preoperative Imaging | Intraoperative Imaging | Detection Rate |
|---|---|---|---|---|---|---|
| Darr et al. [9] | 10 | Prostatectomy (not RA) | 68Ga-PSMA | PET/CT | Cerenkov luminescence imaging LightPath CLI system (Lightpoint Medical Ltd.). | 3 positive surgical margins/2 detected with CLI Sensitivity 66% |
| Chen et al. [58] | 16 | RA prostatectomy | 68Ga-P3 | PET/CT | Firefly fluorescence imaging (Intuitive Surgical) | Sensitivity 79.1%, specificity 90.4%, PPV 81.5%, NPV 89.0% |
| Eder AC et al. [59] | 1 | RA prostatectomy | PSMA-11-derived hybrid molecule PSMA-914 (68Ga-Glu-ureaLys-(HE)3-HBED-CC-IRDye800CW) | PET/CT | Firefly fluorescence imaging (Intuitive Surgical) | NA |
| Aras et al. [60] | 10 | RA prostatectomy | [18F]-BF3-Cy3-ACUPA | PET/CT 6 patient PET/CT/MRI | Custom-made fluorescence imager | NA |
| Sentinel Node Studies | N | Surgical Procedure | Tracer | Hybrid Yes/No | Preoperative Imaging | Intraoperative Imaging | Detection Rate |
|---|---|---|---|---|---|---|---|
| Michalik B et al. [61] | 10 | Prostatectomy + ePLND No robot surgery | Superparamagnetic iron oxide nanoparticles (SPION) and indocyanine green | Yes | MRI | NIR optical imaging system (QUEST SPECTRUM 3, Olympus, Hamburg, Germany) Handheld magnetometer probe (Sentimag, Endomag, Cambridge, UK) | 70% concordance for preoperative MRI vs. magnetometer-guided PLND 88% concordance for magnetic vs. fluorescent SLN detection. Sensitivity and specificity NA |
| Wit EMK et al. [10] | 138 | RA prostatectomy + SLNB | ICG-99mTc-nancolloid Vs. 99mTc-nancolloid + “free ICG” | Yes | SPECT/CT | Firefly fluorescence imaging, Intuitive Surgical | NA |
| KleinJan GH et al. [3] | 55 | RA prostatectomy + SLNB | ICG-99mTc-nancolloid | Yes | SPECT/CT | Firefly fluorescence imaging, Intuitive Surgical | Sensitivity 92.9% FNR 7.1% |
| KleinJan GH et al. [14] | 40 | RA prostatectomy + SLNB | ICG-99mTc-nancolloid | Yes | SPECT/CT | Karl Storz laparoscopes +lap gamma probe | Sensitivity 75% FNR 14% |
| Van der Poel et al. [5] | 11 | RA prostatectomy + SLNB | ICG-99mTc-nancolloid | Yes | SPECT/CT | Karl Storz laparoscopes + lap gamma probe | NA |
| Reference | N | Surgical Procedure | Tracer | Hybrid Yes/No | Preoperative Imaging | Intraoperative Imaging | Detection Rate |
|---|---|---|---|---|---|---|---|
| Hekman MC et al. [41] | 8 | Open and laparoscopic nephrectomy | Indium-111-DOTA-gerentuximab-IRDye800CW | Yes | SPECT 111-Indium | Fluorescence laparoscope Storz D-light P | Sensitivity 100% |
| Sulek et al. [74] | 10 | RA Partial nephrectomy | OTL38 | No | NA | Firefly fluorescence imaging, Intuitive Surgical | Safety and effectiveness study Negative contrast |
| Tobis et al. [71] | 19 | RA Partial nephrectomy | ICG | No | NA | Endoscopic SPY Imaging System | NA 11 patients 7 hypo-fluorescent 3 iso fluorescent |
| Manny et al. [72] | 100 | RA Partial nephrectomy | ICG | No | NA | Firefly fluorescence imaging, Intuitive Surgical | Sensitivity 84% Specificity 57%. PPV 87% NPV 52%, |
| Reference | N | Surgical Procedure | Tracer | Hybrid Yes/No | Preoperative Imaging | Intraoperative Imaging | Detection Rate |
|---|---|---|---|---|---|---|---|
| Rietbergen et al. [75] | 20 | Open and RA cystectomy SLNB | ICG-99mTc-nanocolloid | Yes | SPECT/CT | Gamma probe Firefly fluorescence imaging, Intuitive Surgical | SPECT/CT 53% Sensitivity NA |
| Van Gennep et al. [16] | 30 | Open and RA cystectomy SLNB | ICG-99mTc-nanocolloid | Yes | SPECT/CT | Gamma probe + drop-in gamma probe Firefly fluorescence imaging, Intuitive Surgical | Sensitivity 85.7% FNR14.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
KleinJan, G.H.; van Gennep, E.J.; Postema, A.W.; van Leeuwen, F.W.B.; Buckle, T. Hybrid Surgical Guidance in Urologic Robotic Oncological Surgery. J. Clin. Med. 2025, 14, 6128. https://doi.org/10.3390/jcm14176128
KleinJan GH, van Gennep EJ, Postema AW, van Leeuwen FWB, Buckle T. Hybrid Surgical Guidance in Urologic Robotic Oncological Surgery. Journal of Clinical Medicine. 2025; 14(17):6128. https://doi.org/10.3390/jcm14176128
Chicago/Turabian StyleKleinJan, Gijs H., Erik J. van Gennep, Arnoud W. Postema, Fijs W. B. van Leeuwen, and Tessa Buckle. 2025. "Hybrid Surgical Guidance in Urologic Robotic Oncological Surgery" Journal of Clinical Medicine 14, no. 17: 6128. https://doi.org/10.3390/jcm14176128
APA StyleKleinJan, G. H., van Gennep, E. J., Postema, A. W., van Leeuwen, F. W. B., & Buckle, T. (2025). Hybrid Surgical Guidance in Urologic Robotic Oncological Surgery. Journal of Clinical Medicine, 14(17), 6128. https://doi.org/10.3390/jcm14176128






