Nonlinear Relationship Between Myeloperoxidase Levels and Helicobacter pylori Infection Risk in Chinese Adults: A Population-Based Cross-Sectional Study
Abstract
1. Introduction
2. Methods
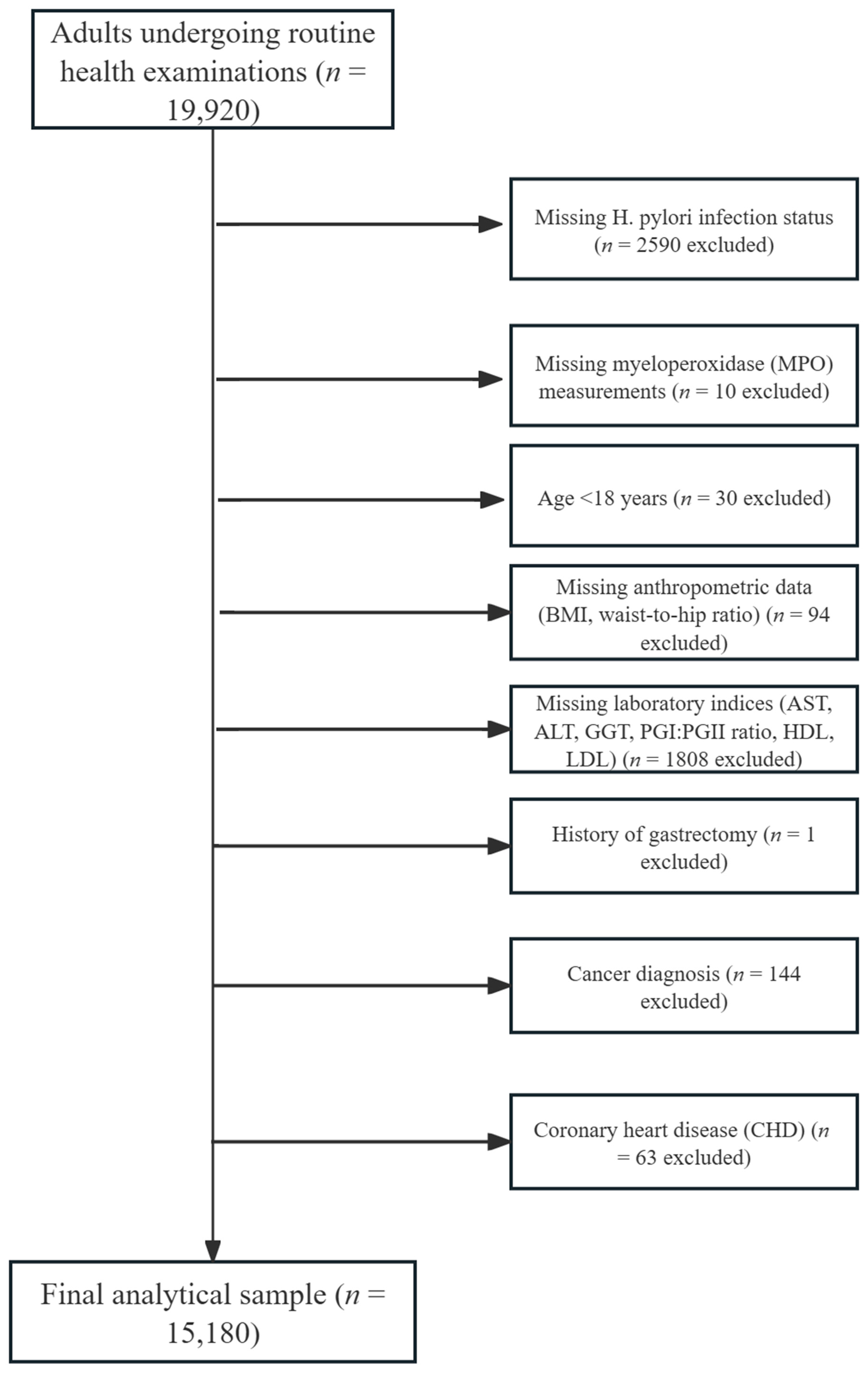
2.1. Study Population
2.2. Demographic and Anthropometric Measurements
2.3. MPO and Other Laboratory Measurement
2.4. H. pylori Infection Detection
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Association Between MPO Levels and H. pylori Infection/DPM Values
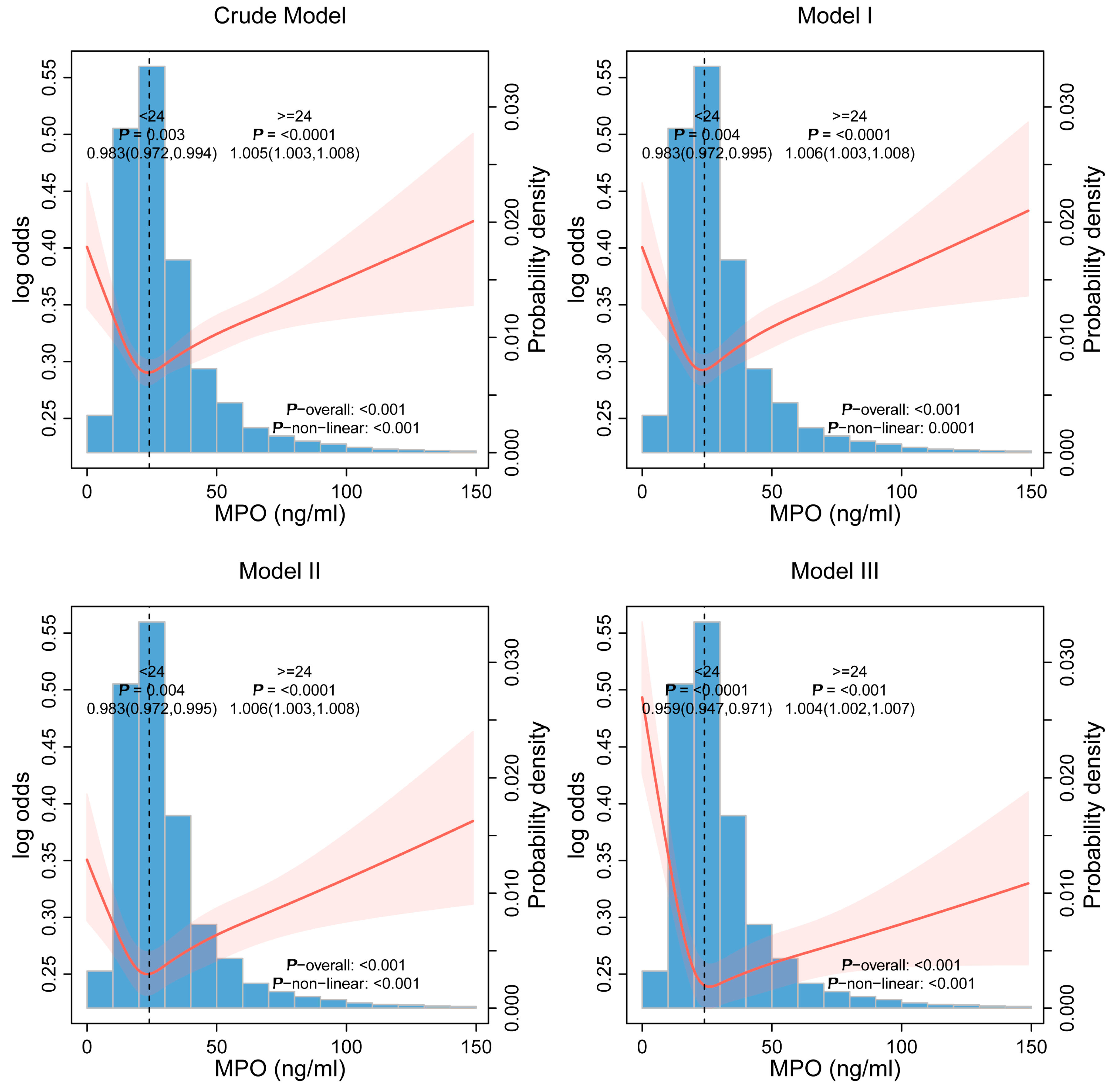
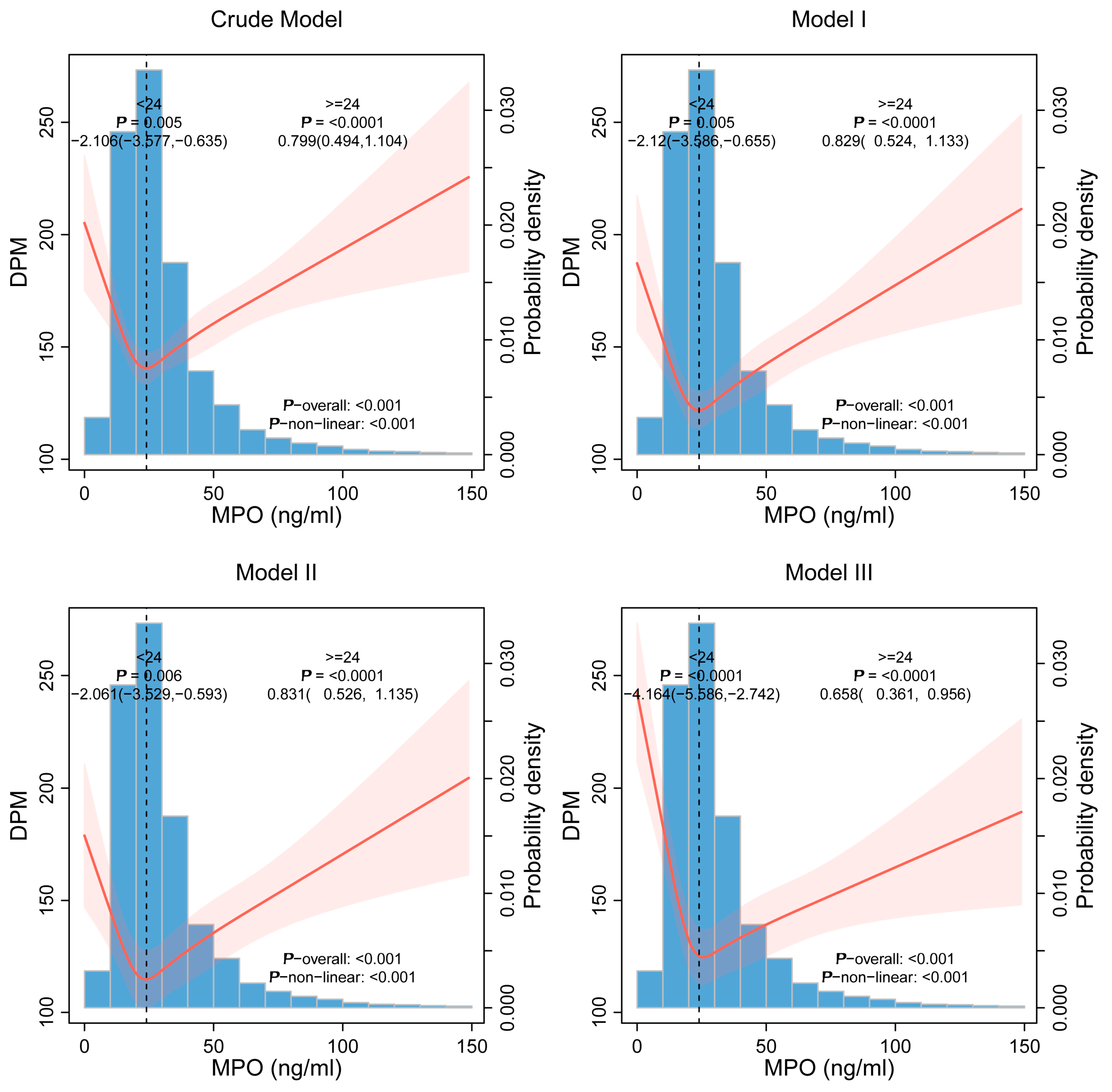
3.3. Nonlinear Threshold Effects
3.4. Subgroup and Interaction Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Shakir, S.M.; Shakir, F.A.; Couturier, M.R. Updates to the Diagnosis and Clinical Management of Helicobacter pylori Infections. Clin. Chem. 2023, 69, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Z.; Lyu, N.H.; Zhu, H.Y.; Cai, Q.C.; Kong, X.Y.; Xie, P.; Zhou, L.Y.; Ding, S.Z.; Li, Z.S.; Du, Y.Q.; et al. Large-scale, national, family-based epidemiological study on Helicobacter pylori infection in China: The time to change practice for related disease prevention. Gut 2023, 72, 855–869. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, Y.; Dou, Y.; Xu, D. Helicobacter pylori and gastric cancer: Mechanisms and new perspectives. J. Hematol. Oncol. 2025, 18, 10. [Google Scholar] [CrossRef]
- Shah, S.C.; Camargo, M.C.; Lamm, M.; Bustamante, R.; Roumie, C.L.; Wilson, O.; Halvorson, A.E.; Greevy, R.; Liu, L.; Gupta, S.; et al. Impact of Helicobacter pylori Infection and Treatment on Colorectal Cancer in a Large, Nationwide Cohort. J. Clin. Oncol. 2024, 42, 1881–1889. [Google Scholar] [CrossRef]
- Yan, L.; Chen, Y.; Chen, F.; Tao, T.; Hu, Z.; Wang, J.; You, J.; Wong, B.C.Y.; Chen, J.; Ye, W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report From a Randomized Controlled Trial With 26.5 Years of Follow-up. Gastroenterology 2022, 163, 154–162.e3. [Google Scholar] [CrossRef]
- Moss, S.F.; Shah, S.C.; Tan, M.C.; El-Serag, H.B. Evolving Concepts in Helicobacter pylori Management. Gastroenterology 2024, 166, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.J.; Du, L.L.; Diao, Y.L.; Wen, J.; Meng, X.B.; Gao, J.; Shao, C.L.; Wang, W.Y.; Zhu, X.Y.; Tang, Y.D. Association of dietary inflammatory index with helicobacter pylori infection and mortality among US population. J. Transl. Med. 2023, 21, 538. [Google Scholar] [CrossRef]
- Qiu, J.; Fang, H.; Liu, D.; Lai, Q.; Xie, J.; Wang, Y.; Chen, S.; Xie, Y. Accelerated biological aging mediates the association between inflammatory markers with Helicobacter pylori infection and mortality. J. Transl. Med. 2025, 23, 174. [Google Scholar] [CrossRef]
- Zhang, Y.; Seeburg, D.P.; Pulli, B.; Wojtkiewicz, G.R.; Bure, L.; Atkinson, W.; Schob, S.; Iwamoto, Y.; Ali, M.; Zhang, W.; et al. Myeloperoxidase Nuclear Imaging for Epileptogenesis. Radiology 2016, 278, 822–830. [Google Scholar] [CrossRef]
- Tao, Y.; Zhao, Y.; Wang, L.; Huang, J.; Chen, Y.; Huang, Q.; Song, B.; Li, H.Y.; Chen, J.; Liu, H. Flexible Amperometric Immunosensor Based on Colloidal Quantum Dots for Detecting the Myeloperoxidase (MPO) Systemic Inflammation Biomarker. Biosensors 2023, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.A.; Liu, Y.C.; Du, S.Y.; Lin, C.W.; Fu, H.W. Helicobacter pylori neutrophil-activating protein promotes myeloperoxidase release from human neutrophils. Biochem. Biophys. Res. Commun. 2008, 377, 52–56. [Google Scholar] [CrossRef]
- Nazligul, Y.; Aslan, M.; Horoz, M.; Celik, Y.; Dulger, A.C.; Celik, H.; Erel, O. The effect on serum myeloperoxidase activity and oxidative status of eradication treatment in patients Helicobacter pylori infected. Clin. Biochem. 2011, 44, 647–649. [Google Scholar] [CrossRef]
- Zhou, J.; Li, R.; Bao, T.; Jiang, W.; Huang, Y. Association between serum 25-hydroxyvitamin d and myeloperoxidase: A cross-sectional study of a general population in China. Front. Nutr. 2022, 9, 948691. [Google Scholar] [CrossRef]
- Bielanski, W.; Konturek, S.J.; Dobrzanska, M.J.; Pytko-Polonczyk, J.; Sito, E.; Marshall, B.J. Microdose 14C-urea breath test in detection of Helicobacter pylori. J. Physiol. Pharmacol. 1996, 47, 91–100. [Google Scholar]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Day, B.J. The science of licking your wounds: Function of oxidants in the innate immune system. Biochem. Pharmacol. 2019, 163, 451–457. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Hanifin, J.; Cline, M.J. Defective bactericidal activity in myeloperoxidase-deficient human neutrophils. Nature 1969, 223, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Motowaki, T.; Tamura, N.; Aratani, Y. Myeloperoxidase deficiency enhances zymosan phagocytosis associated with up-regulation of surface expression of CD11b in mouse neutrophils. Free Radic. Res. 2016, 50, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.D.; Kim, H.H.; Hong, K.W. Inhibitory effect of rebamipide on the neutrophil adherence stimulated by conditioned media from Helicobacter pylori-infected gastric epithelial cells. J. Pharmacol. Exp. Ther. 1999, 288, 133–138. [Google Scholar] [CrossRef]
- Perkins, A.; Tudorica, D.A.; Amieva, M.R.; Remington, S.J.; Guillemin, K. Helicobacter pylori senses bleach (HOCl) as a chemoattractant using a cytosolic chemoreceptor. PLoS Biol. 2019, 17, e3000395. [Google Scholar] [CrossRef]
- Painsipp, E.; Wultsch, T.; Shahbazian, A.; Edelsbrunner, M.; Kreissl, M.C.; Schirbel, A.; Bock, E.; Pabst, M.A.; Thoeringer, C.K.; Huber, H.P.; et al. Experimental gastritis in mice enhances anxiety in a gender-related manner. Neuroscience 2007, 150, 522–536. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, W.; Ye, J.; Sun, L.; Zhou, S.; Zheng, Q.; Shi, Y.; Chen, Y.; Yao, J.; Wang, L.; et al. The crucial role of neutrophil extracellular traps and IL-17 signaling in indomethacin-induced gastric injury in mice. Sci. Rep. 2025, 15, 12109. [Google Scholar] [CrossRef]
- Roumeguere, T.; Noyon, C.; Van Antwerpen, P.; Poelvoorde, P.; Bar, I.; Abdulsater, F.; Rousseau, A.; Delporte, C.; Vanhamme, L.; Vanhaeverbeek, M.; et al. Role of Myeloperoxidase in ROS Generation and Inflammation Response on Prostate Epithelial Cells. Inflammation 2023, 46, 1859–1870. [Google Scholar] [CrossRef]
- Oriuchi, M.; Lee, S.; Uno, K.; Sudo, K.; Kusano, K.; Asano, N.; Hamada, S.; Hatta, W.; Koike, T.; Imatani, A.; et al. Porphyromonas gingivalis Lipopolysaccharide Damages Mucosal Barrier to Promote Gastritis-Associated Carcinogenesis. Dig. Dis. Sci. 2024, 69, 95–111. [Google Scholar] [CrossRef]
- Nishizawa, T.; Suzuki, H.; Nakagawa, I.; Minegishi, Y.; Masaoka, T.; Iwasaki, E.; Hibi, T. Early Helicobacter pylori eradication restores sonic hedgehog expression in the gastric mucosa of Mongolian gerbils. Digestion 2009, 79, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Kamali-Sarvestani, E.; Farsiani, H.; Shamoon Pour, M.; Bazargani, A.; Lankarani, K.; Taghavi, A.R.; Saberifiroozi, M. Association of myeloperoxidase -463 G/A polymorphism with clinical outcome of Helicobacter pylori infection in Iranian patients with gastrointestinal diseases. Iran. J. Immunol. 2007, 4, 155–160. [Google Scholar] [PubMed]
- Saxena, K.; Deshwal, A.; Pudake, R.N.; Jain, U.; Tripathi, R.M. Recent progress in biomarker-based diagnostics of Helicobacter pylori, gastric cancer-causing bacteria. Biomark. Med. 2023, 17, 679–691. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 15,180) | T1 <= 20.6 (n = 5061) | T2, (20.6, 31] (n = 5059) | T3, >=31 (n = 5060) | p Value | |
|---|---|---|---|---|---|
| Age | 45.66 ± 11.51 | 46.65 ± 10.98 | 46.01 ± 11.50 | 44.31 ± 11.92 | <0.0001 |
| Sex | <0.001 | ||||
| Female | 7223 (47.58) | 2309 (45.42) | 2417 (47.92) | 2497 (49.43) | |
| Male | 7957 (52.42) | 2775 (54.58) | 2627 (52.08) | 2555 (50.57) | |
| BMI (kg/m2) | 0.01 | ||||
| <24 | 5077 (33.45) | 1705 (33.54) | 1761(34.91) | 1611 (31.89) | |
| [24, 28) | 8709 (57.37) | 2936 (57.75) | 2821(55.93) | 2952 (58.43) | |
| >=28 | 1394 (9.18) | 443 (8.71) | 462(9.16) | 489 (9.68) | |
| Waist-to-hip ratio | 0.85 ± 0.08 | 0.85 ± 0.08 | 0.85 ± 0.08 | 0.84 ± 0.08 | <0.0001 |
| Smoke | 0.07 | ||||
| Current | 3546 (23.36) | 1219 (23.98) | 1157 (22.94) | 1170 (23.16) | |
| Former | 667 (4.39) | 249 (4.90) | 222 (4.40) | 196 (3.88) | |
| Never | 10,967 (72.25) | 3616 (71.13) | 3665 (72.66) | 3686 (72.96) | |
| Drink | <0.0001 | ||||
| Current | 6713 (44.22) | 2398 (47.17) | 2170 (43.02) | 2145 (42.46) | |
| Former | 125 (0.82) | 36 (0.71) | 46 (0.91) | 43 (0.85) | |
| Never | 8342 (54.95) | 2650 (52.12) | 2828 (56.07) | 2864 (56.69) | |
| Hypertension | 0.21 | ||||
| No | 14,204 (93.57) | 4766 (93.75) | 4695 (93.08) | 4743 (93.88) | |
| Yes | 976 (6.43) | 318 (6.25) | 349 (6.92) | 309 (6.12) | |
| Diabetes | <0.001 | ||||
| No | 14,833 (97.71) | 4935 (97.07) | 4934 (97.82) | 4964 (98.26) | |
| Yes | 347 (2.29) | 149 (2.93) | 110 (2.18) | 88 (1.74) | |
| Hyperlipidemia | 0.04 | ||||
| No | 14,944 (98.45) | 4991 (98.17) | 4962 (98.37) | 4991 (98.79) | |
| Yes | 236 (1.55) | 93 (1.83) | 82 (1.63) | 61 (1.21) | |
| AST(U/L) | 21.00 (17.00, 26.00) | 21.00 (18.00, 25.00) | 21.00 (17.00, 26.00) | 21.00 (17.00, 25.00) | 0.64 |
| ALT (U/L) | 20.00 (14.00, 30.00) | 20.00 (14.00, 30.00) | 20.00 (14.00, 30.00) | 19.00 (14.00, 30.00) | 0.28 |
| GGT (U/L) | 22.00 (14.00, 38.00) | 22.00 (14.00, 38.00) | 22.00 (14.00, 37.00) | 22.00 (14.00, 37.00) | 0.10 |
| PGI/PGII | 8.04 ± 2.90 | 8.49 ± 2.91 | 7.85 ± 2.78 | 7.77 ± 2.97 | <0.0001 |
| HDL-C (mmol/L) | 1.51 ± 0.42 | 1.50 ± 0.42 | 1.51 ± 0.41 | 1.53 ± 0.42 | <0.01 |
| LDL-C (mmol/L) | 2.96 ± 0.80 | 3.02 ± 0.81 | 2.97 ± 0.80 | 2.90 ± 0.79 | <0.0001 |
| WBC (×109/L) | 5.82 ± 1.56 | 5.50 ± 1.36 | 5.73 ± 1.43 | 6.23 ± 1.78 | <0.0001 |
| Platelet (×109/L) | 205.09 ± 60.06 | 198.72 ± 58.75 | 204.46 ± 59.06 | 212.13 ± 61.60 | <0.0001 |
| Lymphocyte (×109/L) | 1.63 ± 0.59 | 1.53 ± 0.57 | 1.65 ± 0.58 | 1.70 ± 0.60 | <0.0001 |
| Neutrophil (×109/L) | 3.48 ± 1.23 | 3.22 ± 1.04 | 3.41 ± 1.10 | 3.82 ± 1.44 | <0.0001 |
| Monocyte (×109/L) | 0.35 ± 0.12 | 0.32 ± 0.11 | 0.35 ± 0.12 | 0.38 ± 0.13 | <0.0001 |
| MPO (ng/mL) | 31.31 ± 26.98 | 15.19 ± 3.59 | 25.32 ± 2.90 | 53.51 ± 37.10 | <0.0001 |
| DPM | 35.00 (0.00, 150.00) | 40.00 (0.00, 160.00) | 25.00 (0.00, 130.00) | 35.00 (0.00, 170.00) | <0.0001 |
| H. pylori infection | <0.001 | ||||
| No | 10,481 (69.04) | 3470 (68.25) | 3590 (71.17) | 3421 (67.72) | |
| Yes | 4699 (30.96) | 1614 (31.75) | 1454 (28.83) | 1631 (32.28) |
| T2, (20.6, 31] | T1 <= 20.6 | T3, >=31 | |||
|---|---|---|---|---|---|
| H. pylori infection | OR (95% CI) | p value | OR (95% CI) | p value | |
| crude model | ref | 1.15 (1.06, 1.25) | 0.001 | 1.18 (1.08, 1.28) | <0.001 |
| Model 1 | ref | 1.14 (1.05, 1.24) | 0.002 | 1.19 (1.10, 1.30) | <0.0001 |
| Model 2 | ref | 1.14 (1.05, 1.24) | 0.002 | 1.19 (1.10, 1.30) | <0.0001 |
| Model 3 | ref | 1.36 (1.24, 1.49) | <0.0001 | 1.12 (1.02, 1.22) | 0.02 |
| DPM | β (95% CI) | p value | β (95% CI) | p value | |
| crude model | ref | 22.45 (11.63, 33.27) | <0.0001 | 24.75 (13.91, 35.59) | <0.0001 |
| Model 1 | ref | 22.87 (12.07, 33.67) | <0.0001 | 25.61 (14.78, 36.44) | <0.0001 |
| Model 2 | ref | 22.41 (11.60, 33.22) | <0.0001 | 25.67 (14.84, 36.50) | <0.0001 |
| Model 3 | ref | 37.1 (26.66, 47.53) | <0.0001 | 19.27 (8.81, 29.72) | <0.001 |
| Crude Model | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| OR (95% CI) p Value | OR (95% CI) p Value | OR (95% CI) p Value | OR (95% CI) p Value | |
| two-piecewise linear regression | ||||
| MPO < 24 | 0.983 (0.972, 0.994) 0.003 | 0.983 (0.972, 0.995) 0.004 | 0.983 (0.972, 0.995) 0.004 | 0.959 (0.947, 0.971) <0.0001 |
| MPO ≥ 24 | 1.005 (1.003, 1.008) <0.0001 | 1.006 (1.003, 1.008) <0.0001 | 1.006 (1.003, 1.008) <0.0001 | 1.004 (1.002, 1.007) <0.001 |
| p for Log-likelihood ratio | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Crude Model | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| β (95% CI) p Value | β (95% CI) p Value | β (95% CI) p Value | β (95% CI) p Value | |
| two-piecewise linear regression | ||||
| MPO < 24 | −2.106 (−3.577, −0.635) 0.005 | −2.12 (−3.586, −0.655) 0.005 | −2.061 (−3.529, −0.593) 0.006 | −4.164 (−5.586, −2.742) <0.0001 |
| MPO ≥ 24 | 0.799 (0.494, 1.104) <0.0001 | 0.829 (0.524, 1.133) <0.0001 | 0.831 (0.526,1.135) <0.0001 | 0.658 (0.361, 0.956) <0.0001 |
| p for Log-likelihood ratio | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| T2, (20.6, 31] | T1, <=20.6 | T3, >=31 | p for Interaction | |
|---|---|---|---|---|
| Age (years) | 0.195 | |||
| >=45 | ref | 1.197 (1.072, 1.336) | 1.097 (0.976, 1.233) | |
| <45 | ref | 1.108 (0.967, 1.269) | 1.237 (1.087, 1.407) | |
| Sex | 0.694 | |||
| Male | ref | 1.197 (1.066, 1.346) | 1.165 (1.033, 1.314) | |
| Female | ref | 1.114 (0.983, 1.263) | 1.151 (1.017, 1.303) | |
| BMI (kg/m2) | 0.513 | |||
| <24 | ref | 1.107 (0.987, 1.241) | 1.135 (1.011, 1.273) | |
| [24, 28) | ref | 1.257 (1.087, 1.454) | 1.248 (1.076, 1.448) | |
| >=28 | ref | 1.115 (0.844, 1.474) | 1.007 (0.764, 1.328) | |
| Smoke | 0.223 | |||
| Current | ref | 1.091 (0.918, 1.296) | 1.144 (0.959, 1.363) | |
| Never | ref | 1.165 (1.052, 1.289) | 1.130 (1.020, 1.251) | |
| Former | ref | 1.547 (1.027, 2.346) | 1.880 (1.228, 2.892) | |
| Drink | 0.195 | |||
| Current | ref | 1.198 (1.057, 1.359) | 1.146 (1.006, 1.306) | |
| Never | ref | 1.117 (0.993, 1.256) | 1.152 (1.026, 1.294) | |
| Former | ref | 2.320 (0.762, 7.343) | 4.074 (1.377, 13.022) | |
| Hypertension | 0.056 | |||
| No | ref | 1.163 (1.065, 1.271) | 1.185 (1.084, 1.296) | |
| Yes | ref | 1.116 (0.806, 1.546) | 0.839 (0.597, 1.177) | |
| Diabetes | 0.375 | |||
| No | ref | 1.171 (1.074, 1.277) | 1.163 (1.066, 1.269) | |
| Yes | ref | 0.805 (0.471, 1.377) | 0.998 (0.541, 1.838) | |
| Hyperlipidemia | 0.36 | |||
| No | ref | 1.163 (1.067, 1.268) | 1.166 (1.069, 1.272) | |
| Yes | ref | 1.012 (0.502, 2.053) | 0.592 (0.254, 1.327) |
| T2, (20.6, 31] | T1, <=20.6 | T3, >=31 | p for Interaction | |
|---|---|---|---|---|
| Age (years) | 0.182 | |||
| >=45 | ref | 33.123 (18.761, 47.484) | 24.826 (9.597, 40.055) | |
| <45 | ref | 11.202 (−5.376, 27.781) | 20.864 (5.010, 36.719) | |
| Sex | 0.678 | |||
| Male | ref | 20.562 (8.087, 33.036) | 19.182 (6.337, 32.027) | |
| Female | ref | 27.016 (8.711, 45.321) | 26.613 (8.417, 44.808) | |
| BMI (kg/m2) | 0.53 | |||
| <24 | ref | 21.492 (6.172, 36.813) | 24.471 (8.973, 39.970) | |
| [24, 28) | ref | 29.142 (11.707, 46.576) | 27.133 (9.311, 44.955) | |
| >=28 | ref | 16.546 (−12.135, 45.227) | −1.679 (−29.876, 26.517) | |
| Smoke | 0.312 | |||
| Current | ref | 6.499 (−12.034, 25.033) | 14.377 (−4.579, 33.333) | |
| Never | ref | 27.181 (13.625, 40.738) | 23.327 (9.686, 36.968) | |
| Former | ref | 61.504 (19.426, 103.582) | 59.337 (14.643, 104.031) | |
| Drink | 0.456 | |||
| Current | ref | 17.424 (3.110, 31.739) | 17.417 (2.557, 32.277) | |
| Never | ref | 29.064 (13.027, 45.102) | 26.298 (10.371, 42.226) | |
| Former | ref | 18.875 (−71.539, 109.288) | 99.605 (8.929, 190.281) | |
| Hypertension | 0.252 | |||
| No | ref | 23.95 (12.667, 35.233) | 24.594 (13.163, 36.026) | |
| Yes | ref | 24.185 (−15.064, 63.433) | −4.765 (−44.505, 34.974) | |
| Diabetes | 0.822 | |||
| No | ref | 24.392 (13.401, 35.382) | 23.039 (11.939, 34.138) | |
| Yes | ref | 14.365 (−54.467, 83.197) | 14.451 (−65.562, 94.464) | |
| Hyperlipidemia | 0.892 | |||
| No | ref | 24.23 (13.259, 35.200) | 23.108 (12.013, 34.203) | |
| Yes | ref | 15.5 (−54.624, 85.623) | −15.091 (−93.198, 63.017) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Kong, Q.; Liu, X.; Huang, Y. Nonlinear Relationship Between Myeloperoxidase Levels and Helicobacter pylori Infection Risk in Chinese Adults: A Population-Based Cross-Sectional Study. J. Clin. Med. 2025, 14, 6019. https://doi.org/10.3390/jcm14176019
Zhou J, Kong Q, Liu X, Huang Y. Nonlinear Relationship Between Myeloperoxidase Levels and Helicobacter pylori Infection Risk in Chinese Adults: A Population-Based Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(17):6019. https://doi.org/10.3390/jcm14176019
Chicago/Turabian StyleZhou, Junteng, Qihang Kong, Xiaojing Liu, and Yan Huang. 2025. "Nonlinear Relationship Between Myeloperoxidase Levels and Helicobacter pylori Infection Risk in Chinese Adults: A Population-Based Cross-Sectional Study" Journal of Clinical Medicine 14, no. 17: 6019. https://doi.org/10.3390/jcm14176019
APA StyleZhou, J., Kong, Q., Liu, X., & Huang, Y. (2025). Nonlinear Relationship Between Myeloperoxidase Levels and Helicobacter pylori Infection Risk in Chinese Adults: A Population-Based Cross-Sectional Study. Journal of Clinical Medicine, 14(17), 6019. https://doi.org/10.3390/jcm14176019






