Ischaemic Stroke in Patients with Known Atrial Fibrillation: A Snapshot from a Large University Hospital Experience
Abstract
1. Introduction
- (1)
- under-prescription, particularly among the elderly even without clear contraindication
- (2)
- suboptimal management, i.e., difficulties in keeping INR in range for patients treated with VKA or wrong dosage/poor compliance for patients treated with DOAC
- (3)
- a failure of OAT (i.e., patients facing ischaemic stroke due to AF despite an adequate conducted anticoagulant therapy), is a well-recognized phenomenon in the literature. This condition has been referred to as ‘resistant stroke’ (RS) [5], and in this study we will adopt this term to describe such cases. The mechanisms underlying RS remain poorly understood, and consequently, secondary prevention in these patients is largely empirical.
- To describe the primary and secondary prevention strategies undertaken in patients with known AF and acute ischaemic cerebral event.
- To estimate the percentages of RS and its management.
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
- –
- demographic variables: age and sex
- –
- cardiovascular risk factors: history of hypertension, diabetes, smoking habit, dyslipidaemia, history of heart failure
- –
- previous cerebrovascular events: TIA, ischaemic or haemorrhagic stroke
- –
- echocardiographic data: ejection fraction, left atrial enlargement, valve defects and relative grade, presence of prosthetic heart valves. Ejection fraction was considered normal when ≥50%; left atrial enlargement was dichotomised as yes or no and was defined as an increase of diameter or area or volume, valve defects were indicated as stenosis (mild, moderate or severe), insufficiency or combined
- –
- cervical and intracranial vessels hemodynamic stenosis assessed by CT angiography or doppler ultrasonography
- –
- site and size of ischaemic cerebral lesion on cerebral CT scan and/or cerebral MRI
- –
- etiopathogenesis of ischaemic cerebrovascular events was classified according to TOAST criteria [6]. Embolic stroke, after exclusion of alternative etiologies, were classified as cardioembolic based on the known AF. When more than one potential embolic source was identified, the stroke etiology was classified as “undetermined”. Etiology classification was performed by expert vascular neurologists.
- –
- CHA2DS2-VASc and HAS-BLED score for evaluating thromboembolic and haemorrhagic risk respectively [7]
- –
- Pre-event disability assessed by modified Rankin Scale (mRS)
- –
- Type of preventive therapy on admission, and at discharge:
- (1)
- OAT: VKA or DOAC
- (2)
- Left Atrial Appendage Occlusion (LAAO)
- (3)
- No preventive therapy
2.3. Definition of Adequate OAT
- -
- For patients on VKA, therapy was considered adequate when the INR at hospital admission was ≥1.7, which corresponds to the internationally accepted cut-off for eligibility to systemic thrombolysis [8].
- -
- For patients on DOAC, adequacy was defined when both the indication and dosage were in accordance with the current European guidelines [9] and when good adherence was either reported by the patient/caregiver or confirmed by plasmatic drug level measurement. When information about compliance was unavailable, patients were classified in an “uncertain” group.
2.4. Definition of RS
2.5. Follow-Up
- –
- Adoption of secondary prevention therapy for cardioembolic stroke recommended at hospital discharge
- –
- Recurrence of ischaemic or haemorrhagic stroke
- –
- Death and its cause.
3. Statistical Analysis
4. Results
4.1. Population Characteristics
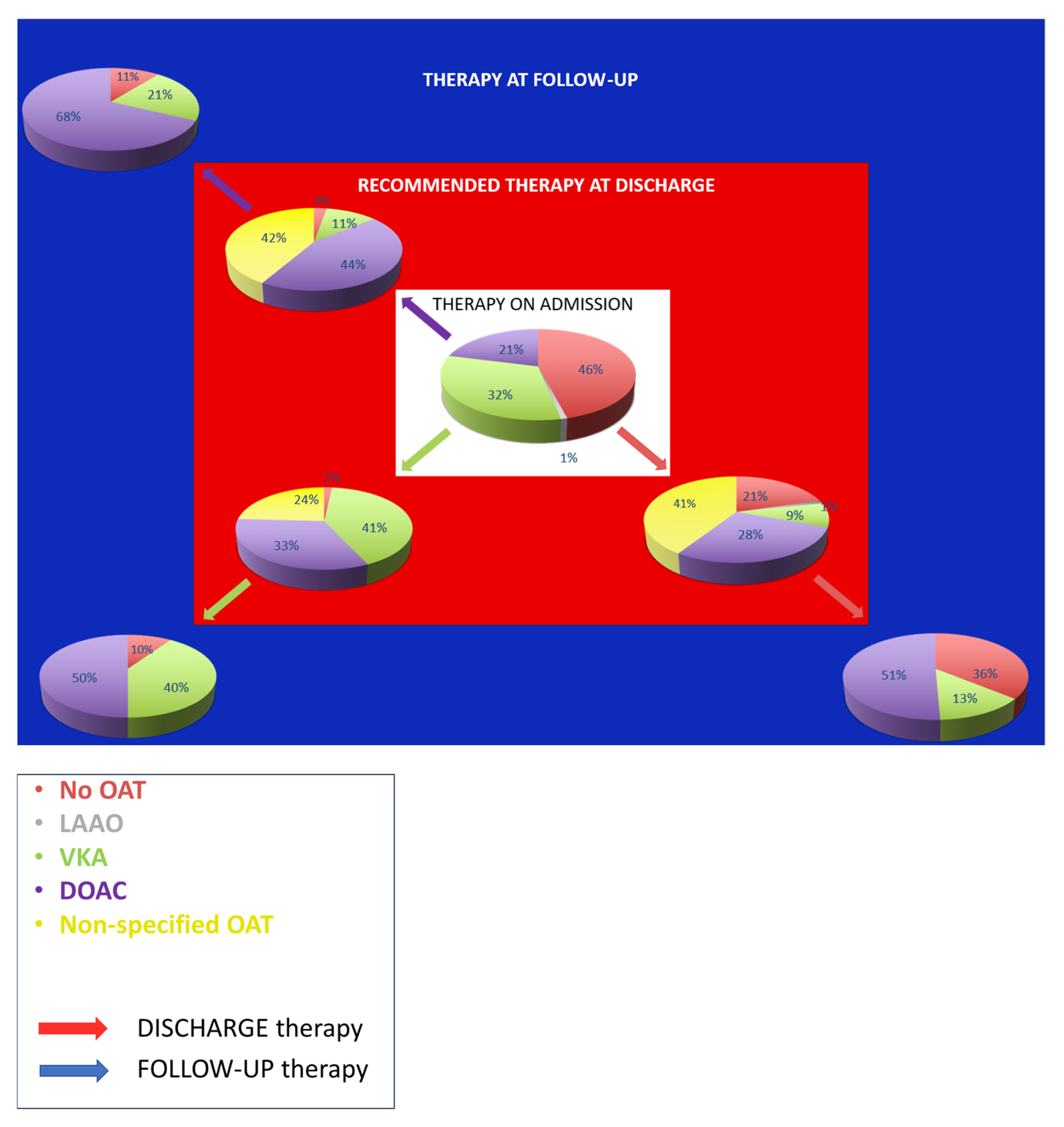
4.2. Preventive Therapy at Admission
4.3. Secondary Prevention Therapy at Discharge and Follow-Up
4.4. In-Hospital Outcomes
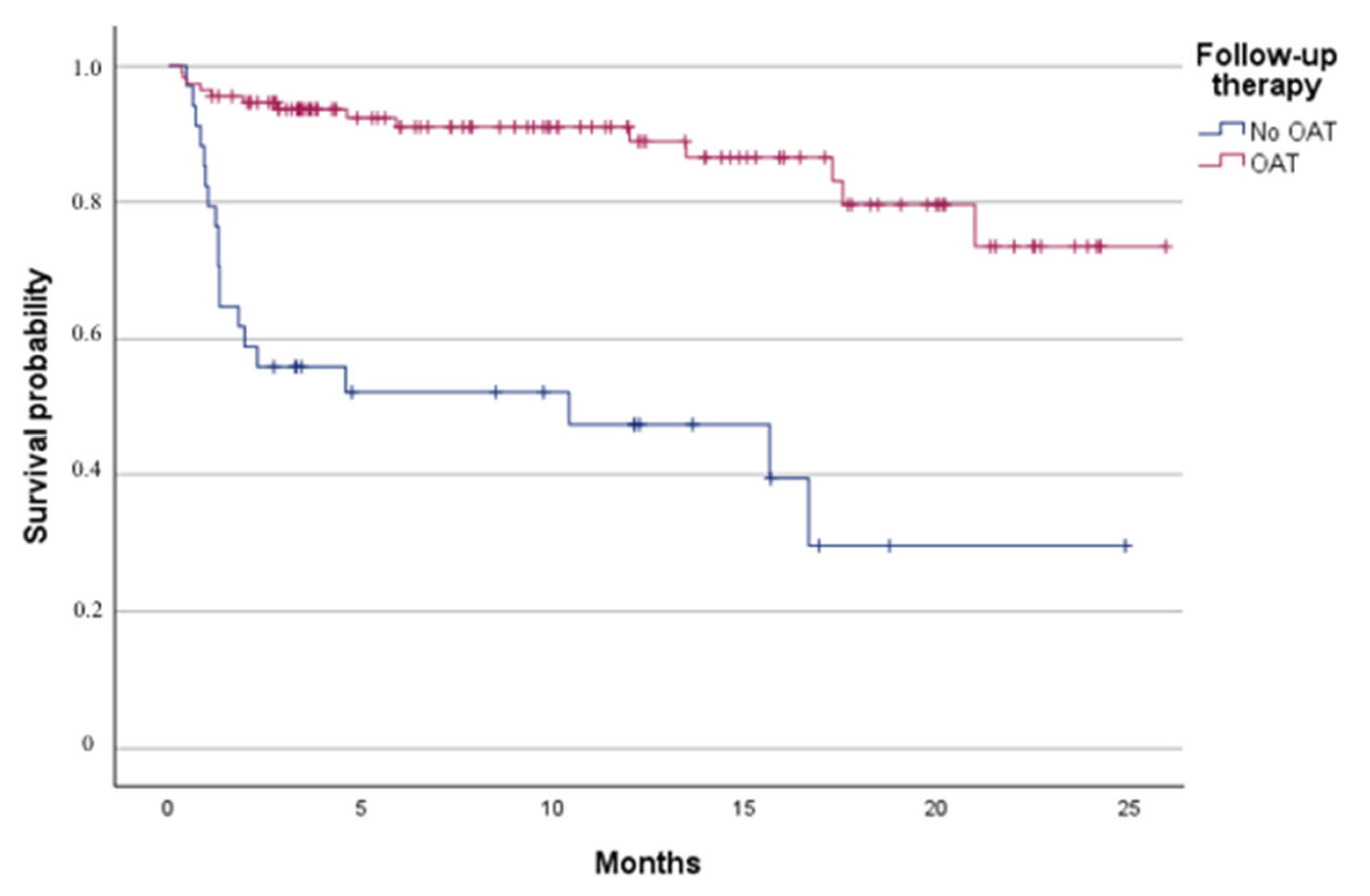
4.5. Follow-Up Outcomes
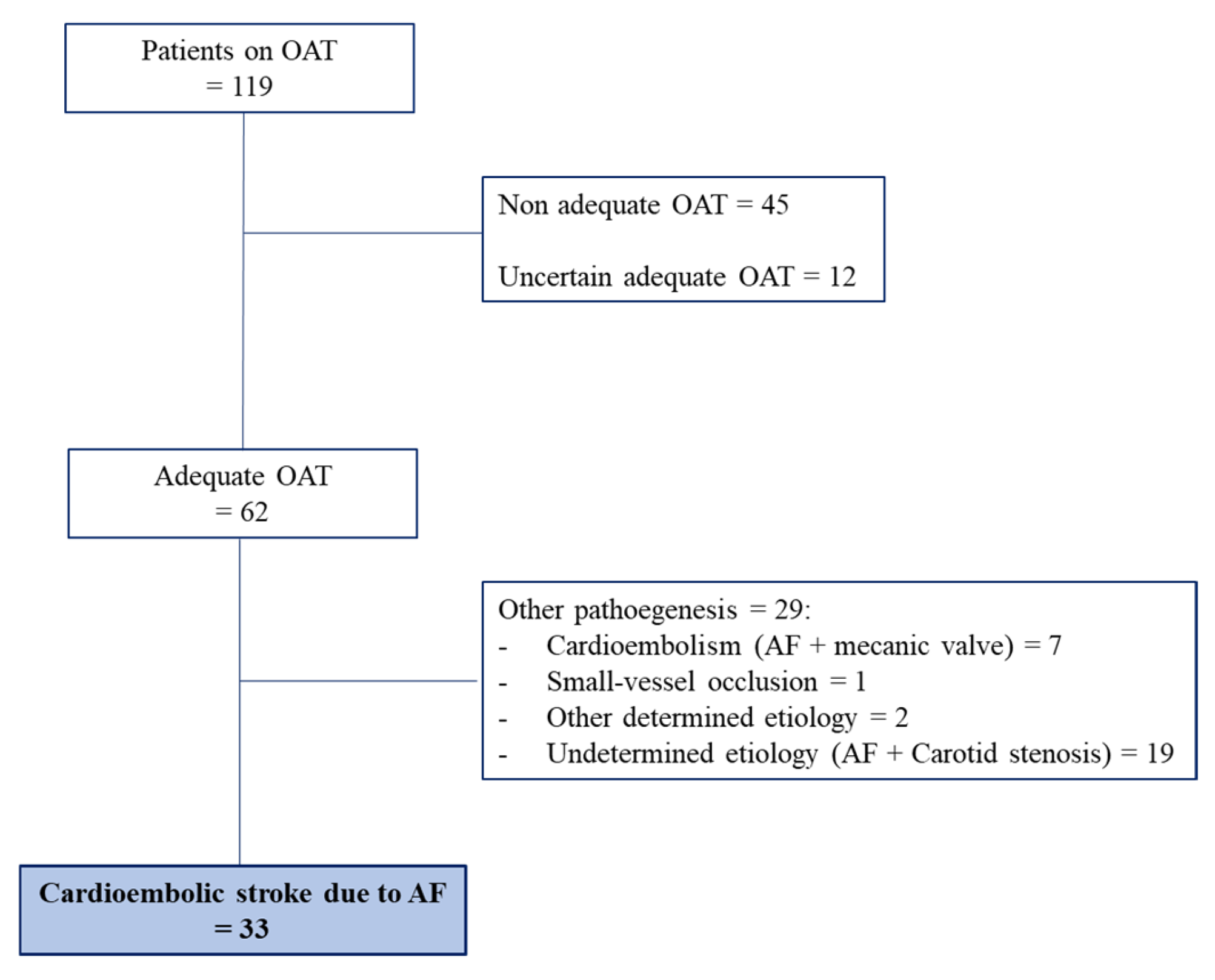
4.6. Adequacy of OAT and Resistant Stroke
5. Discussion
6. Conclusions
- (1)
- the suboptimal adoption of international recommendations regarding OAT and the challenges clinicians faces in balancing its risks and benefits, underscoring the need for evaluation in specific centres. This is particularly evident in elderly patients, where both under prescription and inappropriate underdosing remain common, often driven by an overestimation of bleeding risk. A multidisciplinary approach involving neurologists, cardiologists, geriatricians, and primary care physicians may further enhance outcomes in this population.
- (2)
- RS is not an uncommon condition with an as-yet-unknown mechanism, hence leading to an empirical therapeutic approach. Further research should focus on systematic etiological workups in RS, including LAA morphology. Such approaches could help identify RS patients at higher residual risk and guide more tailored preventive strategies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AF | Atrial Fibrillation |
| DOAC | Direct Oral Anticoagulant |
| IS | Ischemic Stroke |
| LAA | Left Atrial Appendage |
| LAAO | Left Atrial Appendage Occlusion |
| OAT | Oral Anticoagulant Therapy |
| RS | Resistant Stroke |
| TIA | Transient Ischemic Attack |
| VKA | Vitamin K Antagonist |
References
- Seiffge, D.J.; Cancelloni, V.; Räber, L.; Paciaroni, M.; Metzner, A.; Kirchhof, P.; Fischer, U.; Werring, D.J.; Shoamanesh, A.; Caso, V. Secondary stroke prevention in people with atrial fibrillation: Treatments and trials. Lancet Neurol. 2024, 23, 404–417. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial Fibrillation as an Independent Risk Factor for Stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef]
- Suomalainen, O.P.; Martinez-Majander, N.; Broman, J.; Mannismäki, L.; Aro, A.; Curtze, S.; Pakarinen, S.; Lehto, M.; Putaala, J. Stroke in Patients with Atrial Fibrillation: Epidemiology, Screening, and Prognosis. J. Clin. Med. 2023, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Developed by the task force for the management of atrial fibrillation of the European Society of C. Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Cruz-González, I.; González-Ferreiro, R.; Freixa, X.; Gafoor, S.; Shakir, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; et al. Left atrial appendage occlusion for stroke despite oral anticoagulation (resistant stroke). Results from the Amplatzer Cardiac Plug registry. Rev. Espanola Cardiol. (Engl. Ed.) 2020, 73, 28–34. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of Subtype of Acute Ischemic Stroke. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Lip, G.Y. Implications of the CHA 2 DS 2 -VASc and HAS-BLED Scores for Thromboprophylaxis in Atrial Fibrillation. Am. J. Med. 2011, 124, 111–114. [Google Scholar] [CrossRef]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, I–LXII. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Seiffge, D.J.; De Marchis, G.M.; Koga, M.; Paciaroni, M.; Wilson, D.; Cappellari, M.; Macha, K.; Tsivgoulis, G.; Ambler, G.; Arihiro, S.; et al. Ischemic Stroke despite Oral Anticoagulant Therapy in Patients with Atrial Fibrillation. Ann. Neurol. 2020, 87, 677–687. [Google Scholar] [CrossRef]
- Díez-Villanueva, P.; Cosín-Sales, J.; Roldán-Schilling, V.; Barrios, V.; Riba-Artés, D.; Gavín-Sebastián, O. Use of Direct Acting Oral Anticoagulants in Elderly Patients with Atrial Fibrillation: A Multicenter, Cross-Sectional Study in Spain. J. Clin. Med. 2023, 12, 1224. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack; A guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, E364–E467. [Google Scholar] [CrossRef]
- Song, D.; Xu, D.; Zhang, K.; Lou, Y.; Du, Y.; An, Y.; Badr, A.; Rigamonti, D.; Cistola, D.; Yan, D.; et al. Stroke Mortality Risk Factors: Global Trends and Regional Variations (1990–2021). J. Am. Heart Assoc. 2025, 14, e042107. [Google Scholar] [CrossRef]
- Paciaroni, M.; Agnelli, G.; Caso, V.; Silvestrelli, G.; Seiffge, D.J.; Engelter, S.; De Marchis, G.M.; Polymeris, A.; Zedde, M.L.; Yaghi, S.; et al. Causes and Risk Factors of Cerebral Ischemic Events in Patients with Atrial Fibrillation Treated with Non–Vitamin K Antagonist Oral Anticoagulants for Stroke Prevention: The RENo Study. Stroke 2019, 50, 2168–2174. [Google Scholar] [CrossRef]
- Klijn, C.J.; Paciaroni, M.; Berge, E.; Korompoki, E.; Kõrv, J.; Lal, A.; Putaala, J.; Werring, D.J. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur. Stroke J. 2019, 4, 198–223. [Google Scholar] [CrossRef] [PubMed]
- Paciaroni, M.; Caso, V.; Agnelli, G.; Mosconi, M.G.; Giustozzi, M.; Seiffge, D.J.; Engelter, S.T.; Lyrer, P.; Polymeris, A.A.; Kriemler, L.; et al. Recurrent Ischemic Stroke and Bleeding in Patients with Atrial Fibrillation Who Suffered an Acute Stroke While on Treatment with Nonvitamin K Antagonist Oral Anticoagulants: The RENO-EXTEND Study. Stroke 2022, 53, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Polymeris, A.A.; Meinel, T.R.; Oehler, H.; Hölscher, K.; Zietz, A.; Scheitz, J.F.; Nolte, C.H.; Stretz, C.; Yaghi, S.; Stoll, S.; et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J. Neurol. Neurosurg. Psychiatry 2022, 93, 588–598. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Wang, Y.; DI Biase, L.; Horton, R.P.; Nguyen, T.; Morhanty, P.; Natale, A. Left atrial appendage studied by computed tomography to help planning for appendage closure device placement. J. Cardiovasc. Electrophysiol. 2010, 21, 973–982. [Google Scholar] [CrossRef]
- Lee, J.M.; Seo, J.; Uhm, J.; Kim, Y.J.; Lee, H.; Kim, J.; Sung, J.; Pak, H.; Lee, M.; Joung, B. Why is left atrial appendage morphology related to strokes? an analysis of the flow velocity and orifice size of the left atrial appendage. J. Cardiovasc. Electrophysiol. 2015, 26, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, L.; Santangeli, P.; Anselmino, M.; Mohanty, P.; Salvetti, I.; Gili, S.; Horton, R.; Sanchez, J.E.; Bai, R.; Mohanty, S.; et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J. Am. Coll. Cardiol. 2012, 60, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2020, 16, 217–221. [Google Scholar] [CrossRef]
| OAT | ||||
|---|---|---|---|---|
| n = 226 | Yes (n = 119) | No (n = 103) | p | |
| Age, years (median, IQR) | 84.04 (77.9–88.6) | 82.9 (77.3–87.6) | 85.3 (78.1–90.0) | 0.077 |
| Sex (F) | 128/226 (61%) | 72/119 (60.5%) | 64/103 (62.1%) | 0.803 |
| Hypertension | 172/224 (76.7%) | 31/118 (26.3%) | 20/103 (19.4%) | 0.228 |
| Diabetes | 46/223 (20,6%) | 26/117 (22.2%) | 20/103 (19.4%) | 0.610 |
| Dyslipidaemia | 66/152 (43.4%) | 33/80 (41.3%) | 33/70 (47.1%) | 0.468 |
| Actual smoking habit | 10/158 (6.3%) | 3/86 (3.5%) | 7/71 (9.9%) | 0.104 |
| Previous smoking habit | 62/158 (39.2%) | 40/86 (46.5%) | 32/71 (45.1%) | 0.875 |
| Previous ischaemic stroke | 41/220 (18.6%) | 16/62 (25.8%) | 7/44 (15.9%) | 0.223 |
| Previous haemorrhagic stroke | 14/221 (6.3%) | 3/62 (4.8%) | 1/44 (2.3%) | 0.495 |
| Heart failure | 63/219 (28.8%) | 21/62 (33.9%) | 16/44 (36.4%) | 0.791 |
| CHA2DS2-VASc (median, IQR) | 4.5 (3–6) | 4 (3–6) | 5 (3–6) | 0.774 |
| HAS-BLED (median, IQR) | 3 (2–3) | 3 (2–3) | 2 (2–3) | 0.007 |
| mRS pre-stroke (median, IQR) | 2 (0–3) | 1 (0–3) | 2 (0–3) | 0.794 |
| Ischaemic cerebrovascular event TIA Ischaemic Stroke | 8/226 (3.5%) 218/226 (96.5%) | 3/229 (2.5%) 116/119 (97.5%) | 5/103 (4.9%) 98/103 (97.5%) | 0.352 |
| TOAST Cardioembolic Small-artery occlusion Other determined cause Indeterminate (AF + atherothrombosis) | 159/226 5/226 (2.2%) 2/226 (0.8%) 60/226 (26.5%) | 74/119 (62.2%) 3/119 (2.5%) 9/119 (7.6%) 33/119 (27.7%) | 74/103 (71.8%) 2/103 (1.9%) 0/103 (0.0%) 27/103 (26.2%) | 0.034 |
| NIHSS on admission (median, IQR) | 15 (6–21) | 15.5 (6–21) | 13.5 (5–21) | 0.271 |
| Systemic thrombolysis | 49/216 (22.7%) | 1/59 (1.7%) | 12/43 (27.9%) | <0.001 |
| Mechanical thrombectomy | 78/216 (36.1%) | 27/59 (45.8%) | 13/43 (30.2%) | 0.113 |
| Death During Follow-Up | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Yes (n = 57) | No (n = 125) | OR (95% CI) | p | OR (95% CI) | p | |
| Age, years (median, IQR) | 88.1 (82.3–90.8) | 86.2 (74.4–90.5) | 1.13 (1.07–1.19) | <0.001 | 1.22 (1.10–1.36) | <0.001 |
| Sex (F) | 40.4% (23/57) | 42.4% (53/125) | 1.09 (0.58–2.06) | 0.795 | ||
| NIHSS on admission | 18 (10–22) | 9 (3.5–17.5) | 1.09 (1.04–1.14) | <0.001 | 1.13 (1.06–1.21) | <0.001 |
| Hypertension | 84.2% (48/57) | 73.6% (92/125) | 1.91 (0.85–4.32) | 0.119 | ||
| Diabetes | 29.8% (17/57) | 16.8% (21/125) | 2.11 (1.01–4.39) | 0.048 | 5.72 (1.39–23.57) | 0.016 |
| Dyslipidaemia | 31.6% (12/38) | 47.8% (44/92) | 0.50 (0.23–1.12) | 0.092 | ||
| mRS pre-stroke | 2 (0–4) | 0 (0–3) | 1.33 (1.04–1.68) | 0.022 | 0.96 (0.67–1.39) | 0.840 |
| CHA2-DS2-VASC | 5 (4–6) | 4 (3–6) | 1.40 (1.31–1.74) | 0.002 | 1.03 (0.65–1.64) | 0.891 |
| HAS-BLED | 3 (2–3) | 2 (2–3) | 1.60 (1.12–2.29) | 0.010 | 1.88 (0.88–3.96) | 0.103 |
| Adequate OAT | |||
|---|---|---|---|
| Yes (n = 62) | No (n = 45) | p | |
| Age, years (median, IQR) | 82.2 (73.9–87.0) | 84.1 (79.2–88.4) | 0.118 |
| Sex (F) | 23/62 (37.1%) | 21/45 (21%) | 0.321 |
| Hypertension | 44/62 (72.6%) | 32/44 (72.7%) | 0.987 |
| Diabetes | 14/62 (22.6%) | 8/44 (18.2%) | 0.582 |
| Dyslipidaemia | 23/43 (53.5%) | 8/33 (24.2%) | 0.010 |
| Actual smoking habit | 2/42 (4.8%) | 1/36 (2.8%) | 0.650 |
| Previous smoking habit | 23/42 (54.8%) | 16/36 (44.4%) | 0.364 |
| Obesity | 4/27 (14.8%) | 4/13 (30.8%) | 0.237 |
| Previous ischaemic stroke | 16/62 (25.8%) | 7/44 (15.9%) | 0.223 |
| Previous haemorrhagic stroke | 3/62 (4.8%) | 1/44 (2.3%) | 0.495 |
| Heart failure | 21/62 (33.9%) | 16/44 (36.4%) | 0.791 |
| CHA2DS2-VASc (median, IQR) | 5 (3–6) | 4 (3–6) | 0.437 |
| HAS-BLED (median, IQR) | 3 (2–3) | 3 (2–3) | 0.951 |
| Ischaemic cerebrovascular event TIA Ischaemic Stroke | 1/62 (1.6%) 61/62 (98.4%) | 2/45 (4.4%) 43/45 (95.6%) | 0.381 |
| TOAST Cardioembolic AF AF + mechanic prothesis Small-artery occlusion Other determined cause Indeterminate (AF + atherothrombosis) | 40/62 (64.5%) 33/40 (82.5%) 7/40 (17.5%) 1/62 (1.6%) 2/62 (3.2%) 19/62 (30.6%) | 33/45 (73.9%) 31/33(93.9%) 2/33 (6.1%) 1/45 (2.2%) 0/45 (0.0%) 11/45 (24.4%) | 0.540 |
| NIHSS on admission (median, IQR) | 11 (5–20) | 16 (6–21) | 0.357 |
| Systemic thrombolysis | 1/59 (1.7%) | 12/43 (27.9%) | <0.001 |
| Mechanical thrombectomy | 27/59 (45.8%) | 13/43 (30.2%) | 0.113 |
| Transthoracic echocardiography | 50/62 (80.6%) | 40/45 (88.9%) | 0.250 |
| Atrial enlargement | 40/45 (88.9%) | 35/40 (87.5%) | 0.843 |
| Normal ejection fraction | 31/46 (67.4%) | 26/36 (72.2%) | 0.637 |
| Mitral valvulopathy Stenosis Insufficiency Steno-insufficiency | 0/50 (0.0%) 34/50 (68.0%) 2/50 (4.0%) | 1/39 (2.6%) 27/39 (69.2%) 2/39 (5.1%) | 0.629 |
| Aortic Valvulopathy Stenosis Insufficiency Steno-insufficiency | 4/50 (8.0%) 15/50 (30.0%) 2/50 (4.0%) | 2/39 (5.1%) 19/39 (46.2%) 5/39 (10.3%) | 0.189 |
| Mitral prosthetic heart valves Mechanic Biologic | 5/62 (8.1%) 7/62 (11.3%) | 2/43 (4.5%) 0/43 (0.0%) | 0.052 |
| Aortic prosthetic heart valves Mechanic Biologic | 3/62 (4.8%) 10/62 (16.1%) | 2/43 (4.7%) 1/43 (2.3%) | 0.074 |
| Recommended Therapy at Discharge | ||||
|---|---|---|---|---|
| VKA | DOAC | Non-Specified OAT | ||
| Therapy on Admission | VKA (n = 20) | 45% (9/20) | 45% (9/20) | 10% (2/20) |
| DOAC (n = 12) | 16.7% (2/12) | 66.6% * (8/12) | 16.7% (2/12) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scrima, G.D.; Sarti, C.; Pracucci, G.; Nistri, R.; Rapillo, C.M.; Piccardi, B.; Stolcova, M.; Ristalli, F.; Mattesini, A.; Nozzoli, C.; et al. Ischaemic Stroke in Patients with Known Atrial Fibrillation: A Snapshot from a Large University Hospital Experience. J. Clin. Med. 2025, 14, 6012. https://doi.org/10.3390/jcm14176012
Scrima GD, Sarti C, Pracucci G, Nistri R, Rapillo CM, Piccardi B, Stolcova M, Ristalli F, Mattesini A, Nozzoli C, et al. Ischaemic Stroke in Patients with Known Atrial Fibrillation: A Snapshot from a Large University Hospital Experience. Journal of Clinical Medicine. 2025; 14(17):6012. https://doi.org/10.3390/jcm14176012
Chicago/Turabian StyleScrima, Giulia Domna, Cristina Sarti, Giovanni Pracucci, Rita Nistri, Costanza Maria Rapillo, Benedetta Piccardi, Miroslava Stolcova, Francesca Ristalli, Alessio Mattesini, Carlo Nozzoli, and et al. 2025. "Ischaemic Stroke in Patients with Known Atrial Fibrillation: A Snapshot from a Large University Hospital Experience" Journal of Clinical Medicine 14, no. 17: 6012. https://doi.org/10.3390/jcm14176012
APA StyleScrima, G. D., Sarti, C., Pracucci, G., Nistri, R., Rapillo, C. M., Piccardi, B., Stolcova, M., Ristalli, F., Mattesini, A., Nozzoli, C., Morettini, A., Moggi Pignone, A., Nencini, P., Di Mario, C., Marcucci, R., & Meucci, F., on behalf of Heart and Brain Team, Careggi University Hospital. (2025). Ischaemic Stroke in Patients with Known Atrial Fibrillation: A Snapshot from a Large University Hospital Experience. Journal of Clinical Medicine, 14(17), 6012. https://doi.org/10.3390/jcm14176012






