The Application of a Flowable Composite as a Method for Donor Site Protection After Free Gingival Graft: A Comparative Analysis of Four Techniques
Abstract
1. Introduction
- An absorbable gelatin sponge with sutures (GS).
- An absorbable gelatin sponge with sutures and cyanoacrylate tissue adhesive (GS+CTA).
- Oxidized regenerated cellulose with cyanoacrylate tissue adhesive (ORC+CTA).
- An absorbable gelatin sponge covered by a flowable resin composite and secured with sutures (GS+FRC).
2. Materials and Methods
2.1. Study Population
- Aged over 18 years;
- No history of prior palatal harvesting;
- Less than 2 mm of keratinized mucosa in the vicinity of the implant;
- Generally healthy or with well-managed systemic conditions (e.g., hypertension or hypothyroidism) that do not adversely affect healing or bleeding.
- Pregnancy or lactation;
- History of alcohol or drug abuse;
- Receipt of chemotherapy, corticosteroids, or immunosuppressants within the past 6 months;
- Coagulation disorders or current use of anticoagulant therapy;
- Poorly controlled systemic conditions (e.g., hypertension or type 2 diabetes mellitus) that may impair healing or bleeding;
- Active oral infections (bacterial, viral, or fungal);
- History of radiation therapy to the head and neck region.
2.2. Surgical Procedures
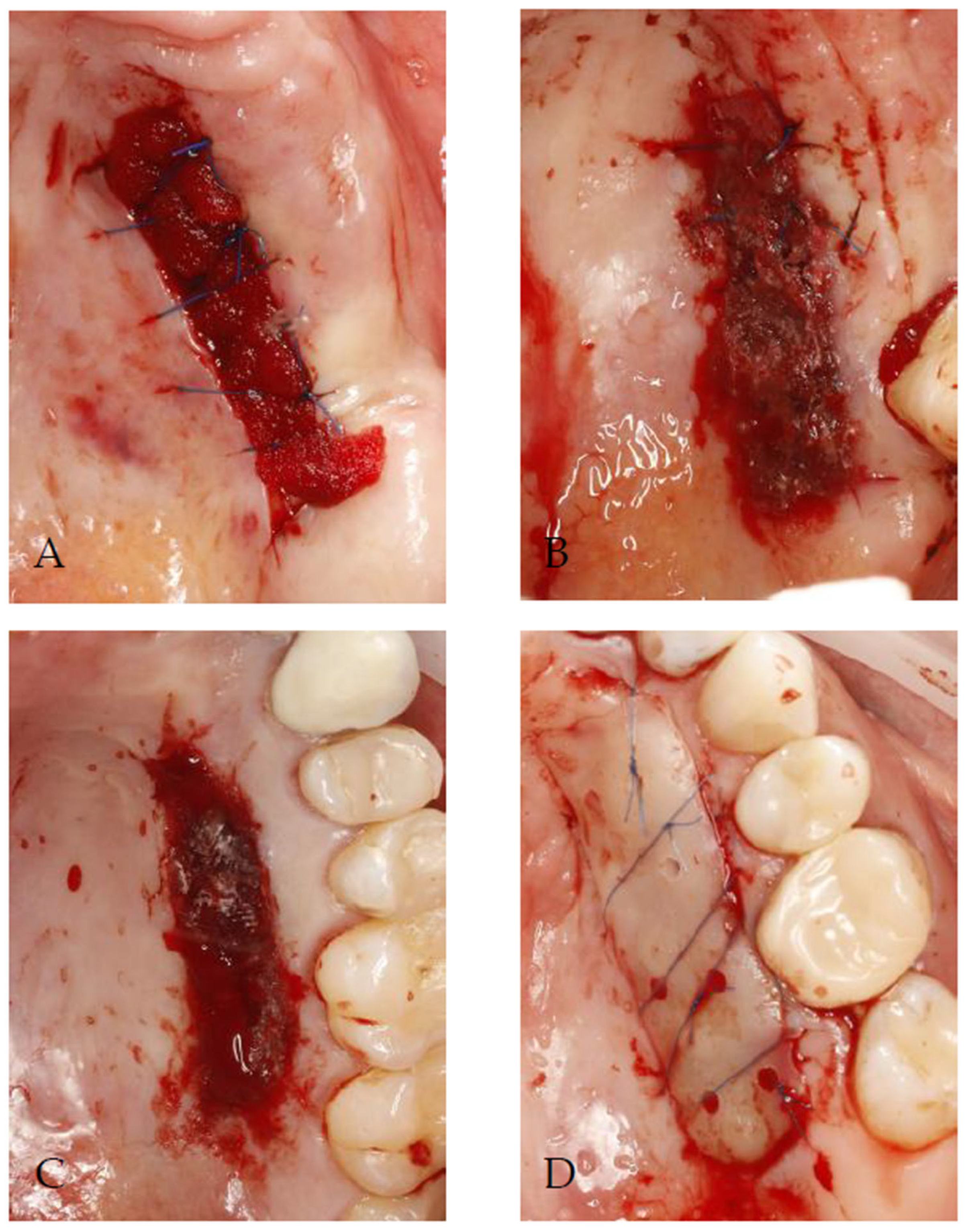
2.3. Donor Site Management
- (1)
- GS—control group (n = 18):
- -
- Absorbable gelatin sponge (Spongostan Dental, Ethicon, Cincinnati, OH, USA) stabilized by 5/0 nylon mattress sutures (Seralon, Serag-Wiessner, Naila, Germany).
- (2)
- GS+CTA—test group I (n = 20):
- -
- Absorbable gelatin sponge stabilized by 5/0 nylon mattress sutures.
- -
- Cyanoacrylate tissue adhesive (PeriAcryl®90, GluStitch, Delta, BC, Canada) as a second layer.
- (3)
- ORC+CTA—test group II (n = 19):
- -
- Oxidized regenerated cellulose (BloodSTOP®iX, LifeScience PLUS Inc., Mountain View, CA, USA).
- -
- Cyanoacrylate tissue adhesive as a second layer.
- (4)
- GS+FRC—test group III (n = 19):
- -
- Before the surgical procedure, the plate made of a flowable resin composite, in which the perforations were made, was prepared and disinfected with 0.1% chlorhexidine digluconate.
- -
- An absorbable gelatin sponge was inserted directly on the wound.
- -
- The composite plate was positioned with 5/0 nylon simple sutures passing through the perforations and stabilized with 5/0 nylon mattress sutures.
- -
- Figure 2 presents the complete procedure.
2.4. Postoperative Care
- To rinse, 0.1% chlorhexidine digluconate mouthwash (Eludril Classic, Pierre Fabre S.A., Paris, France) should be used twice daily for 14 days.
- Preoperative antibiotic prophylaxis involves taking 2000 mg of amoxicillin one hour before surgery, followed by a postoperative dose of 500 mg every eight hours for six days. For patients allergic to penicillin, 600 mg of clindamycin is administered one hour before surgery, followed by a postoperative dose of 300 mg three times a day for six days.
- Painkillers: Both 50 mg of ketoprofen and 500 mg of paracetamol are taken three times a day for three days. For the subsequent days, participants were instructed to use these medications as needed.
- Seven days after surgery, the sutures in the donor area were removed in the control group (GS) and test group I (GS+CTA). In test group III (GS+FRC), the composite plate was removed along with the sutures at the same time.
- In all groups, the sutures at the recipient site were taken out after two weeks.
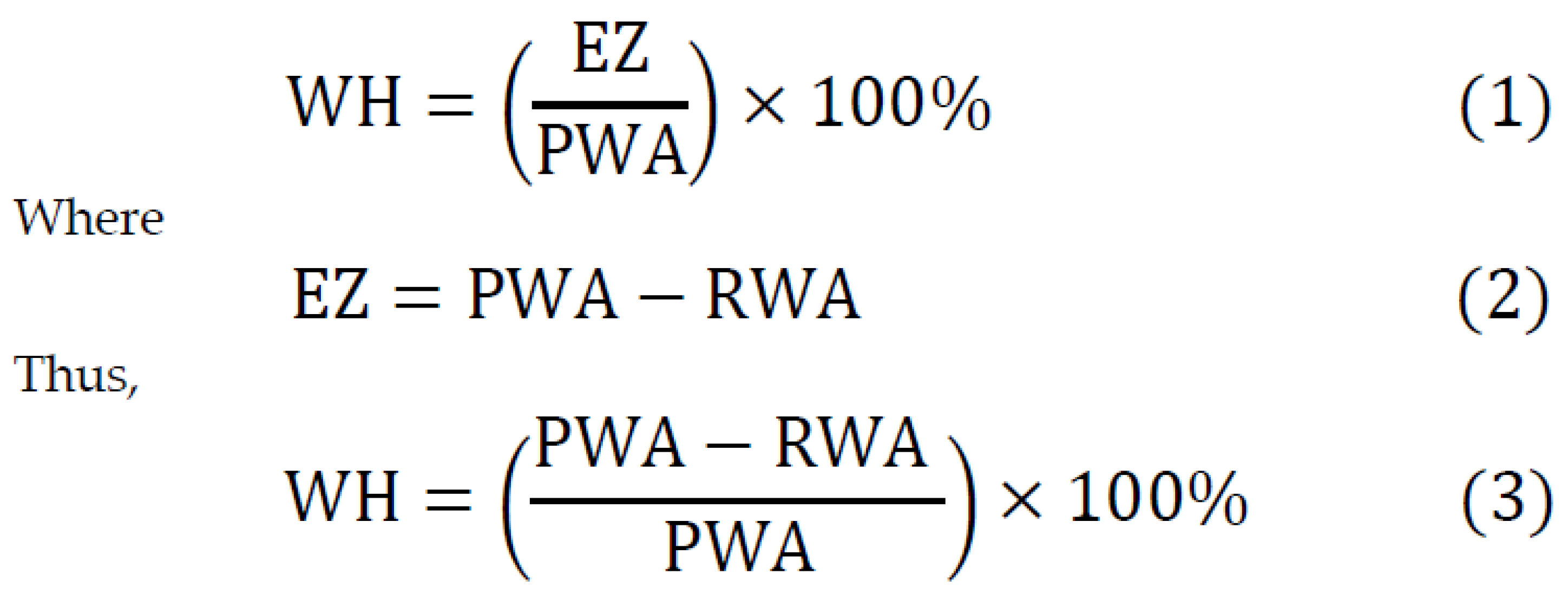
2.5. Outcome Measurements
2.6. Statistical Analysis
2.7. Use of Generative AI Tools
3. Results
4. Discussion
4.1. Pain Perception
4.2. Wound Healing
4.3. Study Limitations and Risk of Bias
5. Conclusions
- (1)
- The use of an absorbable gelatin sponge covered with a flowable resin composite and secured with sutures, despite the presented limitations, appears to be a promising method for palatal wound protection. Although this group consistently demonstrated the lowest mean pain scores, differences in pain intensity among the groups were not statistically significant. Nevertheless, it showed the most favorable outcomes in terms of wound epithelialization.
- (2)
- Suturing a prefabricated composite plate to the palatal soft tissues appears to be a viable approach, as the direct polymerization of a composite material on the palatal wound may exert cytotoxic and allergenic effects on the surrounding soft tissue.
- (3)
- No single method proved superior in preventing secondary bleeding among the evaluated approaches.
- (4)
- Further large-scale research is warranted to investigate the use of a flowable composite for palatal wound protection, particularly given the limited evidence currently available in this area. Additionally, multicenter trials and histological assessments should be considered to validate and expand upon these findings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J. Dimension of the periimplant mucosa. Biological width revisited. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef]
- Agarwal, S.; Sachdev, S.S.; Mistry, L.N.; Agrawal, S.; Deshpande, S.; Sharma, V.; Thorat, R. Soft Tissue Management in Implant Dentistry: A Comprehensive Review. Cureus 2025, 17, e79557. [Google Scholar] [CrossRef] [PubMed]
- Jose, E.P.; Paul, P.; Reche, A. Soft Tissue Management Around the Dental Implant: A Comprehensive Review. Cureus 2023, 15, e48042. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Ayukawa, Y.; Kondo, R.; Oshiro, W.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Soft tissue sealing around dental implants based on histological interpretation. J. Prosthodont. Res. 2016, 60, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ochsenbein, C. Newer Concepts of Mucogingival Surgery. J. Periodontol. 1960, 31, 175–185. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Wang, P.; Zheng, Y.; Wang, Z.; Wang, Z. The Relationship between Adequate Keratinized Mucosa and Peri-Implant Disease: A Systematic Review and Meta-Analysis. BMC Oral Health 2025, 25, 345. [Google Scholar] [CrossRef]
- Bodic, F.; Hamel, L.; Lerouxel, E.; Baslé, M.F.; Chappard, D. Bone loss and teeth. Joint Bone Spine 2005, 72, 215–221. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Li, J.; Yue, Y.; Li, J.; Wang, M.; Wei, N.; Hao, L. Mechanisms of mechanical force in periodontal homeostasis: A review. Front. Immunol. 2024, 15, 1438726. [Google Scholar] [CrossRef]
- Bressan, E.; Zucchelli, G.; Tommasato, G.; Pesce, P.; Canullo, L.; Consensus Meeting Group Iao; Grusovin, M.G. Consensus Report by the Italian Academy of Osseointegration on the Importance of Peri-Implant Soft Tissues. Medicina 2024, 60, 1393. [Google Scholar] [CrossRef]
- Punia, V.; Bhati, J.P.; Porwal, A.; Salgia, P.; Sethia, A.; Malot, S. Timing of Implant Placement in the Esthetic Zone: A Systematic Review and Meta-Analysis Comparing Immediate and Delayed Approaches. J. Popul. Ther. Clin. Pharmacol. 2025, 32, 962–973. [Google Scholar] [CrossRef]
- Wessel, J.R.; Tatakis, D.N. Patient Outcomes Following Subepithelial Connective Tissue Graft and Free Gingival Graft Procedures. J. Periodontol. 2008, 79, 425–430. [Google Scholar] [CrossRef]
- Femminella, B.; Iaconi, M.C.; Di Tullio, M.; Romano, L.; Sinjari, B.; D’Arcangelo, C.; De Ninis, P.; Paolantonio, M. Clinical Comparison of Platelet-Rich Fibrin and a Gelatin Sponge in the Management of Palatal Wounds After Epithelialized Free Gingival Graft Harvest: A Randomized Clinical Trial. J. Periodontol. 2016, 87, 103–113. [Google Scholar] [CrossRef]
- Ehab, K.; Abouldahab, O.; Hassan, A.; El-Sayed, K.M.F. Alvogyl and Absorbable Gelatin Sponge as Palatal Wound Dressings Following Epithelialized Free Gingival Graft Harvest: A Randomized Clinical Trial. Clin. Oral Investig. 2020, 24, 1517–1525. [Google Scholar] [CrossRef]
- Koca-Ünsal, R.B.; Ünsal, G.; Kasnak, G.; Fıratlı, Y.; Özcan, I.; Orhan, K.; Fıratlı, E. Ultrasonographic Evaluation of the Titanium-Prepared Platelet-Rich Fibrin Effect in Free Gingival Graft Procedures. J. Periodontol. 2022, 93, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Parlak, H.M.; Durmaz, M.H.; Bayrak, H.; Yilmaz, B.T.; Keceli, H.G. Cyanoacrylate and Hyaluronic Acid Combination on Palatal Donor Site Management After De-Epithelialized Graft Harvesting. J. Periodontol. 2023, 94, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Bahammam, M.A. Effect of Platelet-Rich Fibrin Palatal Bandage on Pain Scores and Wound Healing After Free Gingival Graft: A Randomized Controlled Clinical Trial. Clin. Oral Investig. 2018, 22, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Patarapongsanti, A.; Bandhaya, P.; Sirinirund, B.; Khongkhunthian, S.; Khongkhunthian, P. Comparison of Platelet-Rich Fibrin and Cellulose in Palatal Wounds After Graft Harvesting. J. Investig. Clin. Dent. 2019, 10, e12467. [Google Scholar] [CrossRef]
- Gatti, F.; Iorio-Siciliano, V.; Scaramuzza, E.; Tallarico, M.; Vaia, E.; Ramaglia, L.; Chiapasco, M. Patient-Reported Outcome Measures of Leucocyte- and Platelet-Rich Fibrin (L-PRF) or Hemostatic Agent Application at Palatal Donor Sites After Free Gingival Graft Harvesting: A Randomized Controlled Clinical Trial. Quintessence Int. 2023, 54, 408–417. [Google Scholar]
- Scott, S.M.H.; Lacy, J.A.; Palaiologou, A.A.; Kotsakis, G.A.; Deas, D.E.; Mealey, B.L. Donor Site Wound Healing Following Free Gingival Graft Surgery Using Platelet-Rich Fibrin: A Randomized Controlled Trial. J. Periodontol. 2024, 95, 632–639. [Google Scholar] [CrossRef]
- Rossmann, J.A.; Rees, T.D. A Comparative Evaluation of Hemostatic Agents in the Management of Soft Tissue Graft Donor Site Bleeding. J. Periodontol. 1999, 70, 1369–1375. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, A.; Puri, K.; Bansal, M.; Khatri, M. Application of Platelet-Rich Fibrin Membrane and Collagen Dressing as Palatal Bandage for Wound Healing: A Randomized Clinical Control Trial. Indian, J. Dent. Res. 2019, 30, 881–888. [Google Scholar] [PubMed]
- Spin, J.R.; Lopes de Oliveira, G.J.P.; Spin-Neto, R.; Herculano, R.D.; Marcantonio, R.A.C. Effect of Natural Latex Membranes on Wound Repair of Palate Donor Areas: A Pilot Randomized Controlled Trial Study, Including the Membranes Characterization. Mater. Today Commun. 2021, 27, 102390. [Google Scholar] [CrossRef]
- Yussif, N.; Wagih, R.; Selim, K. Propylene Mesh versus Acrylic Resin Stent for Palatal Wound Protection Following Free Gingival Graft Harvesting: A Short-Term Pilot Randomized Clinical Trial. BMC Oral Health 2021, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Meza-Mauricio, J.; Mourão, E.R.S.T.; Oliveira Marinho, K.; Vergara-Buenaventura, A.; Mendoza-Azpur, G.; Muniz, F.W.M.G.; Santamaria, M.P.; Faveri, M. Effect of Collagen Sponge and Flowable Resin Composite on Pain Management After Free Gingival Graft Harvesting: A Randomized Controlled Clinical Trial. Eur. J. Oral Sci. 2023, 131, e12935. [Google Scholar] [CrossRef]
- Keskiner, I.; Lutfioğlu, M.; Aydogdu, A.; Saygun, N.I.; Serdar, M.A. Effect of Photobiomodulation on Transforming Growth Factor-β1, Platelet-Derived Growth Factor-BB, and Interleukin-8 Release in Palatal Wounds After Free Gingival Graft Harvesting: A Randomized Clinical Study. Photomed. Laser Surg. 2016, 34, 263–271. [Google Scholar] [CrossRef]
- Bitencourt, F.V.; De David, S.C.; Schutz, J.D.S.; Neto, A.O.K.; Visioli, F.; Fiorini, T. Minimizing Patient Morbidity After Free Gingival Graft Harvesting: A Triple-Blind Randomized-Controlled Clinical Trial. Clin. Oral Implants Res. 2022, 33, 622–633. [Google Scholar] [CrossRef]
- Morshedzadeh, G.; Aslroosta, H.; Vafaei, M. Effect of GaAlAs 940 nm Photobiomodulation on Palatal Wound Healing After Free Gingival Graft Surgery: A Split-Mouth Randomized Controlled Clinical Trial. BMC Oral Health 2022, 22, 202. [Google Scholar] [CrossRef]
- Pekbağrıyanık, T.; Dadaş, F.K.; Enhoş, Ş. Effects of Non-Thermal Atmospheric Pressure Plasma on Palatal Wound Healing of Free Gingival Grafts: A Randomized Controlled Clinical Trial. Clin. Oral Investig. 2021, 25, 6269–6278. [Google Scholar] [CrossRef]
- Miguel, M.M.V.; Mathias-Santamaria, I.F.; Rossato, A.; Ferraz, L.F.F.; Figueiredo-Neto, A.M.; de Marco, A.C.; Casarin, R.C.V.; Wallet, S.M.; Tatakis, D.N.; Mathias, M.A.; et al. Microcurrent Electrotherapy Improves Palatal Wound Healing: Randomized Clinical Trial. J. Periodontol. 2021, 92, 244–253. [Google Scholar] [CrossRef]
- Sullivan, H.C.; Atkins, J.H. Free autogenous gingival grafts. I. Principles of successful grafting. Periodontics 1968, 6, 121–129. [Google Scholar]
- Huskisson, E.C. Measurement of pain. Lancet 1974, 2, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 7 April 2025).
- Esfahani, N.N.; Khorsand, A.; Salehimonfared, S.M. The influence of harvesting free gingival graft on self-reported pain perception: A randomized two-arm parallel clinical trial. J. Dent. Sci. 2021, 16, 410–416. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, F.; Caggiano, M.; Chiacchio, A.; Acerra, A.; Giordano, F. Palatal Graft Harvesting Site Healing and Pain Management: What Is the Best Choice? An Umbrella Review. Appl. Sci. 2024, 14, 5614. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Stefanini, M.; Zucchelli, G.; Giannobile, W.V.; Wang, H.L. Wound Healing Dynamics, Morbidity, and Complications of Palatal Soft-Tissue Harvesting. Periodontol 2000. 2023, 92, 90–119. [Google Scholar] [CrossRef]
- Leite, G.G.; Viana, K.S.S.; Cota, L.O.M.; Abreu, L.G.; Esteves Lima, R.P.; Costa, F.O. Efficacy of Different Interventions on the Morbidity of the Palatal Donor Area after Free Gingival Graft and Connective Tissue Graft: A Systematic Review. Jpn. Dent. Sci. Rev. 2025, 61, 31–40. [Google Scholar] [CrossRef]
- Sousa, F.; Machado, V.; Botelho, J.; Proença, L.; Mendes, J.J.; Alves, R. Effect of A-PRF Application on Palatal Wound Healing after Free Gingival Graft Harvesting: A Prospective Randomized Study. Eur. J. Dent. 2020, 14, 63–69. [Google Scholar] [CrossRef]
- Veríssimo, A.H.; Ribeiro, A.K.C.; Martins, A.R.L.A.; Gurgel, B.C.V.; Lins, R.D.A.U. Comparative Analysis of the Hemostatic, Analgesic, and Healing Effects of Cyanoacrylate on Free Gingival Graft Surgical Wounds in Donor and Recipient Areas: A Systematic Review. J. Mater. Sci. Mater. Med. 2021, 32, 98. [Google Scholar] [CrossRef]
- Silva, C.O.; Ribeiro, E.P.; Sallum, A.W.; Tatakis, D.N. Free Gingival Grafts: Graft Shrinkage and Donor-Site Healing in Smokers and Non-Smokers. J. Periodontol. 2010, 81, 692–701. [Google Scholar] [CrossRef]
- Tawfik, A. Efficacy of Free Gingival Graft in Treatment of Localized Gingival Recession in Smokers and Non-Smokers: Donor Site Healing, Graft Shrinkage and Success. Al-Azhar, J. Dent. Sci. 2020, 23, 401–407. [Google Scholar] [CrossRef]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef]
- Matsuura, T.; Komatsu, K.; Choi, K.; Suzumura, T.; Cheng, J.; Chang, T.-L.; Chao, D.; Ogawa, T. Conditional Mitigation of Dental-Composite Material-Induced Cytotoxicity by Increasing the Cure Time. J. Funct. Biomater. 2023, 14, 119. [Google Scholar] [CrossRef]
- Laguna-Martos, M.; Cascos, R.; Iglesias-Velázquez, Ó.; Gómez-Polo, M.; Vasquez-Ramos, S.; Castro-Calderón, A. Technique to Protect the Palatal Donor Area after Taking a Free Gingival Graft: The Patchwork Technique. J. Oral Implantol. 2025, 51, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Basma, H.S.; Saleh, M.H.A.; Abou-Arraj, R.V.; Imbrogno, M.; Ravida, A.; Wang, H.L.; Li, P.; Geurs, N. Patient-Reported Outcomes of Palatal Donor Site Healing Using Four Different Wound Dressing Modalities Following Free Epithelialized Mucosal Grafts: A Four-Arm Randomized Controlled Clinical Trial. J. Periodontol. 2023, 94, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Ustaoğlu, G.; Ercan, E.; Tunali, M. The role of titanium-prepared platelet-rich fibrin in palatal mucosal wound healing and histoconduction. Acta Odontol. Scand. 2016, 74, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, M.; Ucak, O.; Alkaya, B.; Keceli, S.; Seydaoglu, G.; Haytac, M.C. Effects of Platelet-Rich Fibrin on Palatal Wound Healing after Free Gingival Graft Harvesting: A Comparative Randomized Controlled Clinical Trial. Int. J. Periodontics Restor. Dent. 2017, 37, e270–e278. [Google Scholar] [CrossRef]
- Ustaoglu, G.; Ercan, E.; Tunali, M. Low-Level Laser Therapy in Enhancing Wound Healing and Preserving Tissue Thickness at Free Gingival Graft Donor Sites: A Randomized, Controlled Clinical Study. Photomed. Laser Surg. 2017, 35, 223–230. [Google Scholar] [CrossRef]
- Isler, S.C.; Uraz, A.; Guler, B.; Ozdemir, Y.; Cula, S.; Cetiner, D. Effects of Laser Photobiomodulation and Ozone Therapy on Palatal Epithelial Wound Healing and Patient Morbidity. Photomed. Laser Surg. 2018, 36, 571–580. [Google Scholar] [CrossRef]
- Menchini-Fabris, G.B.; Cosola, S.; Toti, P.; Hwan Hwang, M.; Crespi, R.; Covani, U. Immediate Implant and Customized Healing Abutment for a Periodontally Compromised Socket: 1-Year Follow-Up Retrospective Evaluation. J. Clin. Med. 2023, 12, 2783. [Google Scholar] [CrossRef]
- Perez, A.; Caiazzo, A.; Valente, N.A.; Toti, P.; Alfonsi, F.; Barone, A. Standard vs. customized healing abutments with simultaneous bone grafting for tissue changes around immediate implants: 1-year outcomes from a randomized clinical trial. Clin. Implant Dent. Relat. Res. 2020, 22, 42–53. [Google Scholar] [CrossRef]
- Chokaree, P.; Poovarodom, P.; Chaijareenont, P.; Yavirach, A.; Rungsiyakull, P. Biomaterials and Clinical Applications of Customized Healing Abutment—A Narrative Review. J. Funct. Biomater. 2022, 13, 291. [Google Scholar] [CrossRef]
- Patel, P.V.; Kumar, S.; Vidya, G.D.; Patel, A.; Holmes, J.C.; Kumar, V. Cytological Assessment of Healing Palatal Donor Site Wounds and Grafted Gingival Wounds after Application of Ozonated Oil: An Eighteen-Month Randomized Controlled Clinical Trial. Acta Cytol. 2012, 56, 277–284. [Google Scholar] [CrossRef]

| Parameter | GS (N = 18) | GS+CTA (N = 20) | ORC+CTA (N = 19) | GS+FRC (N = 19) | Total (N = 76) | p | |
|---|---|---|---|---|---|---|---|
| Sex | Female | 9 (50.00%) | 14 (70.00%) | 10 (52.63%) | 13 (68.42%) | 46 (60.53%) | p = 0.461 |
| Male | 9 (50.00%) | 6 (30.00%) | 9 (47.37%) | 6 (31.58%) | 30 (39.47%) | ||
| Age | Mean (SD) | 55.44 (10.07) | 55.85 (9.23) | 50.42 (15.95) | 54 (8.14) | 53.93 (11.23) | p = 0.734 |
| Median (quartiles) | 59.5 (49.25–63.75) | 54 (49.75–64) | 49 (40.5–63.5) | 53 (50.5–61) | 53.5 (48–63) | ||
| Range | 36–67 | 38–72 | 20–72 | 36–66 | 20–72 | ||
| n | 18 | 20 | 19 | 19 | 76 | ||
| Side | Left | 4 (22.22%) | 6 (30.00%) | 14 (73.68%) | 11 (57.89%) | 35 (46.05%) | p = 0.004 * |
| Right | 14 (77.78%) | 14 (70.00%) | 5 (26.32%) | 8 (42.11%) | 41 (53.95%) | ||
| Smoking | Smokers | 2 (11.11%) | 4 (20.00%) | 5 (26.32%) | 3 (15.79%) | 14 (18.42%) | p = 0.709 |
| Non-smokers | 16 (88.89%) | 16 (80.00%) | 14 (73.68%) | 16 (84.21%) | 62 (81.58%) | ||
| Parameter | Method | N | Mean | SD | Median | Min | Max | Q1 | Q3 | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Peak pain intensity | GS | 18 | 4.33 | 2.59 | 5 | 0 | 9 | 2 | 6 | p = 0.608 |
| GS+CTA | 20 | 4.45 | 2.82 | 5 | 1 | 10 | 2 | 6 | ||
| ORC+CTA | 19 | 5.21 | 2.72 | 5 | 0 | 9 | 4 | 7 | ||
| GS+FRC | 19 | 4.11 | 2.16 | 3 | 1 | 8 | 3 | 5.5 | ||
| Average pain intensity Days 1–14 | GS | 18 | 1.75 | 1.45 | 1.39 | 0 | 4.5 | 0.45 | 3.11 | p = 0.44 |
| GS+CTA | 20 | 2.01 | 1.62 | 1.86 | 0.07 | 6.64 | 0.64 | 2.95 | ||
| ORC+CTA | 19 | 2.08 | 1.49 | 1.79 | 0 | 4.29 | 1 | 3.5 | ||
| GS+FRC | 19 | 1.35 | 0.97 | 1.29 | 0.07 | 3 | 0.46 | 2.14 | ||
| Average pain intensity Days 1–7 | GS | 18 | 2.81 | 2.18 | 2.5 | 0 | 6.43 | 0.89 | 4.57 | p = 0.367 |
| GS+CTA | 20 | 3.09 | 2.46 | 3.43 | 0.14 | 9.14 | 0.86 | 4.18 | ||
| ORC+CTA | 19 | 3.35 | 2.23 | 3 | 0 | 7.14 | 1.86 | 5.07 | ||
| GS+FRC | 19 | 2.09 | 1.46 | 1.86 | 0.14 | 4.43 | 0.71 | 3.14 | ||
| Average pain intensity Days 8–14 | GS | 18 | 0.69 | 0.94 | 0.21 | 0 | 3.29 | 0 | 0.96 | p = 0.726 |
| GS+CTA | 20 | 0.93 | 1.05 | 0.64 | 0 | 4.14 | 0.21 | 1.32 | ||
| ORC+CTA | 19 | 0.8 | 0.96 | 0.29 | 0 | 2.86 | 0 | 1.71 | ||
| GS+FRC | 19 | 0.62 | 0.79 | 0.29 | 0 | 3 | 0 | 0.93 |
| Method | N | Wound Healing [%] | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Min | Max | Q1 | Q3 | |||
| GS | 18 | 63.05 | 19.72 | 65.71 | 28.57 | 92 | 57.14 | 76.45 | p = 0.003 * |
| GS+CTA | 20 | 50.36 | 35.84 | 43.65 | 4.76 | 100 | 17.94 | 86.81 | |
| ORC+CTA | 19 | 43.66 | 25.74 | 35.71 | 9.09 | 100 | 26.39 | 56.16 | |
| GS+FRC | 19 | 75.95 | 18.75 | 76.19 | 42.18 | 100 | 61.30 | 92.54 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowski, T.; Jankowska, A.; Kazimierczak, W.; Janiszewska-Olszowska, J. The Application of a Flowable Composite as a Method for Donor Site Protection After Free Gingival Graft: A Comparative Analysis of Four Techniques. J. Clin. Med. 2025, 14, 6009. https://doi.org/10.3390/jcm14176009
Jankowski T, Jankowska A, Kazimierczak W, Janiszewska-Olszowska J. The Application of a Flowable Composite as a Method for Donor Site Protection After Free Gingival Graft: A Comparative Analysis of Four Techniques. Journal of Clinical Medicine. 2025; 14(17):6009. https://doi.org/10.3390/jcm14176009
Chicago/Turabian StyleJankowski, Tomasz, Agnieszka Jankowska, Wojciech Kazimierczak, and Joanna Janiszewska-Olszowska. 2025. "The Application of a Flowable Composite as a Method for Donor Site Protection After Free Gingival Graft: A Comparative Analysis of Four Techniques" Journal of Clinical Medicine 14, no. 17: 6009. https://doi.org/10.3390/jcm14176009
APA StyleJankowski, T., Jankowska, A., Kazimierczak, W., & Janiszewska-Olszowska, J. (2025). The Application of a Flowable Composite as a Method for Donor Site Protection After Free Gingival Graft: A Comparative Analysis of Four Techniques. Journal of Clinical Medicine, 14(17), 6009. https://doi.org/10.3390/jcm14176009








