The Future of Oncology in Psychiatric Medications
Abstract
1. Introduction
2. Methods
3. Advantages and Disadvantages of Using Psychiatric Drugs in Oncology
3.1. Disadvantages
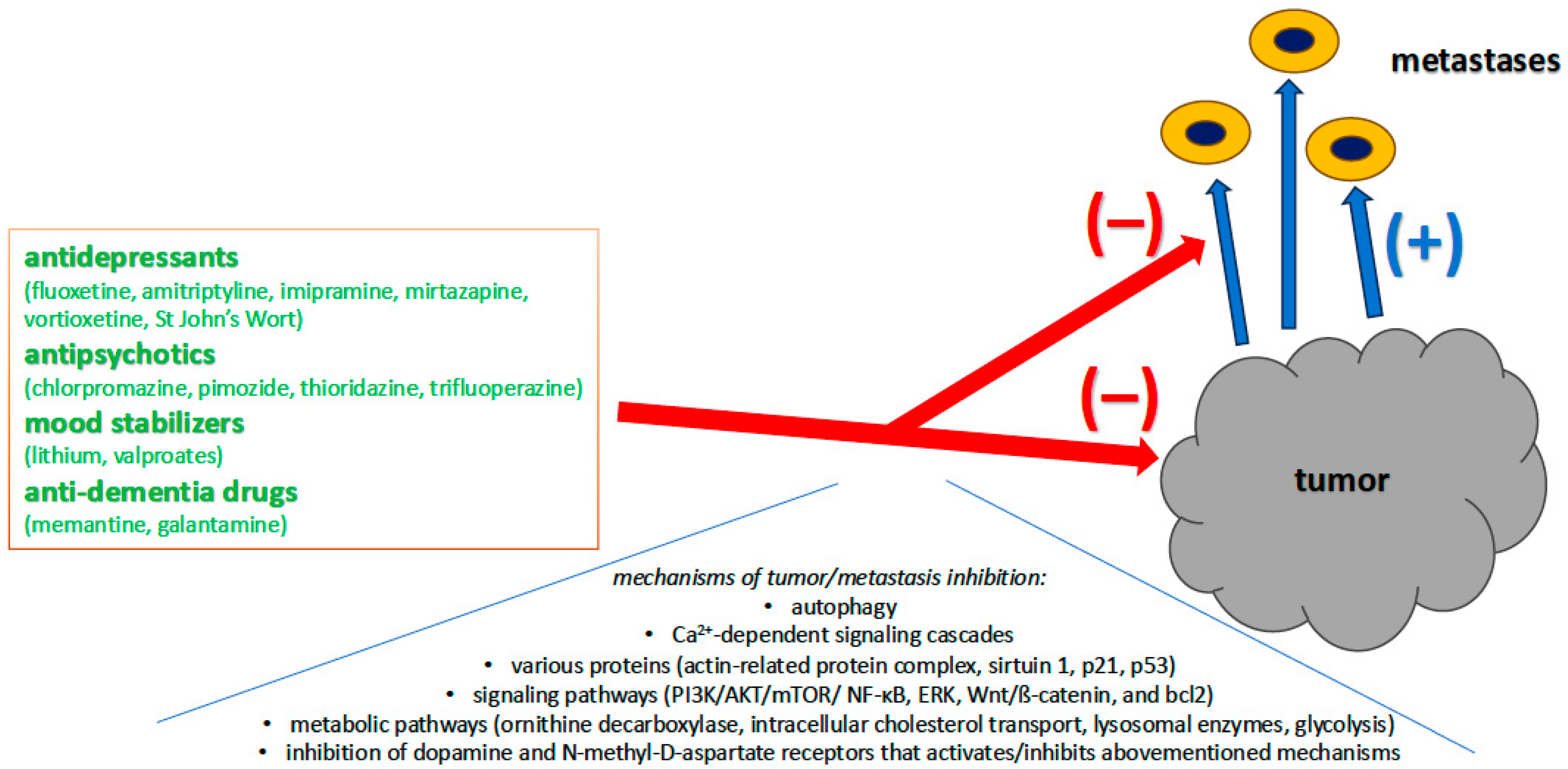
3.2. Advantages: Psychiatric Drugs as a New Frontier in Oncology
| Antidepressive Drug | Psychiatric Disorder Treated | Cancer Treated | Anticancer Mechanism of Psychiatric Drugs | |
|---|---|---|---|---|
| selective serotonin reuptake inhibitors (SSRIs) | fluoxetine |
|
|
|
| paroxetine |
| |||
| sertraline |
| |||
| citalopram |
| |||
| tricyclic antidepressants (TCAs) | amitryptyline |
| ||
| imipramine |
| |||
| serotonin–norepinephrine reuptake inhibitors (SNRIs) | duloxetine |
| ||
| tetracyclic antidepressants | mirtazapine |
| ||
| monoamine oxidase inhibitor (MAOI) | phenelzine |
| ||
| serotonin modulator and stimulator (SMS) | vortioxetine |
|
| |
| herbs | St. John’s Wort |
|
| |
| Antipsychotic Drug | Psychiatric Disorder Treated | Cancer Treated | Anticancer Mechanism of Psychiatric Drugs |
|---|---|---|---|
| aripiprazole |
|
|
|
| brexpiprazole |
| ||
| chlorpromazine |
| ||
| chlorprothixene |
| ||
| clozapine |
| ||
| fluphenazine |
| ||
| flupenthixol |
| ||
| fluspirilene |
| ||
| haloperidol |
| ||
| iloperidone |
| ||
| olanzapine |
| ||
| penfluridol |
| ||
| perphenazine |
| ||
| pimavanserin |
| ||
| pimozide |
| ||
| prochlorperazine |
| ||
| quetiapine |
| ||
| risperidone |
| ||
| sertindole |
| ||
| spiperone |
| ||
| sulpiryde |
| ||
| thioridazine |
| ||
| trifluoperazine |
| ||
| zuclopenthixol |
|
| Psychiatric Drug | Psychiatric Disorder Treated | Cancer Treated | Anticancer Mechanism of Psychiatric Drugs | |
|---|---|---|---|---|
| mood stabilizers | lithium |
|
|
|
| valproates |
|
| ||
| antidementia drugs | memantine |
|
|
|
| galantamine |
|
| ||
4. Repurposing
5. Limitations
6. Future Directions
7. Discussion
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| BAX | Apoptosis regulator protein |
| Bcl2 | B-cell lymphoma 2 protein |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BPNT1 | Bisphosphate 3-primenucleotidase |
| Ca2+ | Calcium ion |
| CBT | Cognitive–behavioral therapy |
| CD133 | antigen (prominin-1) encoded by the PROM1 gene |
| CREB | Camp-Response-Element-Binding Protein |
| c-Myc | A family of regulator genes |
| CYP2D6 | Cytochrome P450 2D6 |
| Eag1 | Ether-à-go-go1 K+ channel |
| ERK | Extracellular signal-regulated kinases |
| ESL-1 | Cysteine-rich FGF receptor and E-selectin-ligand 1 |
| FGFR | Fibroblast growth factor receptor |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| GLG1 | Golgi glycoprotein 1 |
| GSK-3 | Glycogen synthase kinase-3 |
| HDAC | Histone deacetylase |
| HSPGs | Heparan sulfate proteoglycans |
| IFN | Interferon |
| IL | Interleukin |
| IMCP | Individual meaning-centered psychotherapy |
| JNK/c-Jun | c-Jun N-terminal kinases |
| K-Ras | Kirsten rat sarcoma virus protein |
| KLF5 | Krüppel-like factor 5 |
| MBCT | Mindfulness-Based Cognitive Therapy |
| MDM2 | Mouse Double Minute 2 protein |
| MMP-9 | Matrix metallopeptidase 9 |
| mTOR | The mammalian target of rapamycin |
| MYCN | N-myc proto-oncogene protein |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NMDA | N-methyl-D-aspartate |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| NUPR1 | Nuclear Protein 1-transcriptional regulator |
| p53 | Tumor protein p53 |
| PAK 4 | Serine/threonine-protein kinase 4 |
| PI3K/AKT | Phosphoinositide 3-kinase signaling pathway |
| PKM2 | Pyruvate kinase M2 isoform |
| REST | Repressor element 1 silencing transcription factor |
| ROS | Reactive oxygen species |
| SIRT1 | Silent mating type information regulation homolog for Sirtuin |
| SNAI1 | Zinc finger protein |
| SMI | Severe mental illness |
| SOX2 | Single-exon nuclear transcription factor 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TCTP | Translationally controlled tumor protein |
| TGF | Transforming growth factor |
| TIMP1 | Tissue Inhibitor of Metalloproteinases 1 |
| TORC1 | Transducer of regulated CREB activity 1 |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless-related integration site |
References
- Steinhoff, T.; Guyer, D.; Almhanna, K. Is every psychiatrist an oncology psychiatrist?—Special needs for special populations: A scoping review. Ann. Palliat. Med. 2024, 13, 287–300. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef]
- Mehnert-Theuerkauf, A.; Hufeld, J.M.; Esser, P.; Goerling, U.; Hermann, M.; Zimmermann, T.; Reuter, H.; Ernst, J. Prevalence of mental disorders, psychosocial distress, and perceived need for psychosocial support in cancer patients and their relatives stratified by biopsychosocial factors: Rationale, study design, and methods of a prospective multi-center observational cohort study (LUPE study). Front. Psychol. 2023, 14, 1125545. [Google Scholar] [CrossRef]
- Ge, M.W.; Sheng, J.; Shen, L.T.; Hu, F.H.; Jia, Y.J.; Ur-Rehman, A.; Li, W.; Lan, J.Z.; Liu, P.; Chen, H.L. Global Prevalence of Mental Health Problems Among Cancer Survivors: A Meta-Analysis From 31 Countries. Psychooncology 2025, 34, e70077. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Lai, A.G. Cumulative burden of psychiatric disorders and self-harm across 26 adult cancers. Nat. Med. 2022, 28, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Kim, H.C.; Lee, S. Psychosocial impact of cancer patients on their family members. Cancer Res. Treat. 2013, 45, 226–233. [Google Scholar] [CrossRef]
- Miovic, M.; Block, S. Psychiatric disorders in advanced cancer. Cancer 2007, 110, 1665–1676. [Google Scholar] [CrossRef]
- Guy, M.; Stern, T.A. The desire for death in the setting of terminal illness: A case discussion. Prim. Care Companion J. Clin. Psychiatry 2006, 8, 299–305. [Google Scholar] [CrossRef]
- Launders, N.; Scolamiero, L.; Osborn, D.P.J.; Hayes, J.F. Cancer rates and mortality in people with severe mental illness: Further evidence of lack of parity. Schizophr. Res. 2022, 246, 260–267. [Google Scholar] [CrossRef]
- Singh, A.K.; Chatterjee, U.; MacDonald, C.R.; Repasky, E.A.; Halbreich, U. Psychosocial stress and immunosuppression in cancer: What can we learn from new research? BJPsych Adv. 2021, 27, 187–197. [Google Scholar] [CrossRef]
- Prince, M.; Patel, V.; Saxena, S.; Maj, M.; Maselko, J.; Phillips, M.R.; Rahman, A. No health without mental health. Lancet 2007, 370, 859–877. [Google Scholar] [CrossRef] [PubMed]
- Turossi-Amorim, E.D.; Camargo, B.; do Nascimento, D.Z.; Schuelter-Trevisol, F. Potential Drug Interactions Between Psychotropics and Intravenous Chemotherapeutics Used by Patients with Cancer. J. Pharm. Technol. 2022, 38, 159–168. [Google Scholar] [CrossRef]
- Grassi, L.; McFarland, D.; Riba, M.; Ferrara, M.; Zaffarami, G.; Belvederi Murri, M.; Cruciata, M.; Caruso, R. The Challenging Problems of Cancer and Serious Mental Illness. Curr. Psychiatry Rep. 2025, 27, 41–57. [Google Scholar] [CrossRef]
- Anghel, T.; Melania, B.L.; Costea, I.; Albai, O.; Marinca, A.; Levai, C.M.; Hogea, L.M. Review of Psychological Interventions in Oncology: Current Trends and Future Directions. Medicina 2025, 61, 279. [Google Scholar] [CrossRef]
- Skånland, S.S.; Cieślar-Pobuda, A. Off-label uses of drugs for depression. Eur. J. Pharmacol. 2019, 865, 172732. [Google Scholar] [CrossRef]
- Fuentes, A.V.; Pineda, M.D.; Venkata, K.C.N. Comprehension of Top 200 Prescribed Drugs in the US as a Resource for Pharmacy Teaching, Training and Practice. Pharmacy 2018, 6, 43. [Google Scholar] [CrossRef]
- Fava, G.A. May antidepressant drugs worsen the conditions they are supposed to treat? The clinical foundations of the oppositional model of tolerance. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320970325. [Google Scholar] [CrossRef]
- Vlachos, N.; Lampros, M.; Voulgaris, S.; Alexiou, G.A. Repurposing Antipsychotics for Cancer Treatment. Biomedicines 2021, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Lampros, M.; Vlachos, N.; Lianos, G.D.; Bali, C.; Alexiou, G.A. Editorial: Drug repurposing for cancer treatment: Current and future directions. Front. Oncol. 2025, 15, 1550672. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S. Treatment of cancer with antipsychotic medications: Pushing the boundaries of schizophrenia and cancer. Neurosci. Biobehav. Rev. 2022, 141, 104809. [Google Scholar] [CrossRef] [PubMed]
- Varalda, M.; Antona, A.; Bettio, V.; Roy, K.; Vachamaram, A.; Yellenki, V.; Massarotti, A.; Baldanzi, G.; Capello, D. Psychotropic Drugs Show Anticancer Activity by Disrupting Mitochondrial and Lysosomal Function. Front. Oncol. 2020, 10, 562196. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A. Brain drugs can now cross the once impenetrable blood-brain barrier. Nature 2025, 641, 1086–1088. [Google Scholar] [CrossRef]
- Lee, S.; Weiss, T.; Bühler, M.; Mena, J.; Lottenbach, Z.; Wegmann, R.; Sun, M.; Bihl, M.; Augustynek, B.; Baumann, S.P.; et al. High-throughput identification of repurposable neuroactive drugs with potent anti-glioblastoma activity. Nat. Med. 2024, 30, 3196–3208. [Google Scholar] [CrossRef]
- Zheng, Y.; Chang, X.; Huang, Y.; He, D. The application of antidepressant drugs in cancer treatment. Biomed. Pharmacother. 2023, 157, 113985. [Google Scholar] [CrossRef]
- Boursi, B.; Lurie, I.; Mamtani, R.; Haynes, K.; Yang, Y.X. Anti-depressant therapy and cancer risk: A nested case-control study. Eur. Neuropsychopharmacol. 2015, 25, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Lianos, G.D.; Alexiou, G.A.; Rausei, S.; Galani, V.; Mitsis, M.; Kyritsis, A.P. Repurposing antipsychotic drugs for cancer treatment: Current evidence and future perspectives. Expert Rev. Anticancer Ther. 2022, 22, 131–134. [Google Scholar] [CrossRef]
- Kline, C.L.B.; Ralff, M.D.; Lulla, A.R.; Wagner, J.M.; Abbosh, P.H.; Dicker, D.T.; Allen, J.E.; El-Deiry, W.S. Role of Dopamine Receptors in the Anticancer Activity of ONC201. Neoplasia 2018, 20, 80–91. [Google Scholar] [CrossRef]
- Amerio, A.; Gálvez, J.F.; Odone, A.; Dalley, S.A.; Ghaemi, S.N. Carcinogenicity of psychotropic drugs: A systematic review of US Food and Drug Administration-required preclinical in vivo studies. Aust. New Zealand J. Psychiatry 2015, 49, 686–696. [Google Scholar] [CrossRef]
- Villegas-Vázquez, E.Y.; Quintas-Granados, L.I.; Cortés, H.; González-Del Carmen, M.; Leyva-Gómez, G.; Rodríguez-Morales, M.; Bustamante-Montes, L.P.; Silva-Adaya, D.; Pérez-Plasencia, C.; Jacobo-Herrera, N.; et al. Lithium: A Promising Anticancer Agent. Life 2023, 13, 537. [Google Scholar] [CrossRef]
- Aron, L.; Ngian, Z.K.; Qiu, C.; Choi, J.; Liang, M.; Drake, D.M.; Hamplova, S.E.; Lacey, E.K.; Roche, P.; Yuan, M.; et al. Lithium deficiency and the onset of Alzheimer’s disease. Nature 2025. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, X.; Yu, B. Repurposing antidepressants for anticancer drug discovery. Drug Discov. Today 2022, 27, 1924–1935. [Google Scholar] [CrossRef]
- Chen, V.C.; Hsu, T.C.; Lin, C.F.; Huang, J.Y.; Chen, Y.L.; Tzang, B.S.; McIntyre, R.S. Association of Risperidone with Gastric Cancer: Triangulation Method from Cell Study, Animal Study, and Cohort Study. Front. Pharmacol. 2022, 13, 846455. [Google Scholar] [CrossRef]
- Min, H.Y.; Lee, H.Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Fini, E.; Calabrò, P.F.; Carli, M.; Scarselli, M.; Bocci, G. Valproate and lithium: Old drugs for new pharmacological approaches in brain tumors? Cancer Lett. 2023, 560, 216125. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, F.; Hayakawa, S.; Kawashima, S.; Asakura, T.; Oishi, Y. Antitumor effect of memantine is related to the formation of the splicing isoform of GLG1, a decoy FGF-binding protein. Int. J. Oncol. 2022, 61, 80. [Google Scholar] [CrossRef]
- Liu, C.T.; Yang, C.C.; Chien, W.C.; Chung, C.H.; Tsai, C.S.; Tsai, Y.T.; Lin, C.Y.; Lin, Y.C.; Chen, Y.S.; Tzeng, N.S. Association between long-term usage of acetylcholinesterase inhibitors and lung cancer in the elderly: A nationwide cohort study. Sci. Rep. 2022, 12, 3531. [Google Scholar] [CrossRef] [PubMed]
- Obreshkova, D.; Atanasov, P.; Chaneva, M.; Ivanova, S.; Balkanski, S.; Peikova, L. Cytotoxic activity of Galantamine hydrobromide against HeLa cell line. Pharmacia 2023, 70, 619–623. [Google Scholar] [CrossRef]
- Ma, L.; Tan, E.C.K.; Goudey, B.; Jin, L.; Pan, Y. Unraveling the bidirectional link between cancer and dementia and the impact of cancer therapies on dementia risk: A systematic review and meta-analysis. Alzheimer’s Dement. 2025, 21, e14540. [Google Scholar] [CrossRef]
- Pace, A.; Lombardi, G.; Villani, V.; Benincasa, D.; Abbruzzese, C.; Cestonaro, I.; Corrà, M.; Padovan, M.; Cerretti, G.; Caccese, M.; et al. Efficacy and safety of chlorpromazine as an adjuvant therapy for glioblastoma in patients with unmethylated MGMT gene promoter: RACTAC, a phase II multicenter trial. Front. Oncol. 2023, 13, 1320710. [Google Scholar] [CrossRef]
- Fond, G.; Macgregor, A.; Attal, J.; Larue, A.; Brittner, M.; Ducasse, D.; Capdevielle, D. Antipsychotic drugs: Pro-cancer or anti-cancer? A systematic review. Med. Hypotheses 2012, 79, 38–42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waszkiewicz, N. The Future of Oncology in Psychiatric Medications. J. Clin. Med. 2025, 14, 6003. https://doi.org/10.3390/jcm14176003
Waszkiewicz N. The Future of Oncology in Psychiatric Medications. Journal of Clinical Medicine. 2025; 14(17):6003. https://doi.org/10.3390/jcm14176003
Chicago/Turabian StyleWaszkiewicz, Napoleon. 2025. "The Future of Oncology in Psychiatric Medications" Journal of Clinical Medicine 14, no. 17: 6003. https://doi.org/10.3390/jcm14176003
APA StyleWaszkiewicz, N. (2025). The Future of Oncology in Psychiatric Medications. Journal of Clinical Medicine, 14(17), 6003. https://doi.org/10.3390/jcm14176003






